Introduction
Cognitive disorders are found in approximately half of persons with multiple sclerosis (MS; Chiaravalloti & DeLuca, Reference Chiaravalloti and DeLuca2008), and are related to the volume of demyelinating lesions and brain atrophy (Benedict & Zivadinov, Reference Benedict and Zivadinov2011). Typically, correlations between cognitive performance and brain imaging account for 25–30% of the variance, begging the question why some patients with prominent lesion burden and atrophy retain normal cognitive function.
Cognitive reserve theory may explain some of the variance in cognitive abilities in MS. This construct is defined as “individual differences in how people process tasks allow[ing] some to cope better than others with brain pathology” (Stern, Reference Stern2009, page 2016). Additional research suggests that cognitive reserve developed early in life, as measured by lifetime experience metrics such as intelligence and childhood enrichment, is associated with better cognitive performance in MS (Amato et al., Reference Amato, Razzolini, Goretti, Stromillo, Rossi, Giorgio and De Stefano2013; Sumowski, Chiaravalloti, Leavitt, & Deluca, Reference Sumowski, Chiaravalloti, Leavitt and Deluca2012). In our prior work (Schwartz, Snook, Quaranto, Benedict, & Vollmer, Reference Schwartz, Snook, Quaranto, Benedict and Vollmer2013; Schwartz, Quaranto, Healy, Benedict, & Vollmer, Reference Schwartz, Quaranto, Healy, Benedict and Vollmer2013; Schwartz, Snook, Quaranto, Benedict, Rapkin, et al., Reference Schwartz, Snook, Quaranto, Benedict, Rapkin and Vollmer2013), we have distinguished between passive and active aspects of cognitive reserve, the former reflecting cognitive and neural development, and the latter ongoing, possibly post-injury, activities that “keep the brain active and fit” (Schwartz, Snook, Quaranto, Benedict, & Vollmer, Reference Schwartz, Snook, Quaranto, Benedict and Vollmer2013, page 87). MS patients with higher active cognitive reserve report lower perceived disability, better functional health, higher well-being, and increased ability to remain in a positive mindset and focus on improving quality of life (Schwartz, Snook, Quaranto, Benedict, & Vollmer, Reference Schwartz, Snook, Quaranto, Benedict and Vollmer2013; Schwartz, Snook, Quaranto, Benedict, Rapkin, et al., Reference Schwartz, Snook, Quaranto, Benedict, Rapkin and Vollmer2013).
We investigated how changes in recreational activities influence the correlation between brain atrophy and neurocognitive performance. We hypothesized that in patients reporting a decrease in recreational activity participation (active reserve loss), the correlation between atrophy and cognition would be robust, and lower or absent in patients maintaining recreational activities.
Methods
Participants
We enrolled 57 people with MS providing informed consent in accordance with the SUNY Buffalo Institutional Review Board. Patients were predominantly female (43 or 75%) and Caucasian (7 African American, and 1 Latino). Mean age and years of education was 43.0 ± 8.8 and 14.5 ± 2.2, respectively. The median Expanded Disability Status Scale (EDSS) was 2.5, range 0 to 6.5. For descriptive purposes, MS patients were compared to 27 healthy controls matched on demographics as per non-significant t and χ2 tests (17 or 63% female; 24 Caucasian; age 41.9 ± 10.7; mean education 15.26 ± 2.3).
Inclusion criteria were (a) clinically definite MS by Polman et al. criteria (Reference Polman, Reingold, Edan, Filippi, Hartung, Kappos and Wolinsky2005) and relapsing-remitting course (Lublin & Reingold, Reference Lublin and Reingold1996), (b) no history of drug/alcohol abuse or dependence, (c) no history of ADHD or learning disability, (d) no comorbid conditions that may influence cognition or motor function; (e) absence of corticosteroid use or relapse within 4 weeks of assessment; (f) lack of a level of physical impairment that would make cognitive and language testing infeasible, (g) native speaker of the English language, (h) no psychiatric disease predating MS and no current major depression, (i) no current/prior use of antipsychotic medication.
Procedures
All procedures were conducted in an outpatient clinical setting in an urban hospital in the Eastern United States.
