Introduction
Obsessive compulsive disorder (OCD) is a neuropsychiatric disorder associating with persistent intrusive thoughts (obsessions) and/or repetitive behaviors (compulsions), and the lifetime prevalence is estimated to be 2–3% worldwide (Weissman et al. Reference Weissman, Bland, Canino, Greenwald, Hwu, Lee, Newman, Oakley-Browne, Rubio-Stipec and Wickramaratne1994). Empirical findings from family studies have suggested that the first-degree relatives of patients with OCD have almost 3–12 times more risk than the general population of developing OCD (Pauls et al. Reference Pauls, Alsobrook, Goodman, Rasmussen and Leckman1995; Nestadt et al. Reference Nestadt, Samuels, Riddle, Bienvenu, Liang, LaBuda, Walkup, Grados and Hoehn-Saric2000; Hanna et al. Reference Hanna, Veenstra-VanderWeele, Cox, Boehnke, Himle, Curtis, Leventhal and Cook2002). The concordance rate for OCD is also greater among monozygotic twins (80–87%) than dizygotic twins (47–50%) (Inouye, Reference Inouye1965; O'Donnell, Reference O'Donnell1970).
There is increasing evidence to suggest that OCD is associated with altered connectivity within the default mode network (DMN) (Harrison et al. Reference Harrison, Soriano-Mas, Pujol, Ortiz, Lopez-Sola, Hernandez-Ribas, Deus, Alonso, Yucel, Pantelis, Menchon and Cardoner2009; Fitzgerald et al. Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor, Welsh, Hanna and Taylor2010; Jang et al. Reference Jang, Kim, Jung, Choi, Jung, Lee, Choi, Kang and Kwon2010; Sakai et al. Reference Sakai, Narumoto, Nishida, Nakamae, Yamada, Nishimura and Fukui2010; Stern et al. Reference Stern, Fitzgerald, Welsh, Abelson and Taylor2012), which is thought to be the backbone of the intrinsic functional architecture (Vincent et al. Reference Vincent, Patel, Fox, Snyder, Baker, Van Essen, Zempel, Snyder, Corbetta and Raichle2007; Hagmann et al. Reference Hagmann, Cammoun, Gigandet, Meuli, Honey, Wedeen and Sporns2008; Biswal et al. Reference Biswal, Mennes, Zuo, Gohel, Kelly, Smith, Beckmann, Adelstein, Buckner, Colcombe, Dogonowski, Ernst, Fair, Hampson, Hoptman, Hyde, Kiviniemi, Kotter, Li, Lin, Lowe, Mackay, Madden, Madsen, Margulies, Mayberg, McMahon, Monk, Mostofsky, Nagel, Pekar, Peltier, Petersen, Riedl, Rombouts, Rypma, Schlaggar, Schmidt, Seidler, Siegle, Sorg, Teng, Veijola, Villringer, Walter, Wang, Weng, Whitfield-Gabrieli, Williamson, Windischberger, Zang, Zhang, Castellanos and Milham2010; Zuo et al. Reference Zuo, Ehmke, Mennes, Imperati, Castellanos, Sporns and Milham2012) and relevant to brain development (Dosenbach et al. Reference Dosenbach, Nardos, Cohen, Fair, Power, Church, Nelson, Wig, Vogel, Lessov-Schlaggar, Barnes, Dubis, Feczko, Coalson, Pruett, Barch, Petersen and Schlaggar2010; Power et al. Reference Power, Fair, Schlaggar and Petersen2010) and disorders (Broyd et al. Reference Broyd, Demanuele, Debener, Helps, James and Sonuga-Barke2009). This network is more active when the brain is at a ‘resting’ state and has been implicated in the cognitive functions associated with self-referential mental activity (Gusnard et al. Reference Gusnard, Akbudak, Shulman and Raichle2001), the extraction of episodic memory (Cabeza et al. Reference Cabeza, Dolcos, Graham and Nyberg2002), imagining the future (Schacter et al. Reference Schacter, Addis and Buckner2007) and mind wandering (Mason et al. Reference Mason, Norton, Van Horn, Wegner, Grafton and Macrae2007). DMN regions include the posterior cingulate cortex (PCC), precuneus, medial prefrontal cortex, ventral anterior cingulate cortex and lateral parietal cortex (Raichle et al. Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman2001). Several studies have shown that OCD patients demonstrate abnormal DMN functional connectivity (Harrison et al. Reference Harrison, Soriano-Mas, Pujol, Ortiz, Lopez-Sola, Hernandez-Ribas, Deus, Alonso, Yucel, Pantelis, Menchon and Cardoner2009; Fitzgerald et al. Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor, Welsh, Hanna and Taylor2010, Reference Fitzgerald, Welsh, Stern, Angstadt, Hanna, Abelson and Taylor2011; Jang et al. Reference Jang, Kim, Jung, Choi, Jung, Lee, Choi, Kang and Kwon2010; Sakai et al. Reference Sakai, Narumoto, Nishida, Nakamae, Yamada, Nishimura and Fukui2010; Hou et al. Reference Hou, Wu, Lin, Wang, Zhou, Guo, Gu, He, Ahmed, Hu, Qu and Li2012; Stern et al. Reference Stern, Fitzgerald, Welsh, Abelson and Taylor2012). For instance, Harrison et al. (Reference Harrison, Soriano-Mas, Pujol, Ortiz, Lopez-Sola, Hernandez-Ribas, Deus, Alonso, Yucel, Pantelis, Menchon and Cardoner2009) reported that OCD patients had increased functional connectivity along a ventral corticostriatal axis within the DMN and decreased functional connectivity in the dorsal striatum within the lateral prefrontal cortex and the ventral striatum within the midbrain ventral tegmental area by using the striatum seed regions. Jang et al. (Reference Jang, Kim, Jung, Choi, Jung, Lee, Choi, Kang and Kwon2010) found that OCD patients had less functional connectivity with the PCC in the anterior cingulate cortex, middle frontal gyrus, and putamen. Taken together, these findings suggest that abnormal functional connectivity of the DMN exists in OCD patients. However, the heritability property of functional connectivity within the DMN in OCD remains unclear.
Due to their high risk of developing OCD, unaffected siblings of patients with OCD can provide rich genetic information for OCD research, thus helping to disentangle state and trait characteristic of the illness. Thus an important next step in the identification of DMN connectivity as a putative biological marker is to examine the functional connectivity of the DMN in unaffected siblings of OCD patients, providing new insights into genetic risk factors for OCD.
The purpose of this study was to examine the profiles of functional connectivity of the DMN in patients with OCD, their unaffected siblings, and healthy controls. Given the high familial risk of OCD, we expected to observe in unaffected siblings a pattern of functional connectivity within the DMN intermediate between OCD patients and healthy controls.
Method
Participants
The subjects (see Table 1 for details) were recruited through the Guangzhou Psychiatry Hospital and included: (1) patients who met Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for OCD [OCD group; n = 15, 10 males; age (±s.d.) 26.7 ± 4.8 years]; (2) the unaffected siblings of individuals with OCD (SIB group; n = 15, seven males; age 30.0 ± 9.0 years); and (3) healthy control subjects (CON group; n = 28, 21 males; age 27.5 ± 8.4 years). All participants were from the Chinese Han population. Each unaffected sibling was compared with one OCD patient and they came from the same family. The individuals with OCD were all out-patients and had been stabilized on selective serotonin reuptake inhibitors for at least 4 weeks (see online Supplementary Material). Siblings were full siblings, based on self-report. The institutional research and ethics committee of Guangzhou Psychiatry Hospital approved all study protocols. All subjects gave written informed consent before participation.
Table 1. Demographic and clinical characteristics of study subjects

Data are given as mean (standard deviation).
OCD, Obsessive compulsive disorder; SIB, siblings of individuals with OCD; CON, healthy control subjects; IQ, intelligence quotient; YBOCS, Yale–Brown Obsessive Compulsive Scale; OCI-R, Obsessive Compulsive Inventory – Revised; BDI, Beck Depression Inventory; STAI, State-Trait Anxiety Inventory.
