In Tunisia, there are some 100 000 camels (Camelus dromedarius). They are reared under an extensive system in arid and desert regions to produce meat, milk and fibre. Under these conditions, the daily milk production is about 2 l/animal. Nowadays, there is increasing interest in camels’ milk for human nutrition in other population sectors of different parts of the world. It has been proposed as a substitute for cows’ milk in allergic children, as a substitute for mothers’ milk for premature newborns, and as a therapeutic way to repress hyperglycaemia in diabetic patients (Agrawal et al. Reference Agrawal, Swami, Beniwal, Kochar and Kothari2003; Sboui et al. Reference Sboui, Khorchani, Djegham, Agrebi, Elhatemi and Belhadj2009a). However, an extensive production system cannot meet the increasing demand nor guarantee constant milk quantity and quality for urban markets. As a result, many intensive dairy camel farms have been recently created around the world, a majority of them using machine milking (Juhasz & Nagy, Reference Juhasz, Nagy, Nagy, Huszenicza and Juhasz2008; Ayadi et al. Reference Ayadi, Hammadi, Khorchani, Barmat, Atigui and Caja2009; Hammadi et al. Reference Hammadi, Atigui, Ayadi, Barmat, Belgacem, Khaldi and Khorchani2010).
An efficient milking machine should be able to remove milk from the udder as gently, quickly and completely as possible with the minimum of manual intervention (Labussière, Reference Labussière1988). This principle of efficient milking describes the basic characteristics of good ‘milkability’. Usually, ‘milkability’ of an animal is measured as the higher milking speed, the shorter milking duration and the minimum milking labour (Lee & Choudhary, Reference Lee and Choudhary2006). Consequently, if ‘milkability’ results from numerous factors such as teat and udder shape and internal anatomy (Ovesen, Reference Ovesen1972; Labussière, Reference Labussière1988; Rovaï et al. Reference Rovaï, Thomas, Berger and Caja2004; Tilki et al. Reference Tilki, Olak, Ünal and Aulayan2005; Dzidic, Reference Dzidic2009) or temperament of the animals (Lyons, Reference Lyons1989; Knierem, Reference Knierem1991; Murray et al. Reference Murray, Blache and Bencini2009) it can be functionally evaluated by the analysis of milk flow curves during milking. They have been commonly recorded for a long time in experiments (Labussière & Martinet, Reference Labussière and Martinet1964) to evaluate the quality of the milking process and animals’ individual physiological stimulation and milking performance. It has been proved that the pattern of milk emission is typical for each species and has been well studied in dairy cows (Bruckmaier et al. Reference Bruckmaier, Rothenanger and Blum1995; Tancin et al. Reference Tancin, Ipema, Hogewerf and Mačuhovà2006; Sandrucci et al. Reference Sandrucci, Tamburini, Bava and Zucali2007), goats (Bruckmaier et al. Reference Bruckmaier, Schams and Blum1994; Billon et al. Reference Billon, Chastin, Baritaux, Bouvier, Ilahi, Manfredi, Marnet, Zervas and Barillet1999; Marnet et al. Reference Marnet, Billon, Da Ponte, Martin and Manfredi2001), ewes (Labussière, Reference Labussière1988; Mayer et al. Reference Mayer, Weber and Segessemann1989; Marnet et al. Reference Marnet, Combaud, Dano, Zervas and Barillet1999) and buffaloes (Bava et al. Reference Bava, Sandrucci, Tamburini and Zucali2007; Boselli et al. Reference Boselli, Mazzi, Borghese, Terzano, Giangolini, Filippetti, Amatiste and Rosati2010). In general, the milk flow pattern depends on the milk partitioning in the udder, milk ejection reflex and teat anatomy (Labussière, Reference Labussière1988; Marnet & McKusick, Reference Marnet and McKusick2001; Boselli et al. Reference Boselli, Mazzi, Borghese, Terzano, Giangolini, Filippetti, Amatiste and Rosati2010). Milk within the udder can be divided into two fractions: cisternal milk which is immediately extracted by the machine without oxytocin release; and alveolar milk which can only be removed by the active involvement of the animal, when oxytocin release and milk ejection occurs (Bruckmaier & Blum, Reference Bruckmaier and Blum1998). When the animals are physiologically stressed owing to acute events or long-term bad conditions of milking (Tancin & Bruckmaier, Reference Tancin and Bruckmaier2001), we can record physiological responses such as high levels of cortisol and reduced sensitivity to ACTH (Bruckmaier & Wellnitz, Reference Bruckmaier and Wellnitz2007) and catecholamines release (Bruckmaier et al. Reference Bruckmaier, Wellnitz and Blum1997). That leads to a clear partial or total inhibition of milk ejection reflex and a delay in milk ejection and/or reduced milk flow is often described (Wellnitz & Bruckmaier, Reference Wellnitz and Bruckmaier2001). Such a delayed milk ejection causes bimodal milk flow curves (Tancin et al. Reference Tancin, Urhincat, Mačuhovà and Bruckmaier2007). The first peak of milk flow matches the cisternal milk and the second the alveolar milk. Animals with tight streak canals such as buffaloes (Borghese et al. Reference Borghese, Rasmussen and Thomas2007) or within dairy cows (Corbet et al. Reference Corbet, Billon, Allain, Saunier and Huneau2010), or needing a high vacuum to open teat sphincters (Marnet et al. Reference Marnet, Combaud, Dano, Zervas and Barillet1999) are characterised by lower milk flow rate and longer duration of milking.