MRI was obtained using a 3 Tesla General Electric Twin Speed eight-channel scanner. We acquired two-dimensional (2D) multi-planar dual fast spin-echo (SE) PD/T2-weighted images [WI] (TE1/TE2/TR = 9/98/5300 ms, flip angle (FA)=90, echo train length ETL = 14; field-of-view (FOV) of 25.6 × 19.2 cm (256 × 256 matrix with Phase FOV = 0.75), for an in-plane resolution of 1 × 1 mm2; 48 slices of 3 mm thickness and no gap, 2D Fluid-Attenuated Inversion-Recovery (FLAIR) (TE/TI/TR = 120/2100/8500 ms (TI-inversion time), FA = 90, ETL = 24, FOV 25.6 × 19.2 cm (256 × 256 matrix with Phase FOV = 0.75), 1 × 1 mm2, 48 slices of 3 mm no gap) and high resolution 3DT1-WI (fast spoiled gradient-echo with magnetization-prepared inversion recovery (IR-FSPGR), echo time/inversion time/repetition time (TE/TI/TR) = 2.8/900/5.9 ms, FA = 10, FOV 25.6 × 19.2 cm (256 × 256 matrix with Phase FOV = 0.75), 1 × 1 mm2, 128 slices of 1.5 mm). An experienced MRI technician obtained the third ventricle width (3VW) on relevant T2/PD/FLAIR images that were co-registered using a rigid body (6-degrees-of-freedom) registration, as described previously (Benedict et al., Reference Benedict, Bruce, Dwyer, Abdelrahman, Hussein, Weinstock-Guttman and Zivadinov2006). Normalized neocortical volume was segmented by using SIENAX (Smith et al., Reference Smith, Zhang, Jenkinson, Chen, Matthews, Federico and De Stefano2002). Normalized thalamus volumes were obtained by multiplying the estimated volumes from FIRST (Patenaude, Smith, Kennedy, & Jenkinson, Reference Patenaude, Smith, Kennedy and Jenkinson2011) by the volumetric scaling factor from SIENAX. The T2 lesion volume was measured on FLAIR scans using a semi-automated edge detection technique (Zivadinov et al., Reference Zivadinov, Sepcic, Nasuelli, De Masi, Bragadin, Tommasi and Zorzon2001) modified using an in-house developed inpainting technique to avoid the impact of white matter lesions on all gray matter volume measurements.
The Paced Auditory Serial Addition Test (PASAT; Gronwall, Reference Gronwall1977) and the oral version of the Symbol Digit Modalities Test (SDMT; Smith, Reference Smith1982) were combined to create an Information Processing Speed (IPS) index, as in prior work (Benedict, Morrow, Weinstock-Guttman, Cookfair, & Schretlen, Reference Benedict, Morrow, Weinstock-Guttman, Cookfair and Schretlen2010; Sumowski et al., Reference Sumowski, Chiaravalloti, Leavitt and Deluca2012; Sumowski, Chiaravalloti, Wylie, & Deluca, Reference Sumowski, Chiaravalloti, Wylie and Deluca2009). The Beck Depression Inventory – Fast Screen (BDIFS; Beck, Steer, & Brown, Reference Beck, Steer and Brown2000) was used to quantify depression. Passive cognitive reserve was assessed using years of education as well as the North American Adult Reading Test (NAART; Friend & Grattan, Reference Friend and Grattan1998). Active cognitive reserve (loss) was operationalized using the Recreation and Pastimes scale from the Sickness Impact Profile (SIP: Bergner, Bobbitt, Carter, & Gilson, Reference Bergner, Bobbitt, Carter and Gilson1981; Gilson et al., Reference Gilson, Gilson, Bergner, Bobbitt, Kressel, Pollard and Vesselago1975). On this measure, patients respond yes/no to statements reflecting decline in activities since diagnosis. The eight items query hobbies, inactive recreation, entertainment, community activities, and physical recreation.
Statistical Analysis
The active reserve metric was based on the SIP Recreation and Pastimes scale as described above. Exploratory factor analysis revealed an unrotated principle-component analysis, two-factor solution. Factor 1 (eigenvalue 2.78) was composed of items 1, 2, and 5–8. These items ask about hobbies, entertainment, community activities, and exercise. Factor 2 included only item 3, which asks about purely inactive forms of recreation (e.g., TV watching) and as such was excluded. Item 4 was excluded because no subject endorsed it. The active reserve metric was the sum of the remaining items treated as a continuous variable in regression analysis. In addition, we categorized patients reporting a decrease in any activity as reduced reserve, and patients reporting no decrease were placed in the stable group.
Hierarchical regression tested the hypothesis, that active reserve moderates the correlation between brain atrophy and cognition. In step 1 (baseline model), the IPS index was regressed on the MRI metric, demographics, and clinical covariates. Significant predictors were carried to step 2 (passive reserve model) where the passive reserve measures, NAART and education, were added. Again, significant predictors were carried forward to step 3 (active reserve model), where the active reserve variable was added. In the final step 4 model, the atrophy-by-reserve interaction term was added, keeping the sole active reserve variable to gauge moderation effects. This final step was then repeated, including an atrophy interaction term for the most robust passive reserve measure (e.g., 3VW*NAART).