All subjects were diagnosed with DSM-IV criteria for OCD by using the Structured Clinical Interview (SCID) for DSM-IV-TR Axis I disorders (First et al. Reference First, Spitzer, Gibbon and Williams1996). The patients had no history of neurological disorder, Tourette's syndrome, head injury, serious medical condition, or history of drug or alcohol addiction. The SIB and CON subjects were screened by using the SCID for the DSM-IV-TR Axis I disorders, Research Version, Non-Patient edition (SCID-I/NP; First et al. Reference First, Spitzer, Gibbon and Williams2002). Healthy controls with a family history of Axis I or II psychiatric disorders were excluded.
Measures
Clinical and neurocognitive assessments
Estimated intelligence quotient (IQ) was assessed with the Wechsler Adult Intelligence Scale-Revised (Wechsler, Reference Wechsler1981), and subjects with a total score of less than 80 were excluded. The Yale–Brown Obsessive Compulsive Scale (YBOCS) was used to assess illness severity (Goodman et al. Reference Goodman, Price, Rasmussen, Mazure, Fleischmann, Hill, Heninger and Charney1989). The Obsessive Compulsive Inventory – Revised (OCI-R) was used to characterize obsessive and compulsive phenomena, including obsessing, washing, checking, neutralizing, ordering and hoarding (Foa et al. Reference Foa, Huppert, Leiberg, Langner, Kichic, Hajcak and Salkovskis2002; Peng et al. Reference Peng, Yang, Miao, Jing and Chan2011). The Beck Depression Inventory (BDI; Beck & Steer, Reference Beck and Steer1984) was used to assess depressive symptoms, and the State-Trait Anxiety Inventory (STAI; Spielberger, Reference Spielberger1983) was used to measure anxiety symptoms. The Annett Handedness Inventory measured handedness information (Spreen & Strauss, Reference Spreen and Strauss1991). An experienced psychiatrist administered all clinical ratings.
Magnetic resonance imaging acquisition
All scans were performed on a Signa HDe 1.5-T GE Signa scanner (GE Medical Systems, USA) equipped with an eight-channel phased-array head coil at the First Affiliated Hospital of Jinan University. For each subject, 141 resting-state functional images (blood oxygenation level-dependent) were acquired with a single-shot echo-planar imaging sequence [repetition time (TR) = 2000 ms, echo time (TE) = 60 ms, matrix = 64 × 64, field of view (FOV) = 240 mm × 240 mm, 6 mm slice thickness, 22 axial slices, no gap, flip angle = 90°]. All subjects were instructed to relax, stay awake, lie still, keep their eyes closed and not to think of anything in particular. In order to assess the stability of the resting-state scans and the reproducibility of findings, the second resting-state scan (REST2) was obtained following the first resting-state scan (REST1) with the same parameters after 1-min break. We also acquired a high-resolution T1-weighted anatomical image for each subject using a three-dimensional fast spoiled gradient-recalled sequence with 128 contiguous slices (TR = 8 ms, TE = 1.7 ms, flip angle = 20°, FOV = 240 mm × 240 mm, matrix = 256 × 256, slice thickness = 1.0 mm).