The current selection of machine milked she-camels is usually only based on the individual performance of animals and on their behavioural response (aggressiveness) during training to machine milking. Although milk flow recording is one of the most important traits to evaluate dairy animals’ ‘milkability’ with animal morphology of udders and teats, there is no research that highlights milk flow curves of dromedary camels, as far as we know. Without such information, it is difficult to propose some way of improvement of machine milking equipment and settings. The aim of this study was to describe for the first time the milk flow curves and to define milking characteristics of animals in order to evaluate the milking ability in dairy camels under our conditions.
Materials and methods
Animals and their management
A total of 124 milk flow curves were registered during morning milking belonging to 20 dairy Maghrebi dromedary camels from the experimental farm of the Arid Regions Institute (IRA, Chenchou, Tunisia). These camels were selected to have close teat shape and dimension (teat diameter: 3·46±1·11 and 3·58±1·06 cm; teat length 5·48±1·67 and 5·76±1·30 cm for front and rear teats respectively), in order to limit possible effects of bad matching of material to animals.
The trial was conducted during April–December 2011 after a training period. Before the exclusive machine milking period, camels were hand-milked twice (8·00 and 16·00) in a place close to the enclosure reserved for machine milking. Four days prior to start of exclusive machine milking, the milking machine was run at the same time as manual milking to adapt the camels to the noise. Camels were hand-milked only once (morning) the day before being machine-milked. The storage of milk in the udder 24 h after hand-milking increases mammary pressures and may encourage the camel to be milked. On the first day of machine milking, if no milk was ejected, an i.v. injection of oxytocin (10 IU/camel) was given before milking to allow a complete udder emptying and avoid drying. During the following days, oxytocin dosage was reduced progressively until the milk ejection reflex was re-established, which could need about 2–3 weeks depending on the background of the animal (Hammadi et al. Reference Hammadi, Atigui, Ayadi, Barmat, Belgacem, Khaldi and Khorchani2010). Our experiment began after this period.
The camels retained for this experiment were in lactations 1–8 and were 6–19 years old. The milk flow kinetics were recorded monthly between months 4 and 15 of lactation. Since proper machine milking matches the beginning of the fourth month of lactation, we assumed that the period 4–5 months of lactation corresponds to ‘early lactation’, 6–10 months to ‘mid lactation’ and 11–15 months to ‘late lactation’ in this study.
Milking routine
In order to fit with the traditional interval between milking applied by camel breeders in south Tunisia, our camels were routinely milked twice a day (8·00 and 16·00) in a restraining stall using a portable bucket milking machine [Model AM/T115, AGROMILK, 42020 S.Polo d'Enza (Reggio Emilia), Italy] which was set at 48 kPa, 80 pulses/min and 60 : 40 pulsation ratio previously determined to be the best for these animals and this material (Atigui et al. Reference Atigui, Hammadi, Barmat, Khorchani and Marnet2011). DeLaval Clusters (ref of the milking claw: 00100349 S/S Alfa/Laval type 180 cc for cows; reference and characteristics of the rubber: 91000301: length 320 mm, diameter of mouthpiece of 25 mm, found to be the best fit for the udder and teat shape of our camels) were attached after a short (<10 s) teat washing and drying. A machine stripping was performed 15 s after the milk flow decreased to less than 0·1 kg/min, by manual massage and pulling down the milking cluster before the vacuum shut off. After cluster take-off, a teat dipping (Polycide, Laboratoires Interchem, Tunis, Tunisia) was performed. Our camels never received any concentrate feed during milking in this experiment.
