Published online by Cambridge University Press: 18 April 2005
Interatrial communications take several forms. Not all involve directly the atrial septum. Only those produced by deficiencies of the floor of the oval fossa represent true septal defects.1 The defects related to the mouths of the superior and inferior caval veins, the so-called sinus venosus defects, and the defect through the mouth of the coronary sinus, along with the “ostium primum” defect, all unequivocally producing interatrial shunting, are all outside the confines of the normal atrial septum. This is because, in the normal heart, the atrial septum is limited to the floor of the oval fossa and its buttressed muscular antero-inferior rim. On the left atrial aspect, this muscular buttress forms part of the vestibule of the mitral valve. On the right atrial aspect, it is contiguous with the atrial layer of the triangle of Koch, this forming part of the vestibule of the tricuspid valve. It is now common to refer to those defects within the confines of the oval fossa, previously known as “secundum” defect, even though they are produced by deficiencies of the primary atrial septum, as oval fossa defects.
We have now encountered a septal defect in the portion of the atrial septum that separates the vestibules of the mitral and tricupsid valves. To the best of our knowledge, such a septal deficiency has not previously been described. In this correlation, we describe the clinical and morphologic features of the case. We describe the hole as a vestibular defect, since we believe it represents a deficiency of the septal component derived from the embryonic vestibular spine,2 the “spina vestibuli” originally described by Wilhelm His the elder in the latter part of the nineteenth century.3 We also give an account, therefore, of our understanding of the formation of the atrial septum,2, 4 thus putting the anatomico-clinical correlations into their appropriate developmental context.
A female monochorionic twin was born in 1988 at 32 weeks gestation, weighing 1105 g. Birth was by Caesarian section because of fetal distress. At birth, an immediate diagnosis of pulmonary atresia with intact septum was made by echocardiography (Fig. 1). The examination revealed an interatrial communication, which at the time was thought to be within the oval fossa. Retrospective review of the echo subsequent to examination of the autopsied specimen (see below) now shows that this defect was beyond the confines of the oval fossa, being located within its bulbous muscular antero-inferior rim (Figs 2 and 3). The short axis echocardiographic sections also revealed the presence of a mitral valve within the left ventricle (Fig. 4), rather than the left component of a common atrioventricular valve. After diagnosis, patency of the arterial duct was maintained by infusion of prostaglandin. At 2 month of age, a 4 mm right modified Blalock Taussig shunt was constructed. Despite the presence of the shunt, the baby remained dependent on mechanical ventilation. She died of respiratory problems at the aged of 4 months.

Figure 1. This parasternal short axis echocardiographic section shows the atretic pulmonary valve and the hypoplastic right ventricle.
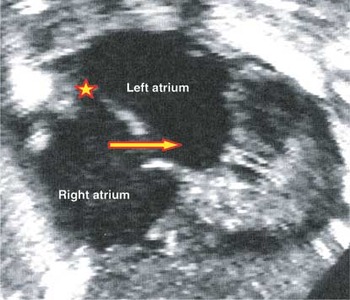
Figure 2. The apical 4 chamber view reveals probe patency of the oval foramen (). A second defect is seen within the muscular antero-inferior rim of the fossa (red and yellow arrow). Having studied the autopsied specimen (see Fig. 5), this hole can be recognised as the vestibular defect.

Figure 3. The subcostal coronal view confirms the probe-patency of the oval foramen, which bows towards the left atrium (). The second interatrial communication (yellow and red arrow) is clearly positioned antero-inferiorly relative to the floor of the oval fossa. The double-headed blue and yellow arrow shows the intact atrioventricular septum between the offset hinges of the atrioventricular valves.

