Introduction
Plant litter plays important, but often overlooked, roles in many processes at the soil–litter interface. At this interface, litter influences a range of processes from nutrient cycling (Frey et al., Reference Frey, Six and Elliott2003) to plant establishment (Facelli and Pickett, Reference Facelli and Pickett1991). Many plant seed banks physically occur at the soil–litter interface. Seeds are mixed into the top layer of soil and are also suspended in the litter directly above the soil. Litter may impact seed banks as a direct physical barrier to germination (Hamrick and Lee, Reference Hamrick and Lee1987) or indirectly by modification of microenvironments affecting germination and establishment (Goldberg and Werner, Reference Goldberg and Werner1983; Facelli and Pickett, Reference Facelli and Pickett1991). Litter can also impact species interactions (i.e. predation, competition and parasitism) within the seed bank. For example, plant litter in central North American grasslands negatively affects seed removal at the soil–litter interface by rodents but not by ants (Reed et al., Reference Reed, Kaufman and Kaufman2006). Presence of litter may indirectly reduce competition in the seed bank. For example, in forests of eastern North America, plant litter reduced density and biomass of the dominant herb Seteria faberii, resulting in increased biomass of weaker seedling competitors (Facelli, Reference Facelli1994). Litter may also impact plant–pathogen species interactions. Seed pathogens have been shown to reduce seed banks in a range of natural ecosystems (Crist and Friese, Reference Crist and Friese1993; Dalling et al., Reference Dalling, Swaine and Garwood1998; Schafer and Kotanen, Reference Schafer and Kotanen2004) and these pathogens may be influenced by the presence of litter (Facelli et al., Reference Facelli, Williams, Fricker and Ladd1999).
Given the importance of seed pathogens in mediating seed-bank dynamics at the soil–litter interface, coupled with the strong direct and indirect influences of plant litter, it is likely that plant litter will have important effects on seed–pathogen interactions. If plant litter facilitates seed pathogen diseases, then greater levels of seed death will occur in seed banks with higher litter density than in those with lower or no litter density. To address this hypothesis, we studied a seed–pathogen system that involved the invasive annual weed cheatgrass (Bromus tectorum L.) and the seed pathogen Pyrenophora semeniperda ((Brittlebank and Adam) Shoemaker (anamorph Drechslera campanulata (Lév.) B. Sutton)), in the semi-arid shrub–steppe community of western North America.
Following the introduction of B. tectorum in the late 19th century, it quickly reached dominance within perennial grasslands and shrublands of the western United States and British Columbia (Mack, Reference Mack1981), and continues to expand its range today (e.g. Colorado Plateau; Floyd et al., Reference Floyd, Hanna, Romme and Crews2006). B. tectorum generates prodigious numbers of seeds and large quantities of litter (Kelrick, Reference Kelrick1991). Seeds drop throughout the summer, creating a dense litter–soil seed bank as seeds become intermixed with the soil and litter from dead stems and leaves of the parent plants. Within this seed bank, large numbers of these seeds can become infected by the generalist fungal pathogen P. semeniperda (Meyer et al., Reference Meyer, Quinney, Nelson and Weaver2007), which occurs naturally in Eurasia (Stewart et al., Reference Stewart, Meyer and Allen2009), United States, Canada, Argentina, Australia, New Zealand and South Africa (Medd et al., Reference Medd, Murray and Pickering2003). Infections of host seeds can occur either aerially during anthesis or from direct contact with other infected seeds. Infected seeds produce black, conidia-laden stromata that can infect other seeds in the seed bank (Beckstead et al., Reference Beckstead, Meyer, Molder and Smith2007). Leaf ring spotting has been observed in some grasses, leading to the suggestion that the fungus may be systemic, with mycelium growing throughout the host (Medd et al., Reference Medd, Murray and Pickering2003) and potentially overwintering in the dead plant material. Due to its efficacy against the invasive B. tectorum within the seed bank, P. semeniperda has been proposed as a potential biological control agent (Meyer et al., Reference Meyer, Quinney, Nelson and Weaver2007), but important questions still remain regarding the role of plant litter in the life cycle of this pathogen and its hosts.
Litter may affect seed mortality by fungal pathogens through direct and indirect mechanisms. Directly, litter could act as an inoculum source for the pathogen and consequently directly kill seeds in the soil–litter seed bank. Medd et al. (Reference Medd, Murray and Pickering2003) hypothesized that plant litter could serve as a secondary infection source in the life cycle of P. semeniperda. Although the fungal mycelium within plant litter has not been shown to produce ascospores and/or conidia, latent mycelium within infected tissues could grow and infect adjacent seeds suspended in the litter (L. Poston and J. Beckstead, unpublished data; P. semeniperda was isolated from 3-week-old leaf tissues of B. tectorum seedlings infected with P. semeniperda). Crop and weed plant debris has been reported as an important source of inoculum for some agricultural diseases (Latorre and Jones, Reference Latorre and Jones1979; Cortesi et al., Reference Cortesi, Gadoury, Ricciolini and Bisiach1997), but little work has addressed the possibility of direct litter infection in natural systems.