Results
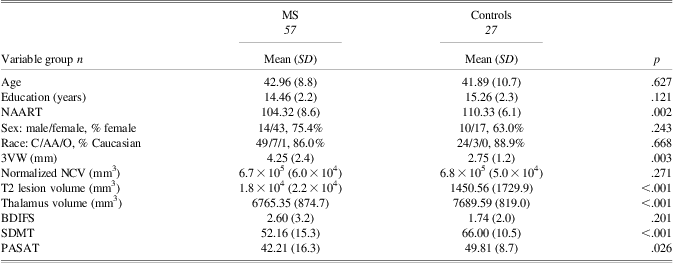
Demographic and clinically relevant information is presented in Table 1. The MS group differed significantly from controls on third ventricle width (3VW; MS 4.3 ± 2.4 mm, controls 2.8 ± 1.2 mm; p = .003; d = 0.7), Symbol Digit Modalities Test (SDMT; MS 52.2 ± 15.3; controls 66.0 ± 10.5; p < .001; d = 1.0), and Paced Auditory Serial Addition Test (PASAT; MS 42.2 ± 16.3, controls 49.8 ± 8.7; p = .026; d = 0.5). These differences confirm expected impairments on brain atrophy and information processing speed (IPS).
Table 1 Between-group comparisons: demographic and clinically relevant data for MS and control groups

Note. NAART = North American Adult Reading Test; 3VW = Third Ventricle Width; NCV = Neocortical Volume; BDIFS = Beck Depression Inventory Fast Screen; SDMT = Symbol Digit Modalities Test; PASAT = Paced Auditory Serial Addition Test.
In the MS group, IPS was negatively correlated with 3VW (r = −.41; p = .002). However, while this correlation was moderate in patients reporting decreased activities (n = 36; r = −.45; p = .006), there was no such correlation in stable patients (n = 21; r = −.05; p = .818) (Figure 1). The absence of significant correlation in stable patients was not an artifact of frequency distribution (Figure 2).

Fig. 1 This figure illustrates the significant moderating effect of active cognitive reserve on brain atrophy and information processing speed. Patients with stable active reserve (shown by the dashed line) had little or no effect of brain atrophy, suggesting that they have the capacity to resist the clinical expression of cerebral injury. In contrast, patients reporting loss in active cognitive reserve (shown by the solid line) had significantly reduced information processing speed with brain atrophy. Information Processing Speed index was computed from the average Z-score for SDMT and PASAT. Active cognitive reserve (loss) was operationalized using the Recreation and Pastimes subscale from the Sickness Impact Profile.

Fig. 2 Scatterplot of information processing scores (IPS) by third ventricle width (3VW) in stable versus declining active reserve participants.
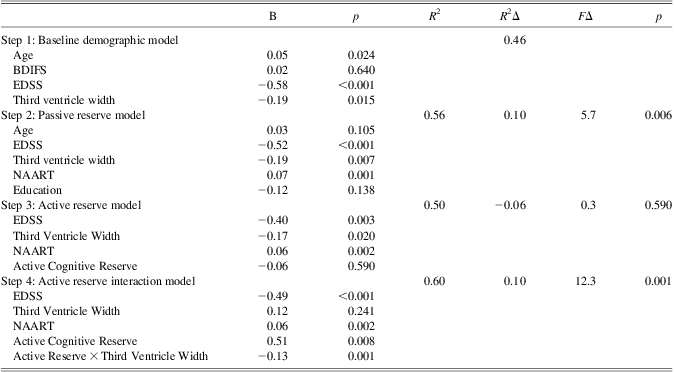
The hierarchical regression results for 3VW are presented in Table 2. In the first model, age, EDSS and 3VW were significant predictors of IPS, and BDIFS was dropped from further consideration. In the second model, NAART predicted additional variance raising the R 2 from 0.46 to 0.56. Age and education were insignificant and dropped from the third model, and the active reserve factor on its own did not contribute significant variance. In the final model, where the moderation effect of 3VW-by-active reserve interaction was assessed, adding the interaction term raised the R 2 by 10.0% of the variance in IPS (p = .001). To control for the possible influence of a 3VW-by-NAART interaction term, we re-ran this final step including both 3VW*active reserve, and 3VW*NAART, and the latter was not significant (p = .195).
Table 2 Multiple regression results for third ventricle width (3VW)

Note. The p values reflect significance of R 2Δ.
