Introduction
The Pd–Ag–S system comprises three binary minerals: vysotskite (PdS), vasilite (Pd16S7), acanthite/argentite (Ag2S) and two ternary minerals: coldwellite (Pd3Ag2S) found in the Marathon Cu–PGE–Au deposit, Colwell Complex, Canada (McDonald et al., Reference McDonald, Cabri, Stanley, Good, Redpath and Spratt2015); and kravtsovite (PdAg2S) found in the Komsomolsky mine in the Talnakh deposit of the Noril'sk district, Russia (Vymazalová et al., Reference Vymazalová, Laufek, Sluzhenikin, Stanley, Kozlov, Chareev and Lukashova2017). Minerals from the Pd–Ag–S system occur, in general, in Cu–Ni–PGE deposits associated with mafic and ultramafic rocks. Furthermore, the occurrences of unnamed phases with compositions that belong to the system Pd–Ag–S have also been reported in the literature, in particular from alluvial deposits related to mafic–ultramafic bedrock sources (e.g. Augé and Legendre Reference Augé and Legendre1992; Tolstykh et al., Reference Tolstykh, Sidorov and Kozlov2009). Therefore, the phase relations within the Pd–Ag–S system are of mineralogical interest. Nevertheless, only the phase relations within the binary subsystems are described in the literature, and the ternary system Pd–Ag–S has not been studied experimentally up to now, only some preliminarily results reported by Vymazalová et al. (Reference Vymazalová, Laufek, Drábek, Kistavchuk, Chareev and Andre Mayer2015a). In this contribution, we present the phase relations in the Pd–Ag–S system and predict possible stable assemblages under natural conditions; we explore the mineralogical implications of our findings. The list of minerals belonging to the Pd–Ag–S system is summarised in Table 1.
Table 1. Minerals in the system Pd–Ag–S and their crystal-structure data.

*The high-temperature form of Ag2S, stable over 177°C.
Previous experiments
The binary Pd–S system
The binary system Pd–S was evaluated by Okamoto (Reference Okamoto1992) based on the data of Matković et al. (Reference Matković, El-Boragy and Schubert1976) and Weibke and Laar (Reference Weibke and Laar1935). The system comprises five intermediate phases: Pd4S, Pd3S (stable in the temperature interval from 555 to 635°C), Pd16S7, PdS and PdS2.
The binary systems Ag–Pd and Ag–S
Karakaya and Thompson (Reference Karakaya and Thompson1988) reported complete solid solution for the Ag–Pd system. In the Ag–S system only one Ag2S phase exists with three polymorphic modifications (α, β and γ) (Sharma and Chang, Reference Sharma and Chang1986).
The ternary system Pd–Ag–S
Phase relations in the ternary system Pd–Ag–S have not been studied to date. Nevertheless, Raub et al. (Reference Raub, Wullhorst and Plate1954) studied experimentally the solubility of Pd in Ag2S in the temperature interval 700 to 900°C. El-Boragy and Schubert (Reference El-Boragy and Schubert1971) studied phases with β-Mn type structures and synthesised the phase denoted as ‘Pd2AgS’ at 550°C. Synthetic phases belonging to the Pd–Ag–S system are listed in Table 2.
Table 2. Synthetic phases in the system Pd–Ag–S and their crystal-structure data.

*Stable in the temperature interval from 555 to 635°C; **Ag2S exists in three allotropic forms (α-Ag2S, β-Ag2S and γ-Ag2S).
Methods and techniques
Experimental
Experiments were performed in evacuated and sealed silica-glass tubes in horizontal tube furnaces. To prevent loss of material to the vapour phase during experiments, the free space in the tubes was reduced by placing closely fitting glass rods over the charge. Charges of 100 to 300 mg were weighed out carefully from the native elements. We used, as starting chemicals, silver powder (Aldrich Chem. Co., 99.9999% purity), sulfur flakes (Aldrich Chem. Co., 99.999% purity) and palladium powder (Aldrich Chem. Co., 99.95% purity). The starting mixtures were heated up slowly to 300°C until all the sulfur was reacted, then the mixture was annealed at 800°C. The resulting run products were ground in an agate mortar under acetone and reheated to 400°C (for 110 days), and at 550°C (for 80 days). After heating, quenching occurred by dropping the capsules in cold water. Phases in the run products were characterised by powder X-ray diffraction; in polished sections examined in reflected light; and with electron-microprobe techniques. A list of representative experimental runs (starting material and products) is given in Table 3.