Subject-level data pre-processing and connectivity analyses
Both structural and functional data pre-processing were conducted with the Connectome Computation System (http://lfcd.psych.ac.cn/ccs.html) developed by the Laboratory for Functional Connectome and Development (Zuo et al. Reference Zuo, Xu, Jiang, Yang, Cao, He, Zang, Castellanos and Milham2013) based on the ‘1000 Functional Connectomes Project’ scripts (Biswal et al. Reference Biswal, Mennes, Zuo, Gohel, Kelly, Smith, Beckmann, Adelstein, Buckner, Colcombe, Dogonowski, Ernst, Fair, Hampson, Hoptman, Hyde, Kiviniemi, Kotter, Li, Lin, Lowe, Mackay, Madden, Madsen, Margulies, Mayberg, McMahon, Monk, Mostofsky, Nagel, Pekar, Peltier, Petersen, Riedl, Rombouts, Rypma, Schlaggar, Schmidt, Seidler, Siegle, Sorg, Teng, Veijola, Villringer, Walter, Wang, Weng, Whitfield-Gabrieli, Williamson, Windischberger, Zang, Zhang, Castellanos and Milham2010), producing the resultant four-dimensional (4D) residual time series for subsequent subject-level functional connectivity analyses. All details of the data pre-processing can be found in our recent work (Wei et al. Reference Wei, Xu, Fan, Dong, Jiang, Li, Yang, Luo and Zuo2013; Zuo et al. Reference Zuo, Xu, Jiang, Yang, Cao, He, Zang, Castellanos and Milham2013). Of note, the Friston-24 model was employed to correct for individual in-scanner head motion (Yan et al. Reference Yan, Cheung, Kelly, Colcombe, Craddock, Di Martino, Li, Zuo, Castellanos and Milham2013). We examined resting-state functional connectivity (RSFC) associated with the hub of the default network (i.e. seed region of a sphere with 6 mm radius): the PCC (x = −8, y = −56, z = 26) in MNI152 standard space (Andrews-Hanna et al. Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner2010). For each subject, we first transferred individual residual 4D data to the Montreal Neurological Institute (MNI152) 2 mm space and extracted the mean time series by averaging across all voxels in each seed region. Using this mean time series, we performed a whole-brain seed correlation analysis for each subject using the AFNI program 3dfim+, carried out in each individual's native space. This analysis produced subject-level correlation maps of all voxels in the brain that were positively or negatively correlated with the seed's time series. Finally, these correlation maps were converted into z value maps using Fisher's r-to-z transformation to improve the data normality.
Group-level default network connectivity analyses
Group-level analyses were implemented as a two-way (factors: group, scan) repeated-measure (scan) analysis of covariance using the Linear Mixed Effect (LME) Matlab toolbox in FreeSurfer 5.1 (Bernal-Rusiel et al. Reference Bernal-Rusiel, Greve, Reuter, Fischl and Sabuncu2012). Cluster-based statistical corrections for multiple comparisons were performed using Gaussian random field theory (z > 2.3; corrected p < 0.05). This group-level analysis produced thresholded z statistic maps showing brain regions with significantly detectable RSFC with the PCC seed regions for OCD, SIB and CON groups, respectively, as well as differences in default network RSFC (i.e. group difference maps indicating OCD versus SIB, SIB versus CON and OCD versus CON comparisons). Of note, according to previous studies showing age, sex, IQ and head micromovement-related changes of default network connectivity (Biswal et al. Reference Biswal, Mennes, Zuo, Gohel, Kelly, Smith, Beckmann, Adelstein, Buckner, Colcombe, Dogonowski, Ernst, Fair, Hampson, Hoptman, Hyde, Kiviniemi, Kotter, Li, Lin, Lowe, Mackay, Madden, Madsen, Margulies, Mayberg, McMahon, Monk, Mostofsky, Nagel, Pekar, Peltier, Petersen, Riedl, Rombouts, Rypma, Schlaggar, Schmidt, Seidler, Siegle, Sorg, Teng, Veijola, Villringer, Walter, Wang, Weng, Whitfield-Gabrieli, Williamson, Windischberger, Zang, Zhang, Castellanos and Milham2010; Tomasi & Volkow, Reference Tomasi and Volkow2012; Zuo et al. Reference Zuo, Ehmke, Mennes, Imperati, Castellanos, Sporns and Milham2012; Yan et al. Reference Yan, Cheung, Kelly, Colcombe, Craddock, Di Martino, Li, Zuo, Castellanos and Milham2013) and the wide age range of OCD patients in the present study, we modeled age, sex, IQ, and mean framewise displacement (Yan et al. Reference Yan, Cheung, Kelly, Colcombe, Craddock, Di Martino, Li, Zuo, Castellanos and Milham2013) as covariates in the group-level LME model.
Test–retest reliability and reproducibility
Despite the reasonable test–retest reliability of the resting-state functional magnetic resonance imaging (R-fMRI) measures reported (Zuo et al. Reference Zuo, Kelly, Adelstein, Klein, Castellanos and Milham2010a ,Reference Zuo, Kelly, Di Martino, Mennes, Margulies, Bangaru, Grzadzinski, Evans, Zang, Castellanos and Milham b , Reference Zuo, Ehmke, Mennes, Imperati, Castellanos, Sporns and Milham2012), previous studies have shown the dynamic and unconstrained nature of R-fMRI (Yan et al. Reference Yan, Liu, He, Zou, Zhu, Zuo, Long and Zang2009; Chang & Glover, Reference Chang and Glover2010). We conducted careful stability analysis to evaluate the test–retest reliability of default network mapping across the two resting-state scans (see online Supplementary Material).