Litter may also affect the seed pathogen–Bromus interaction indirectly by altering microsite conditions. Plant litter alters seed-bank conditions by increasing soil moisture/water availability (Fowler, Reference Fowler1986), decreasing levels of incoming radiation (Facelli and Pickett, Reference Facelli and Pickett1991), and reducing temperature fluctuations (Weaver and Rowland, Reference Weaver and Rowland1952). Compared to bare ground, B. tectorum litter can reduce average maximum daily soil surface temperatures in the spring from 28°C to 19°C, insulate average minimum temperatures from 0°C up to 5°C, and significantly stabilize relative humidity over the diurnal range (Evans and Young, Reference Evans and Young1970). The environmental conditions described under B. tectorum litter align more closely with the optimal sporulation parameters of P. semeniperda (see Campbell et al., Reference Campbell, Medd and Brown2003) than those recorded on adjacent bare ground. B. tectorum litter may provide the ideal suitable environment for disease proliferation in the seed bank at the soil–litter interface.
This study aims to explore how B. tectorum litter affects B. tectorum seed mortality by P. semeniperda. The effects of litter on the B. tectorum seed–pathogen interaction were first investigated with a field seed-bank study, involving the collection of seed-bank samples from low and high B. tectorum litter patches at three study sites. Using a combination of field studies and lab experiments, we aimed to explore the direct and indirect effects of litter on this seed–pathogen interaction, namely: (1) does B. tectorum litter directly determine seed disease in the seed bank via transmission of P. semeniperda; and (2) does B. tectorum litter indirectly affect the seed–pathogen interaction by altering the seed microsite that modifies the disease relationship?
Methods
Field seed-bank study
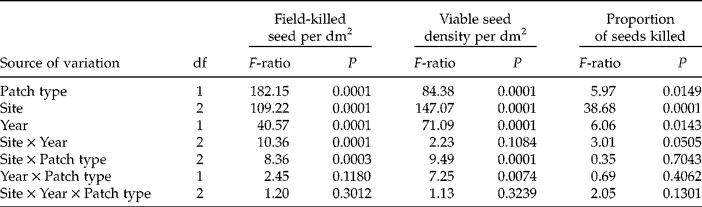
To investigate the effects of litter on the cheatgrass seed–pathogen interaction, soil-core seed-bank samples (for details, see Beckstead et al., Reference Beckstead, Meyer, Molder and Smith2007) were obtained from three cheatgrass monocultures in the western United States: upper Saddle Mountain, Hanford Reach National Monument, Franklin County, Washington [46°47′N, 119°27′W; elevation 582 m above sea level (asl)]; Spokane, Spokane County, Washington (47°37.204′N, 117°18.513′W, elevation 871 m asl) and Whiterocks, Tooele County, Utah (40°N, 109°W; elevation 1839 m asl). Samples (6 cm diameter × 4 cm depth) were taken in two types of cheatgrass-litter patches, low litter (bare ground to < 1 cm litter) and high litter (approximately 2–3 cm of litter) in May prior to the current year's seed dispersal. Forty samples were taken per litter treatment at each of three sites and repeated across 2 years (2009 and 2010). Samples were screened to separate seeds from soil and litter material and then processed to quantify any apparently viable (intact) seeds and also any field-killed seeds with protruding stromata of P. semeniperda. Apparently viable seeds were then incubated for 2 weeks at 20°C and 24-h photoperiod by placing seeds on germination blotters (Anchor Paper, St. Paul, Minnesota, USA) saturated with water within a Petri dish (100 × 15 mm). Petri dishes were stacked randomly in plastic bags closed with wire ties to retard water loss. Seeds that developed stromata during incubation were considered to have been infected and killed in the field; these values were included in the total field-killed densities. The viable seed-bank densities consisted of seeds that germinated during incubation as well as dormant but viable seeds (determined by a cut test; Ooi et al., Reference Ooi, Aulk and Whelan2004). Only 6% of the apparently viable seeds were dormant. At the Spokane site Bromus arvensis (field/Japanese brome; previously Bromus japonica), was intermixed with B. tectorum. Although B. arvensis occurred in low frequencies relative to B. tectorum (1% of the seed bank), it was involved in the disease interaction and was included in the seed-bank analysis.
Seed-bank data were analysed using analysis of variance (ANOVA) for a completely randomized design with year, site and litter patch type as fixed effects (JMP, Version 8.0.1, SAS Institute Inc., Cary, North Carolina, USA). The response variables, field-killed seeds and total seed-bank density, were log transformed to improve homogeneity of variance prior to analysis. Means separations from the LSMEANS statement were examined for each main effect and interactions that were significant (P < 0.05) in ANOVA.