Table 3. Representative experimental runs, starting material and run products (based on XRD and EPMA data).

X-ray diffraction analysis
Each experimental product was primarily studied with powder X-ray diffraction (XRD) using PANalytical X´Pert (CoKα radiation) and Bruker D8 Advance (CuKα radiation, secondary graphite monochromator) diffractometers. The data were collected in the angular range from 10 to 145°2θ. The unit-cell parameters were calculated using the whole-profile pattern fitting method using FullProf (Rodríguez-Carvajal, Reference Rodríguez-Carvajal2006).
Electron-microprobe analyses
Chemical analyses were performed with a CAMECA SX-100 electron probe microanalyser (EPMA) in wavelength-dispersion mode using an electron beam focussed to 1–2 μm. Pure elements were used as standards. Concentrations were quantified on the AgLα, PdLα and SKα (overlap correction PdL b1) with an accelerating voltage of 15 keV, and a beam current of 10 nA. The standards used were pure silver, palladium and sphalerite. In a sample, compositional data were collected from several grains within a polished section (minimum: n = 5) and then averaged. The EPMA data of the studied phases are presented in Table 4.
Table 4. EPMA results for the phases studied*.
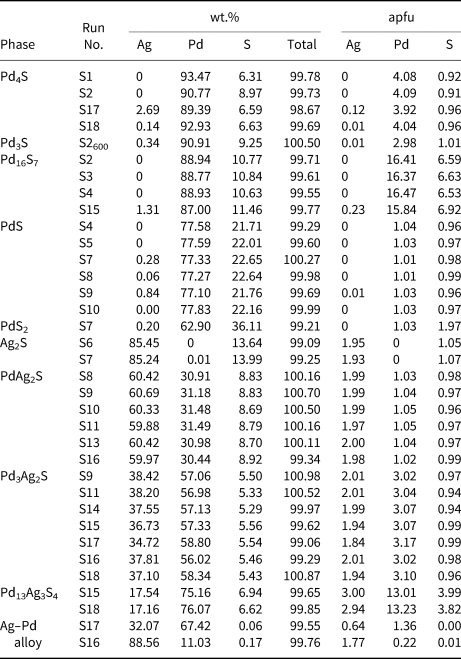
* Compositional data were collected from several grains within a polished section (n = 5) and then averaged. Run products are quenched at 400°C, for S2 (phase Pd3S) at 600°C.
Results and discussion
The system Pd–Ag–S was studied at 400°C and 550°C. Phase assemblages based on EPMA and powder XRD data, are listed in Table 3. The isothermal sections for 400°C and 550°C are shown in Figs 1 and 2, respectively. At 400°C, the system contains three ternary compounds: Pd3Ag2S (coldwellite), PdAg2S (kravtsovite) and Pd13Ag3S4. At 550°C, the only one ternary phase Pd3Ag2S (an analogue of mineral coldwellite) is stable in the system.