Milk flow recording and evaluation
Milk flow was continuously recorded during morning milking using electronic milk flow meters (Lactocorder®, WMB AG, Balgache, Switzerland) especially calibrated to low milk flow rates (<0·05 kg/min; goat calibration). This apparatus had not at this time any ICAR approval for camels’ milk measurement because it was the first study using such a material for milk emission kinetic recording in camels. Nevertheless, we considered this material as well adapted because of several factors: first, the hardware of lactocorder is the same for cow and goats and is proven to be efficient to measure accurately milk production for animals producing 100 ml to more than 40 l of milk and milk flow between 100 ml/min (goat calibration) and more than 12 kg/min (cow calibration) (±2% precision and 2·5% se; ICAR Standard). Camels’ milk production and milk flow are both within these ranges. Second, the measurements made by the associated software lactopro® do not permit the extraction of individual data per time unit and we were obliged to use the data processed by the software. This software was regularly updated for goats and at least for the parameters retained in this study (see below) we were sure of the validity of the data measured (our laboratory has contributed to Lactopro® software improvement with WMB firm for goat use). We also checked by bucket weighting (after minus before milking) and simulatenous time recording that the data of milk quantity (total, machine and stripping milk) and mean milk flow were exact and representative. The evaluation of bimodality of milk ejection by lactopro® software was not used because it seems to be not adapted for camels. We preferred to determine this trait visually when two clear milk flow rises were recorded during the same milking (the first between teat cup attachment and 45 s and the second after 45 s). Third, the milk quality of camels is very close to mean cow milk quality (Sboui et al. Reference Sboui, Khorchani, Djegham and Belhadj2009b) that ensures also a good milk circulation and detection by Lactocorder apparatus. We paid particular attention to avoiding air entry and associated excessive foaming that could modify measurements. It was also the reason why we did not use the automatic sampling device of lactocorder and preferred the sampling of total milk after homogenisation directly in the bucket.
The following milking traits were calculated by Lactopro® software or manually recorded (Fig. 1): milk flow latency (time between the attachment of teat cups and a milk flow of 0·250 kg/min), lag time or time to milk ejection occurrence (from milking cluster attachment till milk ejection occurs; visually determined when teats suddenly swell and manually recorded), total milking duration (from the attachment of the clusters till their removal; manually recorded), effective milking duration (time for main milk fraction recovery), machine milk yield (from when milk flow exceeded 0·250 kg/min until it dropped below 0·100 kg/min), stripping yield (volume collected when milk flow re-exceeded 0·250 kg/min, 15 s after machine milk ceased) and bimodality (visually determined). Overmilking was not considered in the evaluation since it was deliberately caused to better identify stripping milk fraction.