To examine the effects of litter on seed disease independent of seed-bank density, we calculated the proportion of seeds killed by P. semeniperda from the total seed-bank density. Our prediction is that the proportion of seeds killed would be higher in the high-litter treatment, independent of the total seed density. The ANOVA was identical to the analysis above, with the exception that the proportion of seeds killed was transformed with an arcsine transformation.
Direct litter-to-disease experiment
To quantify the direct effects of litter on disease, cheatgrass plant litter was harvested from cheatgrass monocultures from three sites and repeated at three seasonal time intervals. The sites were identical to the field seed-bank study above (Saddle Mountain, Washington; Spokane, Washington; and Whiterocks, Utah), with the exception of the Saddle Mountain, Washington site, which was at a lower elevation on the mountain (46°45′N, 119°28′W, elevation 282 m asl). Standing, dead, above-ground biomass of cheatgrass (hereafter referred to as litter) was collected, rather than dead plants lying on the ground, reducing soil fungal contamination. The experiment was repeated in time to capture temporal variability of any direct fungal disease transmission, which involved early summer litter (late May 2009), late summer litter (August 2009) and the following early spring litter (February 2010). Given that the bulk of carryover litter on the ground is composed primarily of cheatgrass stems, all seeds and leaves were removed and discarded. In addition, all plants infected with the smut fungus Ustilago bullata Berk. were discarded.
To isolate the fungal stage from the litter, the litter was split equally into three treatments. The control consisted of litter fully sterilized by autoclaving for 80 min at 121°C to kill any fungal spores or mycelium. The second treatment was surface-sterilized litter (sequential immersion in 70% ethanol, 10% bleach and deionized water for 1 min each) to remove any fungal conidia on the surface of the litter but to retain mycelium within the litter. The final treatment was unsterilized litter, which retained fungal conidia on the surface and mycelium within the litter. Aseptic technique was followed to limit contamination of materials following treatments. Airborne conidial spore contamination from P. semeniperda was a possibility, especially in the early summer, due to concurrent experiments in the same lab space, although measures were taken to limit this impact.
Twenty-five healthy, dormant cheatgrass seeds (stored at − 18°C), which are highly susceptible to P. semeniperda seed death (Beckstead et al., Reference Beckstead, Meyer, Molder and Smith2007), were placed in Petri dishes (100 × 15 mm) on moist germination blotters (Anchor Paper). Seeds were surface sterilized (1 min in 70% ethanol, 1 min in 0.0525% sodium hypochlorite) and safranin dyed for easy retrieval prior to placing in the Petri dish. Aseptic technique was followed to limit contamination of seeds. Although surface sterilization should remove the majority of P. semeniperda on the surface, there is the possibility of P. semeniperda infection within the seed from floral infection. However, floral infection occurs infrequently in the Intermountain West region (Meyer et al., Reference Meyer, Beckstead, Allen and Smith2008) and is not expected to influence the seed infection rate in this study. For each treatment–location combination, 1 g of litter was placed on top of the seeds in the Petri dish. The number of replicated Petri dishes varied from 10 to 15, based on the availability of litter; most treatment combinations had 15 replicates, with the exception of Whiterock early summer (n = 10) and Whiterock late summer (n = 12). The dishes were sealed with Parafilm® and stacked randomly in plastic bags and incubated at 20°C and 24-h photoperiod for 4 weeks. The dishes were watered as needed, to saturation about once every 3 d.
At the end of the 4 weeks, the total number of seeds killed by P. semeniperda, indicated by fungal stromata on ungerminated seeds, was tallied and the percentage of seeds killed by P. semeniperda was calculated. The total number of viable seeds was established, determined by a cut test on all ungerminated seeds at the end of the incubation period. Each repeated temporal trial of the experiment was analysed independently due to the vastly different environmental conditions the litter experienced in the field. Data were transformed with an arcsine square-root function to improve homogeneity of variance and analysed with a two-way ANOVA (site and litter treatment variables; JMP). Means separations from an LSMEANS statement were examined for each main effect and first-order interaction that was significant (P < 0.05) in the ANOVA.
Indirect litter-to-disease experiment
To quantify the indirect effects of cheatgrass litter on seed death by P. semeniperda, we designed artificial litter–seed banks that varied litter levels. The experiment was repeated at two time intervals, hereafter trials. There were three litter treatments, bare ground (no litter), low litter (1 cm) and a high litter treatment (2.5 cm). The litter was collected from cheatgrass mono-cultures from lower Saddle Mountain, Washington. The litter was saturated in deionized water and then sterilized in the autoclave for 80 min at 121°C to kill any fungal conidia and mycelium.