BDIFS = Beck Depression Inventory Fast Screen, NAART = North American Adult Reading Test, EDSS = Expanded Disability Status Scale. The dependent variable in the regression was the Information Processing Speed (IPS) index computed from the average z-score for Symbol Digit Modalities Test (SDMT) and Paced Auditory Serial Addition Test (PASAT).
The regression analysis was repeated for neocortical volume, thalamus volume, and T2 lesion volume. The final model R 2 for neocortical volume was 0.60, and adding the neocortical volume-by- active reserve interaction term contributed 5% of additional variance (not in Table 2) (p = .014). For thalamus volume, the final model R 2 was 0.58, and adding the thalamus volume-by- active reserve interaction term also contributed 5% of additional variance (not in Table 2) (p = .017). Finally, the T2 lesion volume final model resulted in a total R 2 of 0.59, and the incremental variance was 3% (p = .055).
Discussion
There is a rapidly growing literature supporting the role of cognitive reserve in MS. However, much of this work has used proxies of factors antecedent to disease onset. High premorbid intelligence protects against the neuropsychological effects of brain atrophy (Sumowski et al., Reference Sumowski, Chiaravalloti, Wylie and Deluca2009), and moderates decline in cognition (Benedict et al., Reference Benedict, Morrow, Weinstock-Guttman, Cookfair and Schretlen2010). In this study, we investigated the moderating effects of ongoing stimulating activities, post-disease onset, that may maintain or improve a person's capacity to resist the clinical expression of cerebral injury. The SIP Recreation and Pastimes scale was selected because it queries the extent to which enrichment activities have decreased in frequency. The scope of cognitive reserve is expanding, including, for example, leisure activities (Sumowski, Wylie, Gonnella, Chiaravalloti, & Deluca, Reference Sumowski, Wylie, Gonnella, Chiaravalloti and Deluca2010) and occupational attainment (Ghaffar, Fiati, & Feinstein, Reference Ghaffar, Fiati and Feinstein2012), but this is the first study to report on the moderating effects of recent changes in what we call active cognitive reserve.
Our results show that both passive and active reserve measures contribute to the clinical expression of cognitive impairment in MS. Patients with reported declines in recreational activity demonstrated a significant relationship between neurocognitive functioning and brain atrophy, while those who maintained recreational activities did not. This difference remained significant after controlling for demographics, premorbid IQ and EDSS. The results are in keeping with other research suggesting that maintaining active cognitive reserve is protective against disability progression and that such activities are feasible across the disability spectrum (Schwartz, Snook, Quaranto, Benedict, & Vollmer, Reference Schwartz, Snook, Quaranto, Benedict and Vollmer2013; Schwartz, Quaranto, et al., Reference Schwartz, Quaranto, Healy, Benedict and Vollmer2013; Schwartz, Snook, Quaranto, Benedict, Rapkin, et al., Reference Schwartz, Snook, Quaranto, Benedict, Rapkin and Vollmer2013) and are a potential target for future interventions for cognitive function in MS.
The cross-sectional design of the current study is a limitation; thus, these findings might best be considered hypothesis-generating. Future studies will need to consider the possibility that cognitive impairment is the primary driver of these relationships, reducing activities and thereby lowering cognitive reserve potential. We are presently collecting longitudinal data on this cohort that will afford analysis of change over time and causal inference. Also, the current study suggested moderating effects on all MRI outcomes examined. This is consistent with recent literature emphasizing the detrimental effects of regional and whole-brain atrophy on cognitive function in MS (Benedict & Zivadinov, Reference Benedict and Zivadinov2011). Replicating these findings, however, will be important to confirm these preliminary results. Finally, the present study examined only patients with relapsing-remitting MS, the most common initial course of the illness which is also the most responsive to recovery from demyelinating activity. We would expect cognitive reserve to be more intact in patients with relapsing-remitting, as opposed to progressive course. Sumowski and colleagues (2012) found that passive cognitive reserve is a significant factor in secondary progressive MS. Work done by our group suggests that active cognitive reserve has protective effects against disability progression (Schwartz, Quaranto, et al., Reference Schwartz, Quaranto, Healy, Benedict and Vollmer2013), but the mechanism is currently unknown. Future longitudinal research should investigate whether enhancing patients’ active cognitive reserve can hinder disease progression.
Acknowledgments
Research supported by a grant from the National Multiple Sclerosis Society, PI: R. Benedict. The authors report no conflicts of interest. The authors would like to acknowledge Dr. Marietta Hoogs for her contribution to recruitment and data collection.