Fig. 1. Parameters used for milk ejection curves analysis.
Analysis of milk flow curves during the main milking phase (period of machine milking without any manual intervention) led to 3 typical patterns characterised by specific milk flow traits. Type 1 was characterised by a sharp and high peak flow curve with a continuous increase in the milk flow followed by a declining phase without going through a plateau phase. Type 2 was characterised by milk flow curves with intermediate milk flow rate and a significantly longer plateau phase. Type 3 was characterised by milk flow curves with a low milk flow level and a longer total milking duration (Fig. 2).
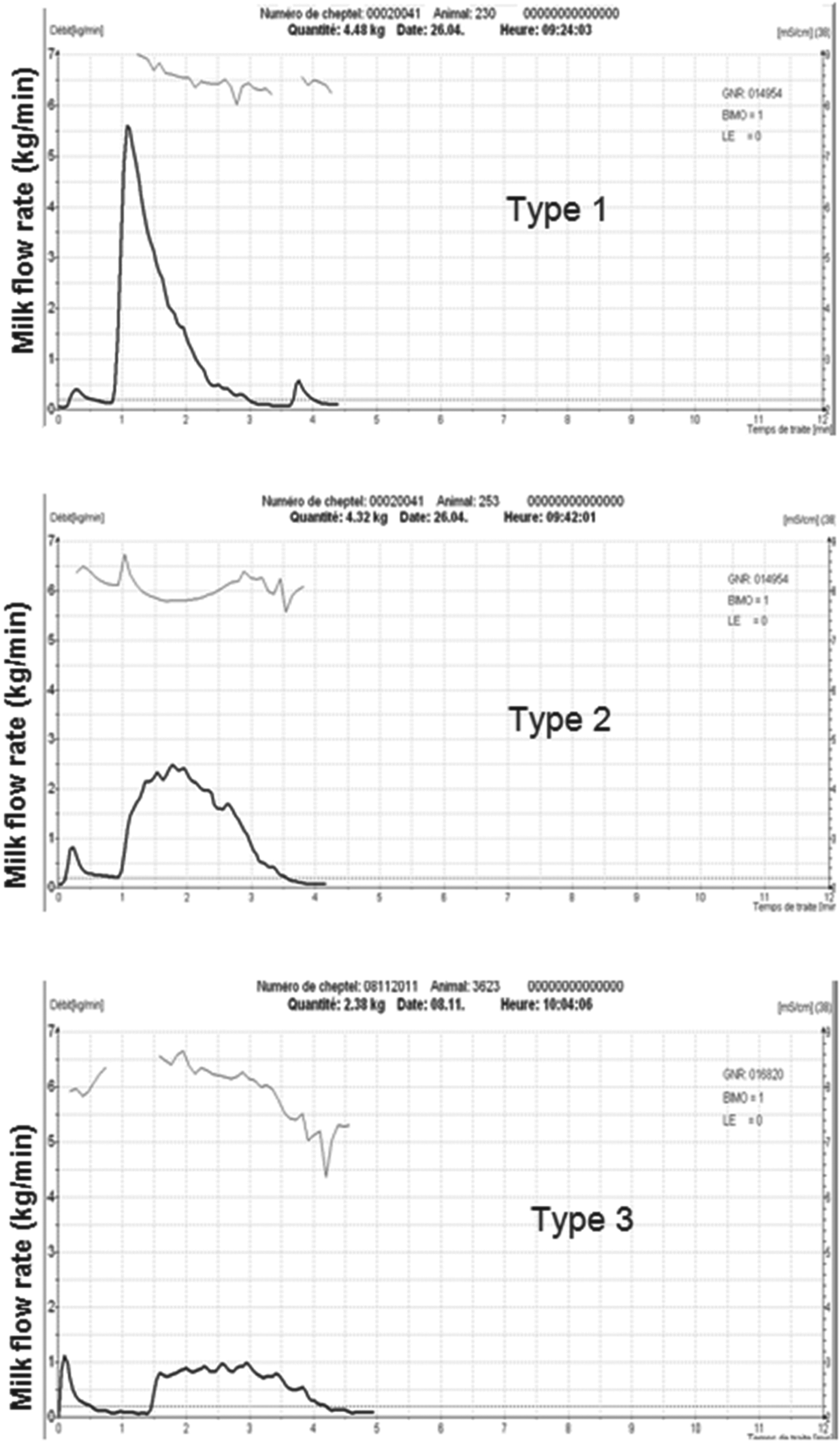
Fig. 2. Different milk flow patterns recordable in dairy camels.
During the study, 160 milk flow curves were recorded. However, many curves were discarded owing to accidents during milking including falling down and slipping of cluster with sudden air entry and excessive foaming, and/or bad correspondence between time of attachment of cluster and start of recording by Lactocorder® apparatus. Thus, only 124 curves were retained for statistical analysis. We gave attention to retaining a sufficient number of recordings per stage of lactation to permit analysis on this point (n=37, 45, 42 for early, middle and late stage of lactation, respectively).
Certainty level
To characterise individual animals’ classification, Mayer et al. (Reference Mayer, Weber and Segessemann1989) defined the certainty level as the relative ratio ‘number of curves of a particular type/all available individual curves’. At levels >50%, animals were attributed to the specific type. This parameter was calculated for each camel.
Determination of cisternal milk fraction
Six of our 20 animals had been injected 3 times throughout lactation with 10 μg/kg body weight of atosiban (i.e. oxytocin receptor blocking agent) to prevent milk ejection due to endogenous oxytocin release. Atosiban was administered 2 min before manual milking of the cisternal milk fraction, without any pre-milking udder preparation. Alveolar milk fraction was machine milked 10 min after injection of a supraphysiological dose of oxytocin (10 IU).
Cisternal milk fraction (%) was defined as (cisternal milk amount × 100)/total milk yield (cisternal milk plus alveolar milk fractions).
Blood sampling and cortisol analysis
To avoid disrupting the camels during sample collection, an intravenous catheter was fitted in the jugular vein of camels some days prior to sampling. Venous blood samples were collected during all the episodes of milking of one day. Blood was collected at −2, −1, 0, 0·5, 1, 1·5, 2, 3, 6 and 12 min (relative to attachment of teat-cups) into heparinised tubes previously cooled in crushed ice and kept at 4 °C following collection. The tubes were centrifuged at 3000 g at 4 °C for 15 min and the plasma removed and stored at −20 °C until the time of assay.
Plasma cortisol was measured by a competitive EIA (method developed in our laboratory). For cortisol assay, B/Bo at 50% (E.D.50) was 1 ng/ml. The intra-assay CV ranged from 24% (0·1 ng/ml) to 9% (1·6 ng/ml). The inter-assay CV ranged from 8% (25 ng/ml) to 5% (1·6 ng/ml).