The artificial seed banks were created by planting 12 dormant cheatgrass seeds in each of 24 plots (8.9 cm square standard flower pots) filled with sterile 2:2:1 sandy loam:peat moss:turface (calcinated clay) mix for each of the three litter treatments, resulting in a total of 24 pots and 288 seeds per trial. Litter treatments were applied to each pot; bare ground (no litter added), low litter (approximately 1 cm; 0.6 g dry litter) and high litter (approximately 2.5 cm; 2.3 g dry litter). Inoculum of P. semeniperda (strain isolated from Whiterocks, Utah; strain no. 0) was applied equally (0.40 g) to all pots in a mixed form of mycelium–conidia–clay carrier (Agsorb Products, Chicago, Illinois, USA). Dormant cheatgrass seeds (collected at Saddle Mountain, Hanford, Washington) were planted into each pot. The seeds were dyed with safranin, glued to toothpicks using Elmer's glue and inserted into each pot. This method allowed for the full recovery of the seeds and to control the vertical placement of the seeds in the litter–seed bank. The pots were placed randomly into a grid design of trenches at a field site in Spokane, Washington (47°37′59.47″N, 117°22′38.68″W, elevation 715 m asl), allowing them to experience autumn conditions for 5 weeks. The repeated trials were separated by 2 weeks and experienced similar rainfall (6.12 cm for Trial 1 and 6.55 cm for Trial 2). In the second trial only one seed out of 288 was killed by the fungus and thus this trial was excluded from the analysis.
Disease incidence was assessed by counting the number of seeds killed by P. semeniperda (seeds that did not germinate and developed the distinctive stromata of P. semeniperda). Data were transformed with a square root transformation and analysed using one-way ANOVA (JMP). To compare the different litter treatments the Tukey–Kramer Honestly Significant Difference (HSD) test was performed.
Results
Field seed-bank study
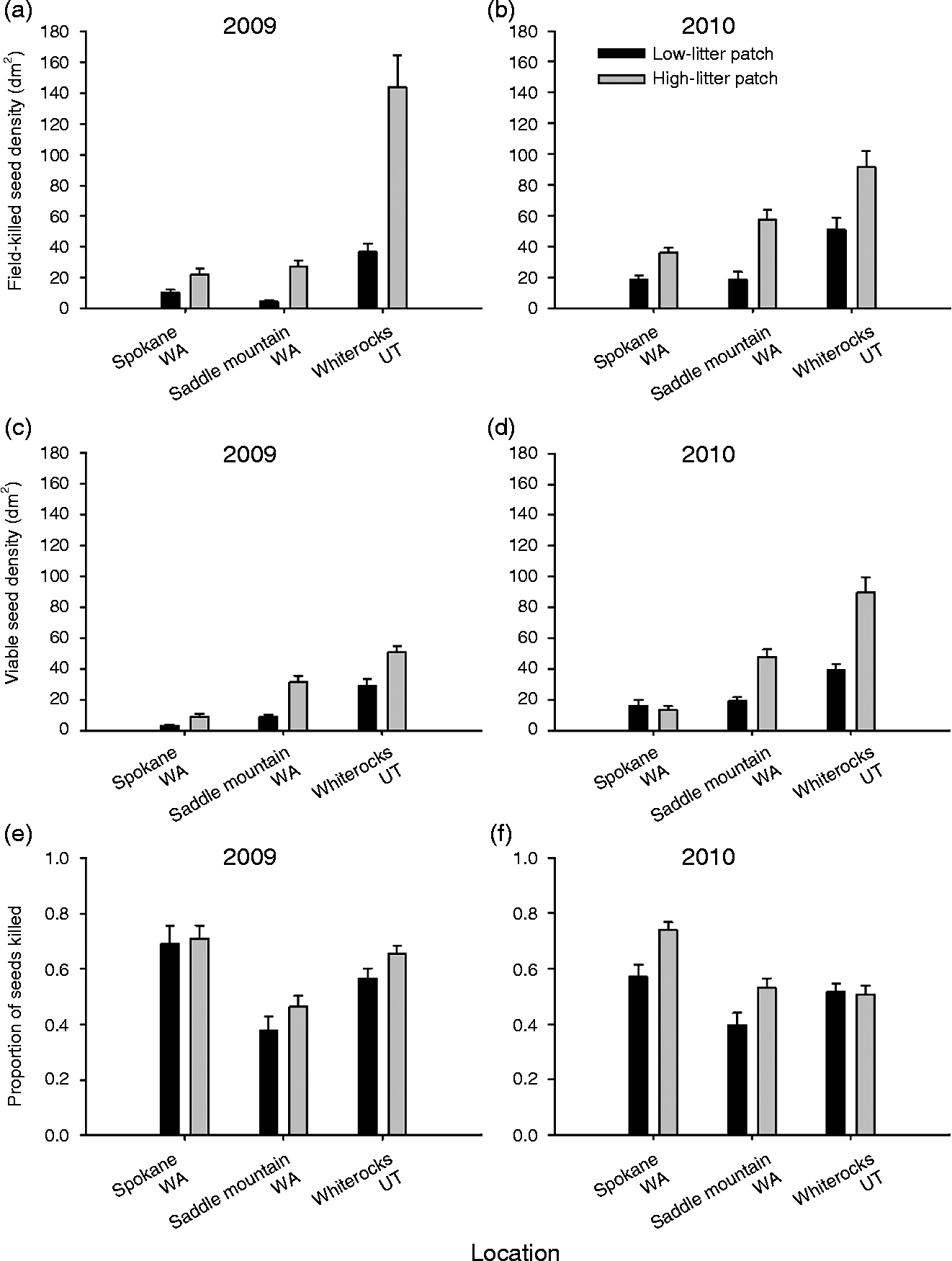
We initially predicted that disease would be higher in seed banks from high-litter patches compared with low-litter patches, indicating that litter facilitates the seed pathogen disease. Our results show that field-killed seed densities were significantly higher in high-litter patches compared with low-litter patches; however, the magnitude of the pattern is variable for a given location and a given year (Table 1 and Fig. 1a and b). The percent difference in field-killed seeds between the high- and low-litter patches varied from a high of 80% (Whiterocks 2009) to a low of 46% (Spokane 2010). In general, 2009 had higher differences between the high- and low-litter patches for all three sites in comparison to 2010 (Fig. 1a and b), but within a given year the site with the largest effect of litter treatment varied.