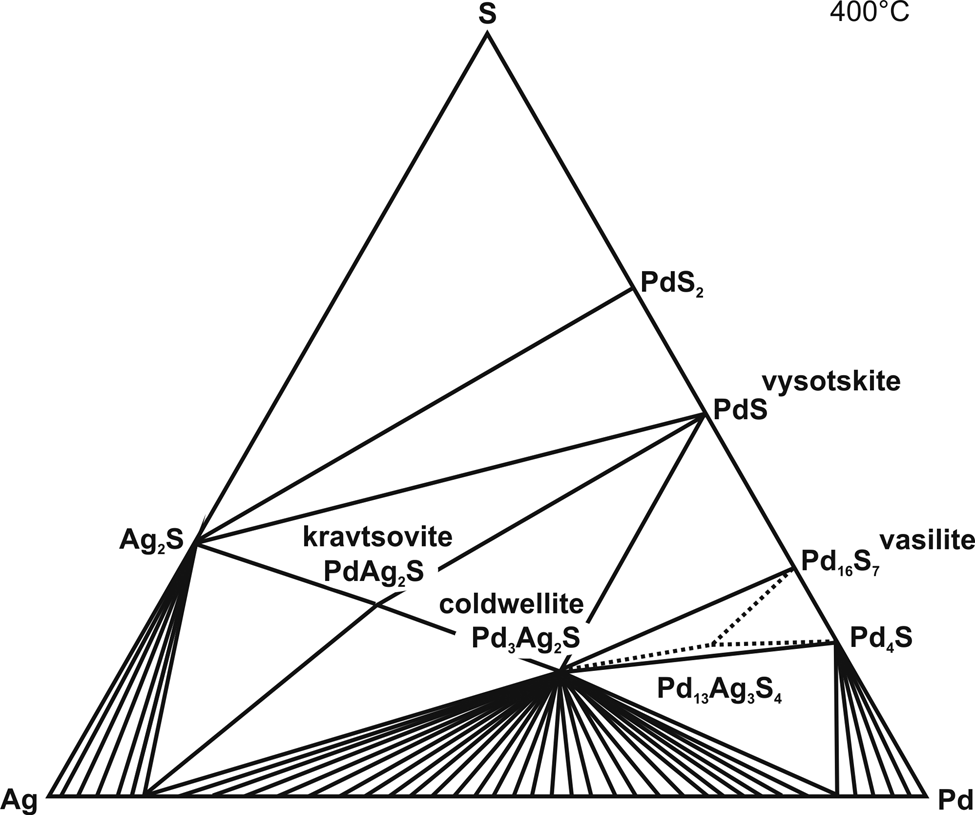
Fig. 1. Isothermal section of the phase diagram of the ternary system Pd–Ag–S system at 400°C.
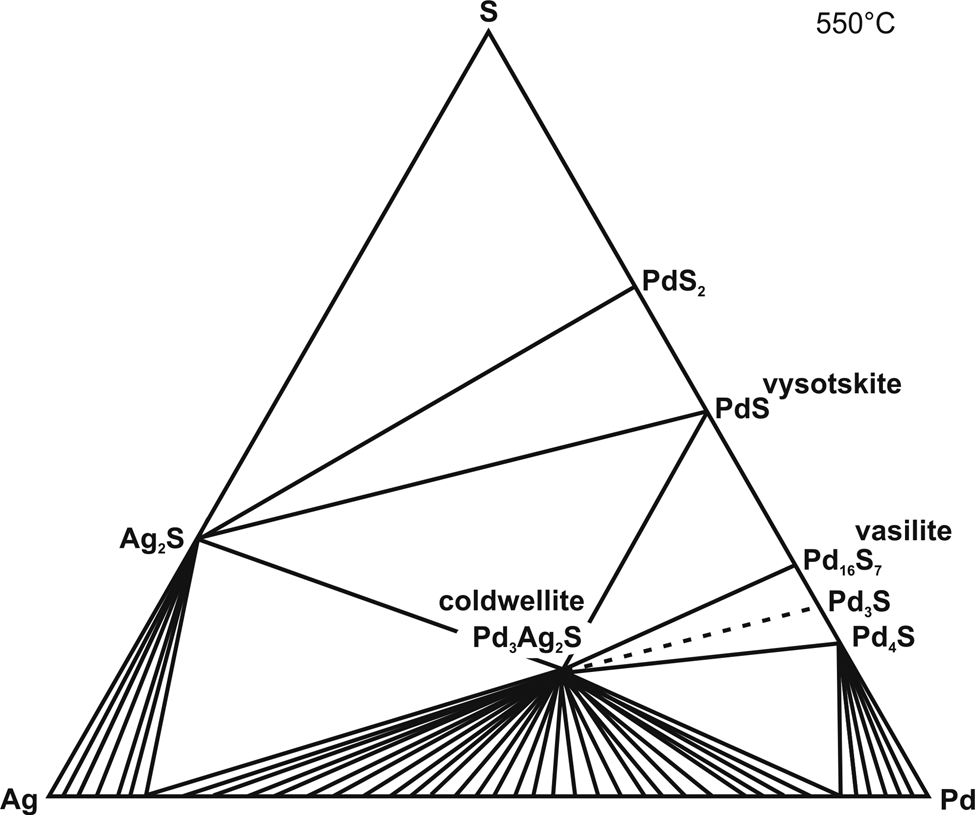
Fig. 2. Isothermal section of the phase diagram of the ternary system Pd–Ag–S system at 550°C.