Statistical analysis
Statistical analysis were carried out using SAS program (SAS version 9.0, SAS Inst. Inc., Cary NC, USA) and results are presented as means±sd. Data were analysed using the MIXED procedure according to the model:
where: Y ijk=individual observation of measured traits: machine milk yield (kg), machine stripping (kg), milk flow latency (min), time to milk ejection (min), total milking duration (min), main milking duration (min), average milk flow (kg/min), peak milk flow (kg/min), time of peak milk flow (min) and % 3 min milk yield, μ=overall mean, CTi=the fixed effect of milk flow type (i=1 to 3), LSj=the fixed effect of lactation stage (j=1 to 3), A k=the random effect of the animal (k=1 to 20), e ijk=random error.
Fixed effects included in the model were estimated using the Least Squares Means methods. Residual error variances were estimated using the REML (Restricted Maximum Likelihood) method. Differences between least squares means were determined with the TDIFF test. Pearson correlation coefficients between traits were also calculated.
The effect of bimodality, lactation stage and their interaction on milk yield were analysed using the MIXED procedure according to the model:
with, μ=overall mean, BIMOi=the fixed effect of bimodality (i=1, 2), LSj=the fixed effect of lactation stage (j=1 to 3), A k=the random effect of the animal (k=1 to 20), e ijk=random error.
The χ2 test was used to evaluate group differences of bimodality trait frequency. Significance was declared as P<0·05 unless otherwise indicated.
Results
Milk flow curves and milkability of camel
A total of 124 milk flow curves were recorded. The ratio of the different milk flow patterns evaluated in this study was 40:38:22% for types 1, 2 and 3, respectively (Fig. 2).
In type 1 curves, milk flow is never bridled during milking procedure resulting in higher peak flow levels and short milking durations which depend also mainly on the amount of milk stored in the udder.
Type 2 curves characterise animals with relatively high milk production and lower milk flow rate, giving a larger plateau phase than type 1 animals. Milk flow fluctuations are sometimes recorded in type 2 curves.
Type 3 profiles show various patterns of milk flow, all characterised by low peak flow rate.
As shown in Table 1, machine milk yield was higher (P<0·0001) in type 1 followed by type 2 and type 3 animals (4·24±1·16, 3·30±0·72, 2·34±0·66 kg/milking, respectively). Machine stripping yield was not influenced by curve type and ranged from 0·027±0·02 to 0·032±0·02 kg/milking. There was no significant difference between milk flow latency. Time to milk ejection was the shortest (P=0·027) for type 1 milk emission curves. Average and peak milk flow were higher (P<0·0001) in type 1 animals followed by type 2 and type 3.
Table 1. Milk flow traits related with the different milk flow patterns

† Time between the attachment of teat cups and a milk flow of 0·250 kg/min
‡ Time from milking cluster attachment till milk ejection occurs (visually determined when teat suddenly swell)
a,b,cMeans in the same line with different superscript letter were significantly different (P<0·05)
Positive and significant correlation was observed between milk yield, and peak milk flow and average milk flow (r=0·83, P<0·0001; r=0·73, P<0·0001, respectively) but no correlation was measured between milk yield and total milking duration and main machine milking duration. A significant correlation was detected between time to peak milk flow occurrence, and total machine milking duration and time to milk ejection occurrence (r=0·58, P<0·0001 and r=0·47, P<0·0001, respectively). Conversely, negative correlations were obtained between time to milk ejection, and machine milk yield, peak and average milk flow rate (r=−0·24, P=0·0060; r=−0·22, P=0·012; r=−0·18, P=0·042, respectively).
Certainty level
This ratio was calculated and we were able to assign a specific profile for 90% of animals. Certainty levels ranged from 63 to 100% for all types. However, 10% of animals showed high individual variability of milk flow pattern and so could not be assigned to any type (Fig. 3).

Fig. 3. Changing type of milk flow kinetics in the same animal.
Cortisol level
Since milk removal can easily be disturbed in dromedary camels, cortisol levels were determined for animals around machine milking time to look at a possible stress that could explain changes in milk flow pattern (Fig. 4). Basal concentration of cortisol remained low during milking (9·6±2·8 ng/ml) in normal conditions, whereas when some animals were clearly disturbed (cry, grumbling, agitation, kicking…), cortisol level was already elevated and remained high (47·1±7·8 ng/ml) during milking and slowly decreased after milk removal ceased. Neverthess, we did not record any significant difference in mean cortisol levels between milk flow pattern groups or stage of lactation.

Fig. 4. Plasma cortisol level during normal (a) and disturbed (b) milk ejection in one representative animal.