Figure 4. The parasternal short axis view shows that the mitral valve has two leaflets, confirming that the heart does not possess a common atrioventricular junction.
There were no significant congenital anomalies other than the cardiac anomaly. There was usual arrangement of the thoraco-abdominal organs, and the spleen was single and left-sided. The heart was located in the left chest, with its apex pointing to the left. There was usual atrial arrangement, with concordant atrioventricular and venticulo-arterial connections, and right hand ventricular topology. The systemic and pulmonary venous connections were normal. The flap valve of the oval fossa remained probe patent at its superior margin and a small fenestration was seen at its antero-inferior rim (Fig. 5). The unusual finding was the presence of an additional defect within the muscular antero-inferior rim of the oval fossa, producing a communication between the vestibules of the right and left atrioventricular junctions (Figs 5 and 6). The mouth of the coronary sinus was dilated, but the sinus itself was intact and of normal dimensions, with no persistent left superior caval vein. The atrioventricular septal area was intact, and the left atrioventricular valve had the bifoliate configuration typical for the mitral valve. The tricuspid valve was morphologically normal but hypoplastic, having a diameter of 8 mm, representing a Z value of −2. The right ventricle was markedly hypertrophied, but it was an easy matter to recognise well-formed inlet, apical trabecular, and outlet components. The pulmonary valve was domed and atretic (Fig. 7). The pulmonary arteries were normal, and the arterial duct was completely occluded. The left ventricle and aorta were normal, the aortic arch being left-sided. The graft placed between the right subclavian artery and the right pulmonary artery remained patent.
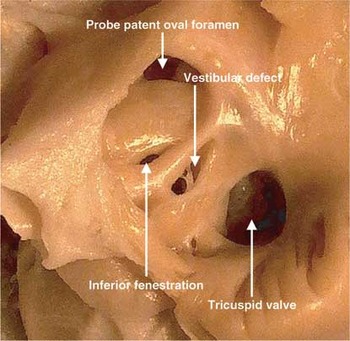
Figure 5. This picture shows the right atrium of the autopsied specimen, revealing the probe patent oval foramen, along with a small fenestration in the inferior margin of the oval fossa. In addition, however, there is a larger defect within the muscular antero-inferior rim of the fossa, immediately superior to the mouth of the coronary sinus.

Figure 6. When viewed from the left side, probe patency of the oval foramen is confirmed. Note the small fenestration at the base of the flap valve, along with the larger muscular defect opening into the vestibule of the mitral valve, and crossed by fibrous strands.

Figure 7. Opening the pulmonary trunk reveals the domed and imperforate pulmonary valve.
During development of the heart, the systemic venous tributaries are present from the outset, and drain more-or-less symmetrically into the primary atrial component of the initial heart tube. At this early stage of development, there is formation neither of the lung buds nor pulmonary veins. The heart tube itself is initially connected to the posterior body wall by the dorsal mesocardium. With disruption of the mesocardium, the ventricular part of the tube loops to the right. At the caudal pole, however, the tube retains its connection to the developing mediastinum through the dorsal mesocardium (Fig. 8). The lungs themselves are formed by bifurcation of the tracheo-bronchial groove, the latter budding forward from the oesophagus, with the pulmonary vasculature developing concomitantly with formation of the lungs. As the lungs are developing, two prominences, the pulmonary ridges, are seen within the cavity of the atrium, albeit that there is no direct communication from the back of the atrial cavity to the mediastinal space. When the lungs form, vascular strands luminise within the posterior mediastinum. This serves to join the developing venous component of the pulmonary vasculature with the atrial cavity, the newly formed vein opening as a solitary channel between the two pulmonary ridges. By this time, there has also been re-arrangement of the systemic venous tributaries, also known as the horns of the sinus venosus, which rotate so as to open exclusively into the right side of the primary atrial component of the heart tube. Only subsequent to this reorientation of the systemic venous tributaries is it possible for a septum to grow from the roof of the primary atrium. This is the primary atrial septum, which grows towards the endocardial cushions, now formed superiorly and inferiorly within the atrioventricular canal (Fig. 9). As the primary septum grows towards the cushions, it carries on its leading edge a prominent cap of mesenchymal tissue (Fig. 10). At the same time, there is expansion of the right pulmonary ridge to produce the structure initially recognised by His,3 and termed the “spina vestibuli” (Fig. 11). Mediastinal tissue grows into the heart through the vestibular spine (Fig. 12). In the mouse, the prominence thus formed develops its own mesenchymal cap.5 With subsequent growth, the two endocardial cushions of the atrioventricular canal fuse together to produce the right and left atrioventricular junctions, and the mesenchymal cap on the primary atrial septum joins with the cushions to obliterate the primary atrial foramen. Long before this, the upper edge of the primary septum has broken down to form the secondary interatrial foramen (Fig. 10). The tissue derived from the vestibular spine then reinforces the right side of the newly formed septum, muscularising to form the antero-inferior rim of the oval fossa (Fig. 13). When the primary atrial foramen is first closed, and at the stage of formation of the antero-inferior buttress, the pulmonary vein drains as a solitary channel inferiorly within the left atrium. Only subsequent to the completion of atrial septation is there incorporation of the pulmonary veins to form the atrial roof. And only subsequent to incorporation of the right pulmonary veins is the infolding produced that is recognisable in the definitive heart as the superior and posterior rims of the oval foramen (Fig. 14).