Table 1 Results of three-way ANOVA on seed-bank measures of field-killed seed density by Pyrenophora semeniperda, total viable seed density, and the proportion of seeds of Bromus sp. killed. The three factors analysed are patch type (low litter and high litter), field site (Saddle Mountain, Washington; Spokane, Washington; and Whiterocks, Utah) and year (2009 and 2010) (n=40). Seed-bank samples from Saddle Mountain and Whiterocks contained only Bromus tectorum seeds, whereas Spokane contained a small amount of B. arvensis intermixed with B. tectorum (see Methods)
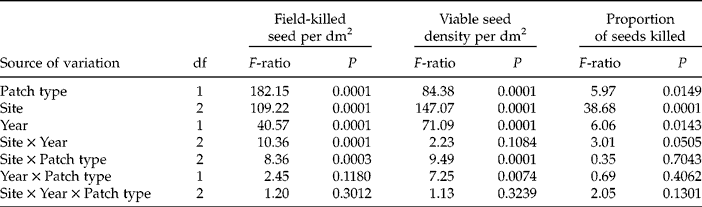
Figure 1 Seed-bank measures of field-killed seed density (i.e. recently killed by Pyrenophora semeniperda in the field and exhibiting stromata) in 2009 and 2010 (a, b), total viable seed density (i.e. living, free of disease) in 2009 and 2010 (c, d), and the proportion of seeds killed (field-killed seed density divided by total viable seed density) in 2009 and 2010 (e, f) for Bromus sp. in each of two litter patches (low-litter patch and high-litter patch) for three site locations (Spokane, Washington; Saddle Mountain, Washington; and Whiterocks, Utah; mean +1 SE; n = 40). Seed-bank samples from Saddle Mountain and Whiterocks contained only Bromus tectorum seeds, whereas Spokane contained a small amount of B. arvensis intermixed with B. tectorum (see Methods).
The total viable seed density was significantly different among seed-bank samples collected from the two litter patch types (Table 1 and Fig. 1c and d). In general, the high-litter patches had significantly more seeds (usually twice as many) compared with the low-litter patches, with the exception of Spokane in 2010. It is likely that high-litter patches with high seed density resulted in greater disease levels due to the higher host density, facilitating the seed pathogen disease.
In addition, we found that litter facilitates the seed pathogen disease even when seed density is accounted for, as in the proportional killed seed data (Table 1 and Fig. 1e and f). High-litter patches had significantly greater proportions of seeds killed (15% increase) compared with the low-litter patches, and this pattern was consistent across sites during most years. These results suggest that some of the litter facilitation of seed disease is independent of seed density and possibly due to microsite modification by the litter during the seed-infection process.