Cisternal milk
In the camels measured for milk repartition, the ratio cisternal/total milk averaged 3·8±0·5%, range 0·3–6·5%. Cisternal milk fraction averaged 230±40 ml, range 14–530 ml. The ratio cisternal/total milk was higher (P<0·05) in early and mid lactation compared with late lactation (4·7±0·4%, 4·9±0·9% and 1·7±0·3%, respectively).
Bimodality
Bimodal curves occurred in 41·9% of total recorded milk flow patterns independent of milk flow type. Bimodality results from initial emptying of milk that has been drained into the teat and gland cisterns in the inter-milking period followed by a second milk emission of alveolar milk discharged during milk ejection reflex (Fig. 5).

Fig. 5. Bimodal and unimodal milk flow curves for the same camel at middle (April) and end (end of October) stage of lactation.
Machine milk yield was higher (P<0·0001) for animals with bimodal milk emission curves compared with animals with unimodal curves (4·09±1·01 kg vs. 2·97±1·03 kg, respectively). The effect of lactation stage on bimodality is shown in Table 2.
Table 2. Influence of lactation stage on occurrence (%) of bimodality

The effect of bimodality on milk yield was more significant in mid and late lactation (3·31±1·06 vs. 4·40±1·20 kg and 2·59±0·86 vs. 3·77±1·26 kg, respectively). Seventy per cent of milk ejection curves were bimodal in early lactation, against only 8·9% in late lactation.
Discussion
One of the explanations of the different milk flow patterns between types 1 and 2 between camels could be teat characteristics. Type 1 could have lower teat sphincter resistance and/or a larger streak canal diameter when totally opened, allowing an easy, rapid and continuous emptying of the udder. Conversely, type2 curves could include dams with some anatomical particularities such as narrow teat canals and/or a greater resistance of the teat sphincter (Marnet et al. Reference Marnet, Combaud, Dano, Zervas and Barillet1999; Weiss et al. Reference Weiss, Weinfurtner and Bruckmaier2004). In type 2 milk flow kinetics, the fluctuations of milk flow recorded are probably caused by acyclic fluctuations of vacuum level due to inappropriate teat cup to teat shape.
Milk flow patterns described in this work are close to milk flow kinetics reported for dairy buffaloes by Boselli et al. (Reference Boselli, Mazzi, Borghese, Terzano, Giangolini, Filippetti, Amatiste and Rosati2010) and Caria et al. (Reference Caria, Boselli, Murgia, Rosati and Pazzona2012). In fact, she-camel and buffalo cow have many similarities such as a restricted cistern size and close milk yield (4·9±0·8% of total milk and 3·40±0·88 kg/ milking; Thomas et al. Reference Thomas, Svennersten-Sjaunja, Bhosrekar and Bruckmaier2004). Boselli et al. (Reference Boselli, Mazzi, Borghese, Terzano, Giangolini, Filippetti, Amatiste and Rosati2010) found that differences between milk flow patterns in dairy buffaloes are basically associated with streak canal length and teat diameter. They reported an overall mean for teat canal length of 19·1; 21·4 and 39·1 mm for type 1, type 2 and type 3 curves, respectively. Likewise, differences between the three types of profiles we described between camels, could be related to differences in teat anatomy.
Nevertheless, we have also measured that an average of 1·02 min after teat-cup attachment was needed to observe milk ejection in our camels. This lag-time is similar to lag-time recorded in cattle that ranges from 1 to 2 min and depends on the degree of udder filling i.e. of the intramammary pressure, which in turn, depends on the interval between milkings and the stage of lactation (Bruckmaier & Hilger, Reference Bruckmaier and Hilger2001). In buffaloes, milk ejection may require more time and effort, up to 3 min of tactile stimulation, which is rarely performed (Borghese et al. Reference Borghese, Rasmussen and Thomas2007; Caria et al. Reference Caria, Boselli, Murgia, Rosati and Pazzona2012). Finally, if streak canal diameter remains a possible explanation for different types of milk flow curves, these data seem to exclude a difference of teat sphincter resistance as the main explanation of different flow pattern between our camels.