Figure 8. This cross-section comes from a human embryo measuring 4 mm in crown-rump length, at stage 12 in the Carnegie classification. It shows the connection persisting at the venous pole between the primary atrium and the mediastinal tissues. This is the so-called “dorsal mesocardium”. Note that, as yet, there is no formation of the lungs.

Figure 9. This section of a human embryo at stage 14 of the Carnegie classification is sectioned in the equivalent of parasternal long axis plane. It shows that the direction of ingrowth of tissues from the posterior mediastinum into the vestibular spine, entering the developing atrial septal area between the left sinus horn (LSH) and the downgoing primary atrial septum. The stars show the atrioventricular cushions, which are positioned superiorly and inferiorly within the atrioventricular canal.
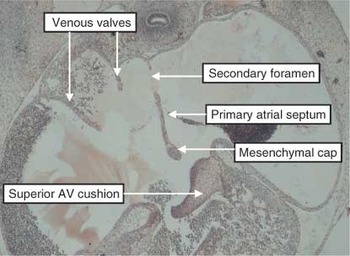
Figure 10. This section, in simulated four chamber plane, is through the atrial tissues of a human embryo at Carnegie stage 16. The primary septum has grown down from the atrial roof, having broken down at its upper edge to form the secondary foramen. There is a mesenchymal cap on its leading edge. The space between this cap and the superior atrioventricular (AV) cushion is the primary atrial foramen.
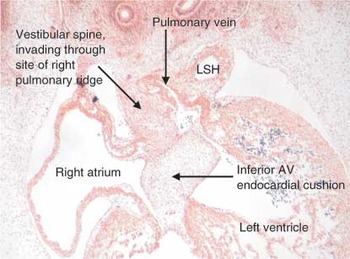
Figure 11. This section, in four-chamber plane, is through the developing atrial chambers of a human embryo at Carnegie stage 16. By this stage, the pulmonary vein has canalised within the tissues of the posterior mediastinum. As shown in this section, it opens initially as a solitary channel close to the atrioventricular (AV) junction, entering the left side of the primary atrium. Note the separate walls of the left sinus horn (LSH), now incorporated within the left atrioventricular junction. The tissues of the posterior mediastinum are invading the heart through the site initially marked by the right pulmonary ridge. This is the area christened by His3 as the “spina vestibuli” (See Fig. 12).

Figure 12. These pictures are scanned and re-labelled from the original publication of Wilhelm His3. They show the location of the structure he named as the “spina vestibuli”, which we have translated to vestibular spine. The pictures show the equivalent four chamber (left) and parasternal long axis (right) projections.

Figure 13. This section, in four chamber plane, is from a human embryo measuring 33 mm from crown to rump, and is in the eighth week of development. It shows how the pulmonary vein is being incorporated into the dome of the left atrium, with there now being two venous orifices. Note that the tissue derived from the vestibular spine has now muscularised to reinforce the base of the atrial septum.