Binary phases
The following binary phases are stable at 400°C: Pd4S, Pd16S7, PdS and PdS2. The phase Pd4S incorporates up to 2.7 wt.% of Ag and phase Pd16S7, an analogue of the mineral vasilite, incorporates up to 1.3 wt.% of Ag.
The β polymorph (cubic) of phase Ag2S, the analogue of the mineral argentite, is unquenchable and reverses to its α variety Ag2S (monoclinic), the analogue of the mineral acanthite. The phase Ag2S does not incorporate Pd.
Ternary phases
Coldwellite Pd3Ag2S
The phase Pd3Ag2S, the analogue of the mineral coldwellite, forms a stable association with vysotskite and vasilite, vysotskite and kravtsovite, ternary phase Pd13Ag3S4 and Pd4S, and a Ag–Pd alloy (Fig. 3). The XRD data for Pd3Ag2S at 400°C are in an agreement with the crystal-structure data determined by McDonald et al. (Reference McDonald, Cabri, Stanley, Good, Redpath and Spratt2015) for natural Pd3Ag2S (Tables 1 and 2). The phase is stable up to 940°C (Raub et al., Reference Raub, Wullhorst and Plate1954). In the study of El-Boragy and Schubert (Reference El-Boragy and Schubert1971) the phase ‘Pd2AgS’ is assumed to have a β-Mn type structure. However the phase ‘Pd2AgS’ synthesised at 550°C by El-Boragy and Schubert (Reference El-Boragy and Schubert1971) is in fact the phase Pd3Ag2S. The phase ‘Pd2AgS’ is not stable at 550°C (Fig. 2).

Fig. 3. Back-scattered electron images illustrating the assemblages: (a) kravtsovite (PdAg2S) + PdS, Run S8, 400°C; (b) coldwellite (Pd3Ag2S) + vasilite (Pd16S7) + phase Pd13Ag3S4, Run S15, 400°C; (c) coldwellite (Pd3Ag2S) + Ag2S + vysotskite (PdS), Run S8, 550°C; and (d) coldwellite (Pd3Ag2S) + Ag2S + vysotskite (PdS), Run S13, 550°C.
Coldwellite was discovered in a heavy-mineral concentrate from the Marathon Cu–PGE–Au deposit, Coldwell Complex, Ontario in Canada (McDonald et al., Reference McDonald, Cabri, Stanley, Good, Redpath and Spratt2015) and also reported by Ames et al. (Reference Ames, Kjarsgaad, McDonald and Good2017) and Good et al. (Reference Good, Cabri and Ames2017) from the same deposit. Prior to full characterisation of coldwellite by McDonald et al. (Reference McDonald, Cabri, Stanley, Good, Redpath and Spratt2015), a mineral with the same composition was also reported by Seversen and Hauck (Reference Seversen and Hauck2003) from the Birch Lake deposit, Duluth complex, Minnesota in USA and by Subbotin et al. (Reference Subbotin, Korchagin and Savchenko2012) from the Federova–Pana layered intrusion, Kola Peninsula in Russia.
Kravtsovite PdAg2S
The ternary phase PdAg2S, an analogue of the mineral kravtsovite, is orthorhombic, has space group (Cmcm) and unit-cell parameters a = 7.983, b = 5.926 and c = 5.745 Å (Vymazalová et al., Reference Vymazalová, Laufek, Sluzhenikin, Stanley, Kozlov, Chareev and Lukashova2017). The phase is isostructural with the phase Na2AuBi (Kim et al., Reference Kim, Miller and Corbett2010) forming the zigzag chains of [S–Pd–S–Pd].
Kravtsovite forms a stable association with Ag2S and vysotskite; vysotskite and coldwellite; and it also coexists with a Ag–Pd alloy (Fig. 3a). Kravtsovite is stable up to 507°C.