Direct litter-to-disease experiment
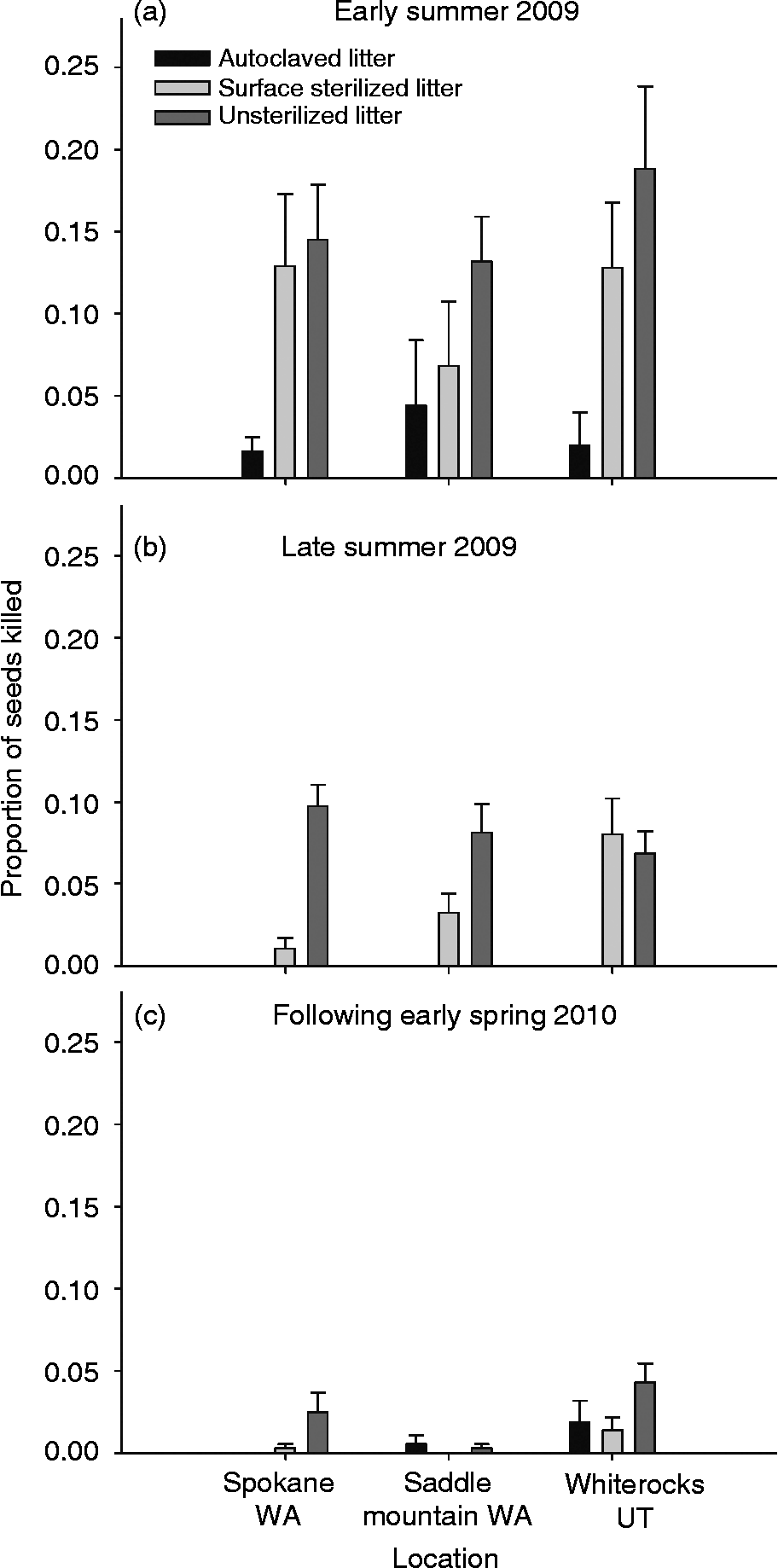
We hypothesized that conidial spores on the outside of the litter or fungal mycelium within the plant were the only possible means of litter acting as a direct inoculum source for seed death by P. semeniperda. The results show that in early summer the litter can act as a direct inoculum source for the pathogen and we found a significant litter treatment effect (Fig. 2a: df = 2, F = 17.93, P < 0.0001). In early summer, high levels of seed death occurred for both the surface-sterilized litter treatment (removal of conidia, but not mycelium) and unsterilized litter treatment (presence of both conidia and mycelium), but not for the autoclaved litter treatment (significance determined by LSMEANS difference Tukey–Kramer HSD). Seed mortality was 64% higher in the surface-sterilized treatment and 75% higher in the unsterilized treatment than in the autoclaved treatment. Although the significant litter treatment effect was consistent across the temporal scale (Fig. 2b: late summer: df = 2, F = 48.80, P < 0.0001; Fig. 2c: early spring: df = 2, F = 6.46, P = 0.002), the high levels of mycelial transmission (via surface-sterilized litter treatment) decreased in importance over time. In the late summer the difference between the autoclaved and surface-sterilized treatments was 79% and between the autoclaved and unsterilized was 88%. There was almost no difference between the autoclaved and surface sterilized treatments by the following early spring, but there was a 52% difference between the autoclaved and the unsterilized treatments. In addition, the proportion of seeds killed by direct litter transmission decreased from a high of 18% mortality in early summer to a high of only 5% in the following spring.