A second possible explanation of the different milk flow pattern, both between camels and within the same camel between milkings all along lactation, could be differences in the occurrence or efficiency of milk ejection reflex. With the highest milking duration and the lowest milk flow and yield, animals of type 3 can be considered as the least suitable to machine milking. In fact, this group included the first milkings of 3 camels probably still not well adapted to milking and with possible reduced milk ejection reflex as described for some dairy ewes by Négrao & Marnet (Reference Négrao and Marnet2003). Animals of type 3 could also have greater sensitivity to stressful milking or environmental factors because some of them showed external signs of anxiety or discomfort (high bleats, agitation…). Furthermore, easily disturbed animals of types 1 and 2 could sometimes show type 3 patterns when exposed to environmental modifications (strange persons, unusual sounds during milking…) and may even inhibit partially or block totally milk let down until large exogenous oxytocin doses injection may reverse blockade (Fig. 3c, d). Our results for mean cortisol concentration in blood around milking showed not significant difference between groups but we observed that one camel, with clear signs of a high stress level, was systematically associated with a very high level of cortisol before as well as during milking, and was always classified in type 3 group. Bruckmaier et al. (Reference Bruckmaier, Pfeilsticker and Blum1996) also reported that emotional stress causes inhibition or delayed milking-related oxytocin release in dairy cows. Even if cortisol itself is not able to inhibit directly the milk ejection reflex as shown by Mayer & Lefcourt (Reference Mayer and Lefcourt1987), it is probable that so high chronic levels of cortisol could be able to feed back negatively to the hypophysis to answer to an ACTH challenge. That could be a bad predictor of the milking ability of animals, because animals with a greater adrenal sensitivity to ACTH had less pronounced inhibition of milk ejection as a consequence of the greater oxytocin release (Macuhova et al. Reference Macuhova, Tancin, Kraetzl, Meyer and Bruckmaier2002).
Ninety per cent of our camels were assigned to a specific milk flow pattern. Marnet et al. (Reference Marnet, Billon, Da Ponte, Martin and Manfredi2001) stated that the repeatability, i.e. correlation between the successive measurements of an animal, of milk flow traits in dairy goats is a characteristic of animals.
Our results for milk partitioning showed a lower cisternal ratio than those found by Caja et al. (Reference Caja, Salama, Fathy, El-Sayed and Salama2011) for manually milked animals in stressful surroundings (7%) but confirmed the extremely small volume of milk available in the udder if milk ejection did not occur. According to this result, it is clear that even in type 3 camels, we obtained alveolar milk assuming that oxytocin was released during all the tested milkings including type 3 animals. Thus, in the type 3 case, it is possible that we found animals in which oxytocin released by milking stimulation could not reach sufficiently the udder because of a reduced blood flow due to the vasoconstrictive action of adrenaline secreted by the adrenal medulla when animals are stressed (Gorewit & Aromando, Reference Gorewit and Aromando1985). This reduction of oxytocin arrival onto myoepithelial cells could decrease synchronous contraction of myo-epithelium in the entire udder, which could reduce intramammary pressure rise and the resulting milk ejection force and milk flow.
The last possible explanation for the intra-animal variability of milk flow pattern could be a sub-optimal cluster adaptation to udder and teats and also to machine settings. Even though we had already tested the best machine settings for our camels (Atigui et al. Reference Atigui, Hammadi, Barmat, Khorchani and Marnet2011) and discarded animals with badly shaped udders, we used non-specific liners and cups and, in some cases, the liner mouthpiece had difficulties in fitting well with the teats and limiting air entry and vacuum fluctuations under the teats. It is probable that with the increasing number of camels being milked, we could soon find more suitable material on the market.
What about the global milkability of our dromedary camels? For our she-camels we recorded an overall of average and peak milk flow of a good level (1·15 and 2·46 kg/min, respectively). It is quite good when compared with Sandrucci et al. (Reference Sandrucci, Tamburini, Bava and Zucali2007) who recorded 3·8 kg/min as maximum milk flow rate for high producing cows, McKusick et al. (Reference McKusick, Marnet, Berger and Thomas2000) and Billon et al. (Reference Billon, Chastin, Baritaux, Bouvier, Ilahi, Manfredi, Marnet, Zervas and Barillet1999) who recorded 1·24 and 1·28 kg/min as maximum milk flow rate respectively for east Friesian dairy ewes and for French dairy goats, and Boselli et al. (Reference Boselli, Mazzi, Borghese, Terzano, Giangolini, Filippetti, Amatiste and Rosati2010) in buffaloes, who recorded an average and peak milk flow of 0·86 and 1·36 kg/min, respectively. All theses results are not fully comparable because we have to keep in mind that the settings of machine milking can affect the milk flow traits recorded by lactocorder as recently shown in buffaloes (Caria et al. Reference Caria, Boselli, Murgia, Rosati and Pazzona2012). These authors reported milk flow traits in buffaloes using a 49 kPa vacuum level, similar to the vacuum level applied in our study (48 kPa). They recorded 0·91 and 1·41 kg/min for average milk flow and peak milk flow, respectively, which confirm that milk flow traits of our camels in our conditions of milking are better than for buffaloes.