Figure 14. This human embryo, again sectioned in four chamber plane, is about one week older than the one shown in Figure 13. Note that the distance between the pulmonary venous orifices has increased with continuing incorporation of the pulmonary venous component. The upper margin of the oval foramen is now folding in to produce the so-called “septum secundum”, in reality the superior interatrial groove.
The definitive atrial septum, therefore, is derived from multiple components. The flap valve is the initial muscular primary atrial septum. The bulbous antero-inferior rim is the muscularised tissue derived from the vestibular spine, together with the mesenchymal cap that initially clothed the leading edge of the primary septum. The so-called “secondary septum” is the infolded supero-posterior rim, which produces a deep groove between the connections of the caval veins to the right atrium and the pulmonary veins to the left, known as either Sondergaard's or Waterston's groove. The defect seen in our case is presumably due to incomplete muscularisation of the tissues derived from the embryonic vestibular spine, or else persistence of a gap between the tissues derived by muscularisation of the spine as opposed to the mesenchymal cap of the primary atrial septum.
We have previously argued that interatrial communications can be divided into those found within the oval fossa, which are true septal defects, and those found elsewhere within the atrial chambers producing shunts outside the confines of the fossa.1 The latter lesions are the so-called sinus venosus defects, which have always in our experience been associated with abnormal pulmonary venous connections, the coronary sinus defect, produced by a fenestration in the walls normally separating the coronary sinus from the left atrium, or the “ostium primum” defect, which is an atrioventricular septal defect seen in the setting of a common atrioventricular junction.1 We now see the need to expand this categorisation, since we had presumed, on our previous experience, that true septal defects would always be found within the confines of the oval fossa. The case reported here shows that true septal defects can also be found within the muscular antero-inferior buttress that contributes to the vestibules of both the mitral and tricuspid valves.
When first seen, however, we had not appreciated that the defect was within the antero-inferior margin of the rim of the oval fossa. Only subsequent to review of the autopsy findings, and retrospective examination of the echocardiographic recordings, did we appreciate the true nature of the septal defect. The correct echocardiographic diagnosis depended on defining the antero-inferior limit of the oval fossa, and showing that the atrioventricular septum was intact. In subcostal view, the orifice of the normally positioned coronary sinus may, on occasion, give the false impression of a defect (Fig. 15). Once the coronary sinus is identified, anglulation of the probe will show that the defect is distant from the venous structure. In the apical four-chamber cut, nonetheless, the defect will be seen very close to the crux of the heart. This might suggest it to be the atrial component of an atrioventricular septal defect with common atrioventricular junction. This was already ruled out at the initial investigation by demonstration of the integrity of the atrioventricular septal area, the short axis cut further showing the normal bifoliate nature of the mitral valve (Fig. 4), not the trifoliate arrangement of the left valve seen with atrioventricular septal defect and common atrioventricular junction.

Figure 15. This cross-sectional subcostal echocardiogram (A) is from a normal heart. It shows how the coronary sinus can, on occasions, give the impression of a defect in the antero-inferior rim of the oval fossa. The accompanying section (B) comes from the same embryo as Figure 14, and shows the relationship of the coronary sinus to the antero-inferior rim of the oval fossa, formed by muscularisation of the vestibular spine. Note the infolded superior rim – the so-called “septum secundum”, filled with echo-dense fibrofatty tissue in the echocardiographic section (A).
It is our belief that the vestibular structure surrounding the defect is produced by muscularisation of the “spina vestibuli” initially described by His.3 We have termed the hole, therefore, a vestibular defect. We are unaware of any previous descriptions of this particular type of atrial septal defect. The existence of the defect, nonetheless, reinforces our conclusions made regarding the development of the atrial septum. We had commented previously on the deficiencies of “classical” theories accounting for septation of the atrial chambers on the basis of downgrowth of separate primary and secondary septums, as had others prior to us.6 The recognition of the role of the vestibular spine now shows that there is, indeed, formation of a secondary atrial septum. This is the muscular buttress that reinforces the right atrial aspect of the base of the primary septum along the line of its fusion with the atrioventricular endocardial cushions. As now demonstrated by the presence of a hole within this structure, this is a true atrial septum. The structure most usually described as the “secondary” septum, however, is the extensive superior and posterior infolding between the attachments of the caval veins to the right atrium, and the right pulmonary veins to the left atrium. This fold is not a true septal structure, albeit that it interposes between the cavities of the right and left atriums (Fig. 15). So as to pass between the atriums across the structure, it is necessary to traverse extracardiac space. This is the space that, in postnatal life, is occupied by fibroadipose tissue. Abnormal growth of such tissue produces the so-called “lipoma of the atrial septum”, in reality a lipoma of the interatrial groove.7
The developmental research of Dr Webb and Professor Anderson is supported by grants from the British Heart Foundation.

This parasternal short axis echocardiographic section shows the atretic pulmonary valve and the hypoplastic right ventricle.