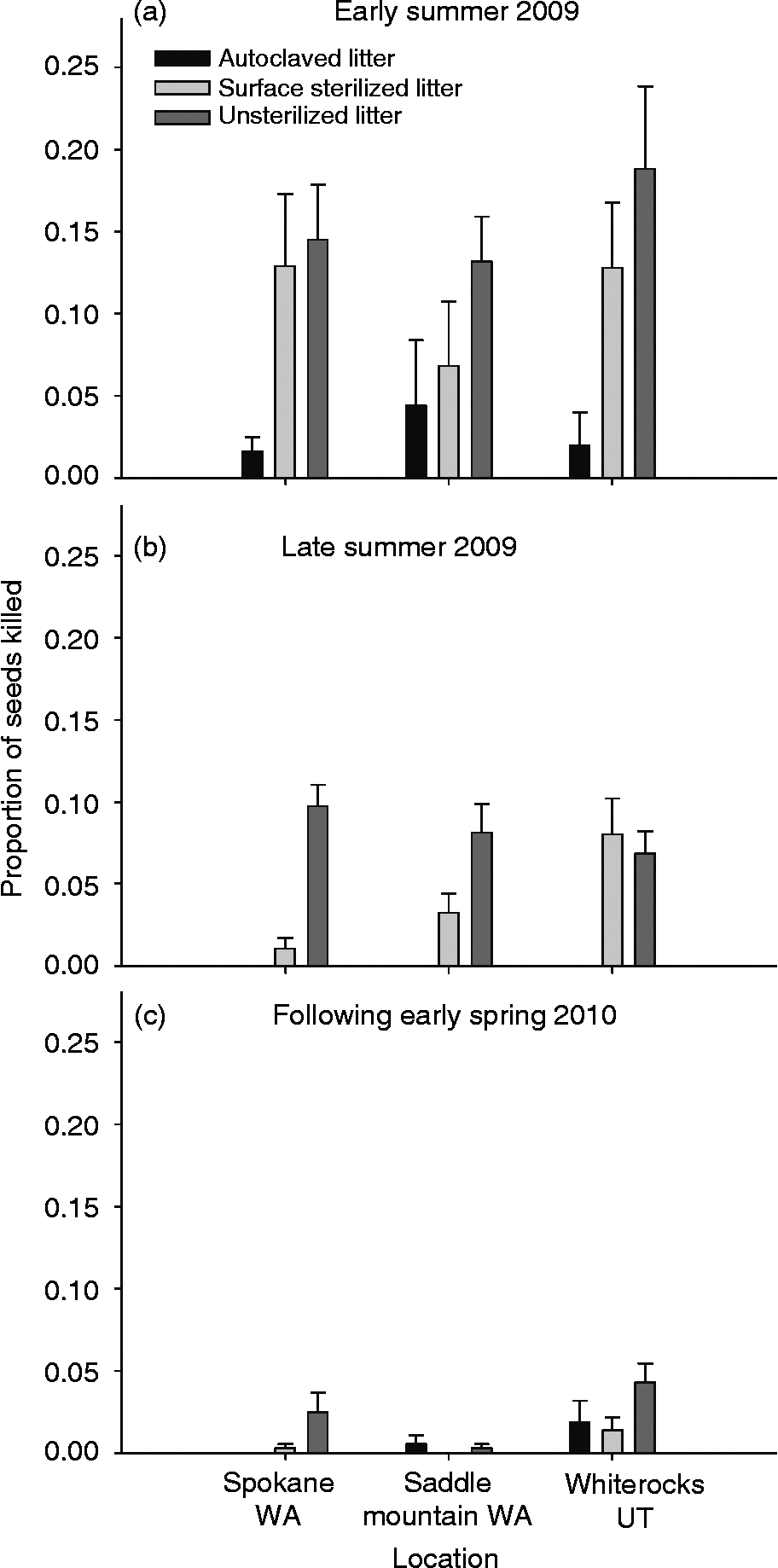
Figure 2 Proportion of Bromus tectorum seeds killed by Pyrenophora semeniperda in early summer 2009 (a), late summer 2009 (b), and the following early spring 2010 (c) in each of three sterilization treatments (mean +1 SE; n = 15, for exceptions see Methods). Sterilization treatments included: autoclaved litter (reduction of conidia on the surface of litter and reduction of mycelium within litter tissues), surface sterilized litter (reduction of conidia on the surface of litter but retained mycelium within litter tissues) and unsterilized litter (retained conidia on litter surface and also retained mycelium within litter tissues).
In early and late summer, there was no significant difference in seed death among the three sites (Fig. 2: early summer, site main effect: df = 2, F = 0.70, P = 0.50; and late summer, site main effect: df = 2, F = 1.40, P = 0.25), but in the following early spring there was a significant difference among the three sites (df = 2, F = 7.31, P = 0.001). The magnitude of seed death derived from litter decreased over time (Fig. 2). The interaction effects of site × litter treatment were not significant for early summer (df = 4, F = 0.09, P = 0.61) or early spring (df = 4, F = 1.90, P = 0.1152) but were significant for late summer (df = 4, F = 5.23, P = 0.0007).