Thus, despite our limited knowledge about the best machine milking equipment and settings and about needs of camels for prestimulation compared with other dairy animals, camels milked under our conditions seem to have a good suitability to machine milking. Hence it is possible that some type 2 animals might turn into type 1 if, with a better stimulation, intramammary pressure increases significantly and they become able to increase milk flow rate.
As reported in Table 1, type 1 animals had the highest milk yield and the best milk flow traits, suggesting that selection on ‘milkability’ traits should also improve the overall milk yield of the herd. Machine stripping yield was minimal for all milk flow profile types, giving clear evidence of a proper and complete udder empting which confirms the occurrence of the milk ejection reflex for all milk flow profile types. In the same way, the negative correlation detected between lag-time and average and peak milk flow confirms the difference in milk ejection efficiency, which might partly explain differences in milk flow profiles.
However, milking characteristics registered during this study showed high variability among animals. For instance, peak milk flow ranged from 1·85 to 5·27 kg/min between animals on the same day in mid lactation, which suggests that a selection on this basis could be carried out in the future.
In dairy cows, bimodality shows delayed milk ejection occurrence once the cisternal milk fraction is detected when the milk flow curve had a flow pattern with two rises separated by a clear drop of milk flow below 0·2 kg/min shortly after the start of milking (Bruckmaier & Blum, Reference Bruckmaier and Blum1996; Dzidic et al. Reference Dzidic, Macuhova and Bruckmaier2004). Since camels have very small cisterns and a normally delayed milk ejection reflex, bimodality occurs when the vacuum level provided by machine milking is able to open the teat sphincter and empty the cistern before the milk ejection takes place. Thus, for the same animal, bimodality is also associated with the amount of milk available in the udder. Obviously, when milk yield is higher, pressure increases in the alveoli and milk is withdrawn to the cistern. Increasing mammary pressure during extended milking intervals (16 h) thus makes easier the opening of teat sphincter and bimodality detection. On the contrary, when curves are not bimodal, it might be due to the absence or very limited cisternal milk volume.
In dairy buffaloes, Borghese et al. (Reference Borghese, Rasmussen and Thomas2007) reported that bimodal curves are most common in high-producing animals. Likewise, our statistical analysis showed that machine milk yield is significantly higher for animals with bimodal curves of milk emission. This is due to the reduction of the cisternal milk fraction as lactation progresses and to a low level of udder filling. In fact, we found that the cisternal milk fraction decreased during lactation when applying a 16-h milking interval. Bruckmaier & Hilger (Reference Bruckmaier and Hilger2001) reported also a decrease of intramammary pressure towards late lactation in dairy cows.
The occurrence of bimodal curves suggests the importance of udder pre-stimulation for dromedary camels. As has been proved for species with small cisternal cavities, such as buffaloes (Ambord et al. Reference Ambord, Stoffel and Bruckmaier2010) and cows (Rasmussen et al. Reference Rasmussen, Frimer, Galton and Petersson1992; Bruckmaier & Hilger, Reference Bruckmaier and Hilger2001), udder pre-stimulation is needed to ensure a higher milk flow rate, faster milking and to reduce occurrence of bimodal curves and should be proposed for dairy camels for better milking efficiency. Because feeding concentrate is well known to help induce milk ejection and facilitate entry of the animals into a good milking routine (Samuelsson et al. Reference Samuelsson, Wahlberg and Svennersten1993; Bruckmaier & Wellnitz, Reference Bruckmaier and Wellnitz2007), such a management tool needs to be tested along with improved udder pre-stimulation as already made for buffaloes (Shahid et al. Reference Shahid, Abdullah, Bhatti, Javed, Babar, Jabbar and Zahid2012).
This study suggests that type 1 and type 2 animals show good machine ‘milkability’ performance even without any special treatments during milking. Conditioning and improving machine milking equipment and settings, teat pre-stimulation and cluster attachment after milk ejection occurrence could rapidly improve milking efficiency of dairy camels. Moreover, the presence on the same farm of subjects showing a high variability of ‘milkability’ traits, led to the assumption that a real selection on this basis must be carried out.
This work was carried out in collaboration between Arid Lands Institute (IRESA, Tunisia) and AGROCAMPUS OUEST-INRA (France). The authors would like to thank the Tunisian and French Authorities for financial support, Mr Ahmed Belgacem and Mohamed Dhaoui for their support in data collection assistance and Mr Bechir Saafi and Mr Lassaad Khalfalli for their careful assistance in animal management and Mr Jacques Portanguen for expert hormonal analysis.