Relationship with clinical and neurocognitive variables
To examine whether default network connectivity can predict neurocognitive function and clinical symptom severity in OCD, we conducted partial correlation analysis by controlling for the effects of age, sex and estimated IQ. Across subjects in the patient group, RSFC in the regions showing high test–retest reliability and reproducibility (see online Supplementary material), OCD versus CON or SIB versus CON differences were averaged to correlate with YBOCS, BDI and STAI scores.
Results
Demographic data
One-way analysis of variance was used to examine if there were any differences in behavior measures among the three groups. The results are summarized in Table 1. Briefly, the three groups did not differ significantly in age, sex, and education or estimated IQ. All subjects were right handed. As a group, our OCD patients were mildly impaired [total YBOCS: 25.7 (s.d. = 6.0); OCI-R: 21.0 (s.d. = 10.2); BDI: 20.5 (s.d. = 13.7); state STAI: 55.1 (s.d. = 20.2); trait STAI: 57.9 (s.d. = 18.7)] compared with SIB and normal CON subjects.
Default network connectivity: OCD versus CON
Compared with healthy controls, OCD patients were found to have significantly decreased RSFC within the PCC. We also observed significantly increased RSFC in the right inferior frontal lobe, insula and superior temporal lobe. Of note, the right superior parietal cortex also showed increased RSFC. We employed the threshold of z > 2.3, corrected p < 0.05 for cluster level statistic in FSL (Oxford University, UK). The significant cluster was still there with the threshold at z > 3.1. These results are shown in Fig. 1.
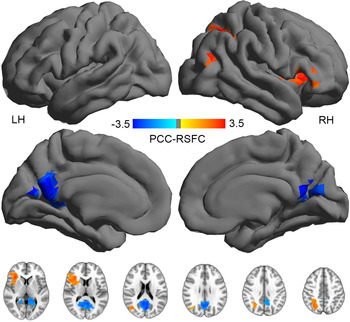
Fig. 1. Group-level default network functional connectivity differences between obsessive compulsive disorder patients and healthy controls. LH, Left hemisphere; PCC, posterior cingulate cortex; RSFC, resting-state functional connectivity; RH, right hemisphere.
Default network connectivity: OCD versus SIB
Compared with SIB subjects, OCD patients exhibited decreased RSFC in the right middle temporal gyrus (Fig. 2).

Fig. 2. Group-level default network functional connectivity differences between obsessive compulsive disorder patients and their first-degree siblings. LH, Left hemisphere; PCC, posterior cingulate cortex; RSFC, resting-state functional connectivity; RH, right hemisphere.
Default network connectivity: SIB versus CON
With the PCC seed (Fig. 3), compared with CON subjects, SIB participants only showed a lower RSFC in the PCC and no increased RSFC was found.

Fig. 3. Group-level default network functional connectivity differences between obsessive compulsive disorder first-degree siblings and healthy controls. LH, Left hemisphere; PCC, posterior cingulate cortex; RSFC, resting-state functional connectivity; RH, right hemisphere.
Test–retest reliable and reproducible findings
For PCC seeded RSFC maps, we measured the test–retest reliability of the default network connectivity across REST1 and REST2 with the intraclass correlation coefficient (ICC). There was fair (ICC = 0.4) to excellent (ICC = 0.9) reproducibility for RSFC within default network regions (online Supplementary Fig. S1).
Default network connectivity predicts clinical scores
To assess if the reduction of short-distance default network connectivity in both OCD patients and their siblings is associated with various clinical or neurocognitive measures, we extracted the mean RSFC within the overlap region for PCC seeds and correlated them with clinical scores. Our RSFC-symptom correlational analyses found that there was a significant negative correlation between RSFC in the overlap region for the PCC and YBOCS obsessive subscale scores (Pearson r = −0.49, p = 0.008).