Kravtsovite was found in the same specimen as the holotype for recently described minerals thalhammerite (Pd9Ag2Bi2S4, Vymazalová et al., Reference Vymazalová, Laufek, Sluzhenikin, Kozlov, Stanley, Plášil, Zaccarini, Garuti and Bakker2018) and vymazalováite (Pd3Bi2S2, Sluzhenikin et al., Reference Sluzhenikin, Kozlov, Stanley, Lukashova and Dicks2018). The sample came from the vein-disseminated pyrite–chalcopyrite–galena ore characterised by lack of Ni minerals, high galena content and Pt–Pd–Ag bearing minerals, in an association of pyrite and chalcopyrite (Talnakh deposit, Noril'sk district, Sluzhenikin, Reference Sluzhenikin2011; Sluzhenikin and Mokhov, Reference Sluzhenikin and Mokhov2015). Kravtsovite was also found in the W Horizon in the Marathon Cu–Pd deposit (Ames et al., Reference Ames, Kjarsgaad, McDonald and Good2017).
The phase Pd13Ag3S4
In the Pd–Ag–S system we observed a new phase Pd13Ag3S4 that coexists with coldwellite and vasilite (Fig. 3b); coldwellite and phase Pd4S; vasilite and phase Pd4S at 400°C, but is not stable at 550°C. However, we were unable to synthesise the pure phase. All attempts to prepare the pure phase resulted in a mixture containing the phase Pd13Ag3S4 and other phases (e.g. Pd3Ag2S, Pd16S7 or Pd4S). Therefore, the phase is depicted tentatively in the phase diagram (Fig. 1).
The most intensive peaks in XRD patterns of this phase can be indexed by a cubic cell with a = 7.236 Å though several weak peaks remained unindexed. Attempts to index all peaks attributed to the phase Pd13Ag3S4 remained unsuccessful. The above-mentioned cubic subcell suggests a possible structural relationship of this phase to coldwellite. However, Rietveld refinement using a coldwellite-like structure model yielded an unacceptable fit. Even various attempts to refine the occupancy parameters in a coldwellite structure model of Pd13Ag3S4 phase did not result in satisfactory agreement factors.
Phase relations
Phase relations confirmed the stable mineral assemblages that are known to occur in nature, in addition they can also explain the mineral sequences of formation and define the mineral assemblages that can be expected to occur in Nature. The following assemblages of unknown phases with minerals can be expected (Fig. 1):
 $$\eqalign{& {\rm S}_l\,+ \,{\rm A}{\rm g}_2{\rm S}\,+ \,{\rm Pd}{\rm S}_2\semicolon \;\cr & {\rm A}{\rm g}_2{\rm S}\,+ \,{\rm Pd}{\rm S}_2\,+ \,{\rm vysotskite} \;\lpar {\rm PdS}\rpar \semicolon \;\cr & {\rm A}{\rm g}_2{\rm S}\,+ \,{\rm kravtsovite} \; \lpar {{\rm PdA}{\rm g}_2{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \semicolon \;\cr & {\rm kravtsovite} \; \lpar {{\rm PdA}{\rm g}_2{\rm S}} \rpar \,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_{2}{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \semicolon \;\cr & {\rm coldwellite} \; \lpar {{\rm P}{\rm d}_3{\rm A}{\rm g}_2{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \,+ \,{\rm vasilite} \; \lpar {{\rm P}{\rm d}_{16}{\rm S}_7} \rpar.} $$
$$\eqalign{& {\rm S}_l\,+ \,{\rm A}{\rm g}_2{\rm S}\,+ \,{\rm Pd}{\rm S}_2\semicolon \;\cr & {\rm A}{\rm g}_2{\rm S}\,+ \,{\rm Pd}{\rm S}_2\,+ \,{\rm vysotskite} \;\lpar {\rm PdS}\rpar \semicolon \;\cr & {\rm A}{\rm g}_2{\rm S}\,+ \,{\rm kravtsovite} \; \lpar {{\rm PdA}{\rm g}_2{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \semicolon \;\cr & {\rm kravtsovite} \; \lpar {{\rm PdA}{\rm g}_2{\rm S}} \rpar \,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_{2}{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \semicolon \;\cr & {\rm coldwellite} \; \lpar {{\rm P}{\rm d}_3{\rm A}{\rm g}_2{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \,+ \,{\rm vasilite} \; \lpar {{\rm P}{\rm d}_{16}{\rm S}_7} \rpar.} $$At 550°C only one ternary phase, coldwellite (Pd3Ag2S) is stable (Fig. 2). Coldwellite coexists with palladium sulfides PdS, and Pd16S7. The binary phase Pd3S appears in the system at 555°C (Matković et al., Reference Matković, El-Boragy and Schubert1976). Subsequently the following assemblages become stable 550°C:
 $$\eqalign{& {\rm Ag}_2{\rm S}\,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_2{\rm S}} \rpar \,+ \,{\rm Ag}{-}{\rm Pd} \;{\rm alloy}\semicolon \;\cr & {\rm Ag}_2{\rm S}\,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_2{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \semicolon \;\cr & {\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_2{\rm S}} \rpar \,+ \,{\rm vasilite} \; \lpar {{\rm Pd}_{16}{\rm S}_7} \rpar.} $$
$$\eqalign{& {\rm Ag}_2{\rm S}\,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_2{\rm S}} \rpar \,+ \,{\rm Ag}{-}{\rm Pd} \;{\rm alloy}\semicolon \;\cr & {\rm Ag}_2{\rm S}\,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_2{\rm S}} \rpar \,+ \,{\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \semicolon \;\cr & {\rm vysotskite} \; \lpar {{\rm PdS}} \rpar \,+ \,{\rm coldwellite} \; \lpar {{\rm Pd}_3{\rm Ag}_2{\rm S}} \rpar \,+ \,{\rm vasilite} \; \lpar {{\rm Pd}_{16}{\rm S}_7} \rpar.} $$The occurrence of coldwellite, Ag2S and vysotskite together in equilibrium reflects the formation of the mineral assemblage above the temperature 507°C.
In comparison with other Ag–Pd chalcogenides, there are no analogues or isostructural ternary phases in the systems Pd–Ag–Se and Pd–Ag–Te (Vymazalová et al., Reference Vymazalová, Chareev, Kristavchuk, Laufek and Drábek2014, Reference Vymazalová, Laufek, Kristavchuk, Chareev and Drábek2015b, respectively).
Conclusions and implications
We have determined phase relations in the system Pd–Ag–S at 400°C and 550°C. Three ternary phases were observed in the system at 400°C: Pd3Ag2S (coldwellite), PdAg2S (kravtsovite) and phase Pd13Ag3S4. At 550°C only one ternary phase Pd3Ag2S (coldwellite) is stable.
Coldwellite, at 400°C forms a stable association with vysotskite and vasillite, coexists with ternary phase Pd13Ag3S4 and Pd4S, and also coexists with kravtsovite and a Ag–Pd alloy. Kravtsovite is stable up to 507°C; the presence of kravtsovite in the mineral assemblage reflects its formation below this temperature. In contrast, the occurrence of coldwellite, vysotskite and Ag2S together in equilibrium reflects the formation of a mineral assemblage above 507°C. Coldwellite is stable up to 940°C and may occur at high-temperature assemblages at natural conditions.
Mineral assemblages defined in this study can be expected in Cu–Ni–PGE mineral deposits, associated with mafic and ultramafic igneous rocks and in particular in those mineralisations where Pd–Ag sulfides are known to occur such as deposits in Noril'sk–Talnakh in Russia and the Marathon deposit in the Coldwell Alkaline Complex in Canada.
Acknowledgements
This work was supported by the Grant Agency of the Czech Republic (project No. 18–15390S) and by the Russian Science Foundation (grant No 17–17–01220). The work of DACh is supported by the program 211 of the Russian Federation Government, agreement No. 02.A03.21.0006, by the Russian Government Program of Competitive Growth of Kazan Federal University. The authors are grateful to Dr. Vlasta Böhmová and Dr. Zuzana Korbelová for carrying out the electron-microprobe analyses, and to Dr. Tatyana B. Shatalova for performing DTA analyses. Constructive comments made by Louis J. Cabri and an anonymous reviewer are greatly appreciated; the editorial handling by Stuart Mills is also acknowledged.