The apical 4 chamber view reveals probe patency of the oval foramen (). A second defect is seen within the muscular antero-inferior rim of the fossa (red and yellow arrow). Having studied the autopsied specimen (see Fig. 5), this hole can be recognised as the vestibular defect.

The subcostal coronal view confirms the probe-patency of the oval foramen, which bows towards the left atrium (). The second interatrial communication (yellow and red arrow) is clearly positioned antero-inferiorly relative to the floor of the oval fossa. The double-headed blue and yellow arrow shows the intact atrioventricular septum between the offset hinges of the atrioventricular valves.

The parasternal short axis view shows that the mitral valve has two leaflets, confirming that the heart does not possess a common atrioventricular junction.

This picture shows the right atrium of the autopsied specimen, revealing the probe patent oval foramen, along with a small fenestration in the inferior margin of the oval fossa. In addition, however, there is a larger defect within the muscular antero-inferior rim of the fossa, immediately superior to the mouth of the coronary sinus.

When viewed from the left side, probe patency of the oval foramen is confirmed. Note the small fenestration at the base of the flap valve, along with the larger muscular defect opening into the vestibule of the mitral valve, and crossed by fibrous strands.

Opening the pulmonary trunk reveals the domed and imperforate pulmonary valve.

This cross-section comes from a human embryo measuring 4 mm in crown-rump length, at stage 12 in the Carnegie classification. It shows the connection persisting at the venous pole between the primary atrium and the mediastinal tissues. This is the so-called “dorsal mesocardium”. Note that, as yet, there is no formation of the lungs.

This section of a human embryo at stage 14 of the Carnegie classification is sectioned in the equivalent of parasternal long axis plane. It shows that the direction of ingrowth of tissues from the posterior mediastinum into the vestibular spine, entering the developing atrial septal area between the left sinus horn (LSH) and the downgoing primary atrial septum. The stars show the atrioventricular cushions, which are positioned superiorly and inferiorly within the atrioventricular canal.

This section, in simulated four chamber plane, is through the atrial tissues of a human embryo at Carnegie stage 16. The primary septum has grown down from the atrial roof, having broken down at its upper edge to form the secondary foramen. There is a mesenchymal cap on its leading edge. The space between this cap and the superior atrioventricular (AV) cushion is the primary atrial foramen.

This section, in four-chamber plane, is through the developing atrial chambers of a human embryo at Carnegie stage 16. By this stage, the pulmonary vein has canalised within the tissues of the posterior mediastinum. As shown in this section, it opens initially as a solitary channel close to the atrioventricular (AV) junction, entering the left side of the primary atrium. Note the separate walls of the left sinus horn (LSH), now incorporated within the left atrioventricular junction. The tissues of the posterior mediastinum are invading the heart through the site initially marked by the right pulmonary ridge. This is the area christened by His3 as the “spina vestibuli” (See Fig. 12).

These pictures are scanned and re-labelled from the original publication of Wilhelm His3. They show the location of the structure he named as the “spina vestibuli”, which we have translated to vestibular spine. The pictures show the equivalent four chamber (left) and parasternal long axis (right) projections.

This section, in four chamber plane, is from a human embryo measuring 33 mm from crown to rump, and is in the eighth week of development. It shows how the pulmonary vein is being incorporated into the dome of the left atrium, with there now being two venous orifices. Note that the tissue derived from the vestibular spine has now muscularised to reinforce the base of the atrial septum.

This human embryo, again sectioned in four chamber plane, is about one week older than the one shown in Figure 13. Note that the distance between the pulmonary venous orifices has increased with continuing incorporation of the pulmonary venous component. The upper margin of the oval foramen is now folding in to produce the so-called “septum secundum”, in reality the superior interatrial groove.

This cross-sectional subcostal echocardiogram (A) is from a normal heart. It shows how the coronary sinus can, on occasions, give the impression of a defect in the antero-inferior rim of the oval fossa. The accompanying section (B) comes from the same embryo as Figure 14, and shows the relationship of the coronary sinus to the antero-inferior rim of the oval fossa, formed by muscularisation of the vestibular spine. Note the infolded superior rim – the so-called “septum secundum”, filled with echo-dense fibrofatty tissue in the echocardiographic section (A).