Discussion
The goal of the present study was to examine differences in functional connectivity within the DMN in OCD patients, their unaffected siblings and matched controls to test the hypothesis that the disruptive connectivity of the DMN in OCD may be a putative marker for this disease. We observed reduced functional connectivity in the PCC within the DMN not only in patients with OCD but also in their unaffected siblings. These disrupted connectivity patterns were present in the two sequential scans with a 1-min interval, implying their test–retest reliability. The presence of abnormalities of the DMN in unaffected siblings suggests that these abnormalities are mainly due to genetic factors rather than to treatment or other secondary environmental factors. Taken together, these findings suggest that OCD is associated with disrupted functional connectivity within the DMN, and may provide new insights into the understanding of the pathophysiology of this disorder.
The present study demonstrates that OCD patients are associated with abnormal functional connectivity in the PCC within the DMN. These findings are consistent with previous studies about resting-state functional connectivity in OCD. Fitzgerald et al. (Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor, Welsh, Hanna and Taylor2010) found that pediatric OCD patients exhibited less functional connectivity within the PCC during the resting state. In addition, previous studies have identified structural and functional abnormalities of the PCC in OCD patients. Neuroanatomically, recent studies demonstrated that OCD patients had reductions of gray matter volume in the PCC (Matsumoto et al. Reference Matsumoto, Ito, Takahashi, Ando, Fujimura, Nakayama, Okubo, Obata, Fukui and Suhara2010), as well as white matter reduction in the PCC (Cannistraro et al. Reference Cannistraro, Makris, Howard, Wedig, Hodge, Wilhelm, Kennedy and Rauch2007). Functionally, the PCC is important for cognitive inhibition (Rubia et al. Reference Rubia, Smith, Brammer, Toone and Taylor2005; Simmonds et al. Reference Simmonds, Pekar and Mostofsky2008) and flexibility (Fox et al. Reference Fox, Barense and Baxter2003; Chamberlain et al. Reference Chamberlain, Menzies, Hampshire, Suckling, Fineberg, del Campo, Aitken, Craig, Owen, Bullmore, Robbins and Sahakian2008; Pearson et al. Reference Pearson, Heilbronner, Barack, Hayden and Platt2011), which are the core cognitive dysfunctions in OCD and suggested as cognitive biological markers for OCD (Chamberlain et al. Reference Chamberlain, Fineberg, Menzies, Blackwell, Bullmore, Robbins and Sahakian2007; Menzies et al. Reference Menzies, Achard, Chamberlain, Fineberg, Chen, del Campo, Sahakian, Robbins and Bullmore2007). Most recently, two resting-state studies both found that OCD patients showed decreased functional connectivity within the DMN when using the PCC as seed, which is similar to our results (Jang et al. Reference Jang, Kim, Jung, Choi, Jung, Lee, Choi, Kang and Kwon2010; Stern et al. Reference Stern, Fitzgerald, Welsh, Abelson and Taylor2012). However, their results did not find that OCD patients had reduced connectivity in the PCC. This difference might be due to the differences of sample heterogeneity or seed selection. Specifically, one study included medicated and unmedicated patient samples (Stern et al. Reference Stern, Fitzgerald, Welsh, Abelson and Taylor2012), and another study defined the PCC seed region using a different labeled template (Jang et al. Reference Jang, Kim, Jung, Choi, Jung, Lee, Choi, Kang and Kwon2010). Relative to healthy controls, OCD patients also showed increased functional connectivity in the inferior frontal lobe and insula, which are core regions of the salience network involved in implementing and maintaining attention to external task demands and detecting salient events (Dosenbach et al. Reference Dosenbach, Fair, Miezin, Cohen, Wenger, Dosenbach, Fox, Snyder, Vincent, Raichle, Schlaggar and Petersen2007). Particularly, the insula may act as an integral hub in mediating dynamic interactions between externally oriented attentional networks and internally oriented self-related networks. In other words, this region is important for switching attention away from an internal focus toward the external environment after detecting potentially harmful situations (Menon & Uddin, Reference Menon and Uddin2010). Our results found that OCD patients had decreased functional connectivity in the DMN, but increased connectivity in the salient network. Based on the causal relationship between the salience network and the DMN (Chiong et al. Reference Chiong, Wilson, D'Esposito, Kayser, Grossman, Poorzand, Seeley, Miller and Rankin2013), it is natural to speculate that OCD patients have difficulties in disengaging from internal mental processes when needing to respond more appropriately to salient external information related to potential risk.
Intriguingly, our findings also demonstrated that the unaffected siblings exhibited impairments in functional connectivity within the PCC similar to OCD patients. Given that the unaffected siblings have not received any medication for the treatment of OCD symptoms, the similar functional connectivity patterns shown in them suggests that such a disrupted functional connection in their OCD probands was not secondary to medication or the effects of the illness. This finding is consistent with the findings of disrupted functional connectivity when the first-degree relatives of OCD patients were asked to perform a functional task. For instance, Chamberlain et al. (Reference Chamberlain, Fineberg, Menzies, Blackwell, Bullmore, Robbins and Sahakian2007, Reference Chamberlain, Menzies, Hampshire, Suckling, Fineberg, del Campo, Aitken, Craig, Owen, Bullmore, Robbins and Sahakian2008) demonstrated that relatives and OCD patients both showed impaired cognitive flexibility and motor inhibition, which were related to the PCC (Rubia et al. Reference Rubia, Smith, Brammer, Toone and Taylor2005; Pearson et al. Reference Pearson, Heilbronner, Barack, Hayden and Platt2011). However, future research is needed to elucidate the precise function and connectivity of the PCC in first-degree relatives. These findings may ultimately contribute to brain-based markers for OCD and aid in determining risk genes.
The observed decreased functional connectivity within the DMN may contribute to the core cognitive symptoms of OCD. The DMN has been implicated in cognitive functions associated with internal thought in contrast to stimulus-based perception (Buckner et al. Reference Buckner, Andrews-Hanna and Schacter2008). The reduced RSFC of the DMN in OCD patients may blur the normal boundary between internal thoughts and external perceptions. Based on difficulty in inhibiting the external stimuli (uncontrolled and recurrent thoughts, impulses or images), OCD patients might pay more attention to the distressing stimuli and attend less on internal thoughts. This may result in an ambiguous integration between one's own thoughts and feeling with external events. Furthermore, OCD patients would seem to be imbued with exaggerated perceptions of external stimuli for attempting to avoid or control the uncontrolled and recurrent thoughts. Indeed, the preoccupations were speculated on contributing to many symptoms of OCD for less sense of self-relevance (Fitzgerald et al. Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor, Welsh, Hanna and Taylor2010). Thus, abnormal functional connectivity of the DMN may be involved in the psychopathological symptoms of OCD.
Limitations and future work
Given different OCD symptom dimensions relating to distinct neural mechanisms (Mataix-Cols et al. Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004), we were unable to divide the OCD patient sample into different subtypes because of the relatively small sample. Second, OCD patients in this study were mostly treated with antidepressants and had long average illness duration, so the effects of the illness duration and medication cannot completely be ruled out in our results. Further studies using different subtypes and drug-naive cohorts are required to confirm our preliminary results. Finally, from a methodological perspective, the current study only focused on the examination of the default network connectivity and may be enhanced with high-resolution full-brain network explorations to gain more insights of brain function under such a psychiatric condition.
Conclusion
In the present study, we used the resting-state functional connectivity approach to show the default networks in OCD patients and their unaffected siblings during rest. The current study provides a unique opportunity to understand the pathophysiology of, and the susceptibility to, OCD. Our findings support the hypothesis that OCD is associated with abnormal functional connectivity of the DMN. The similar abnormal connectivity in the PCC with the DMN in both OCD patients and their unaffected siblings may serve as a neuroimaging marker for the development of OCD. Future studies combining R-fMRI with structural MRI techniques will be vital to explore how the functional alterations shown in this study are related to structural changes, and whether these changes could be used for early screening markers, or detecting disease progression and medication effects in OCD patients.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713002250.
Declaration of Interest
None.