Indirect litter-to-disease experiment
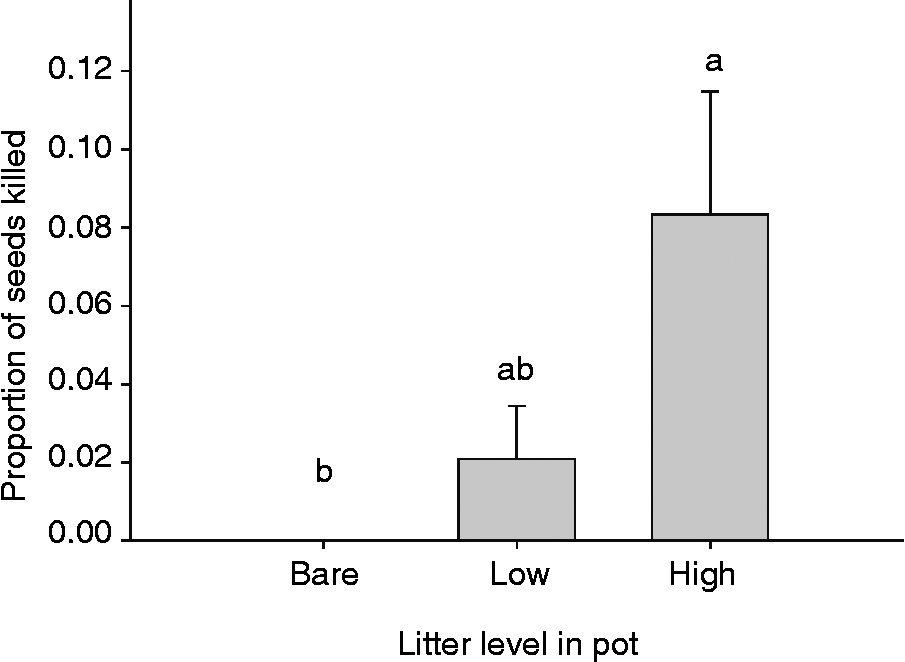
We predicted that cheatgrass litter could indirectly affect the ability of P. semeniperda to kill cheatgrass seeds in artificial litter seed banks. Our data showed a significant difference in seed death among litter levels (Fig. 3: df = 2, F = 5.38, P = 0.01). Overall, the seed death rates were relatively low, less than 10%. The proportion of seeds killed was highest in the high-litter treatments and decreased by 75% in the low-litter treatment and by 100% in the bare ground.
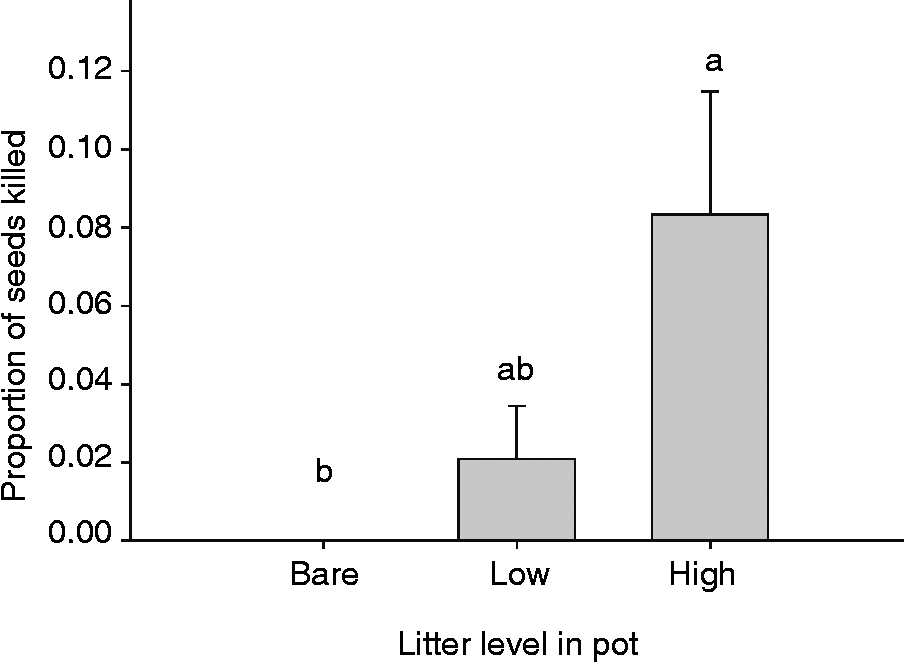
Figure 3 Proportion of Bromus tectorum seeds killed by Pyrenophora semeniperda in pots placed in the field with equal inoculum levels and equal seed densities containing three litter levels (bare, low and high litter; mean +1 SE; n = 8). Different letters represent significant differences (P < 0.05) among treatments, by the Tukey–Kramer HSD multiple comparison test.
Discussion
B. tectorum litter affects seed mortality within the seed bank caused by the pathogen, P. semeniperda. In all three experiments (field seed-bank study, direct litter-to-disease experiment and indirect litter-to-disease experiment) high-litter treatments resulted in higher seed death compared with bare soil or low-litter treatments. Bromus litter has the potential to directly affect seed disease in the seed bank via transmission of the pathogen in early summer, but the influence decreases in the autumn and subsequent spring when the litter is naturally in contact with seeds in the seed bank. Moreover, Bromus litter also indirectly increases seed disease by altering conditions conducive for disease transmission.
Field seed-bank study
We found an increase in pathogen field-killed seed densities in high-litter patches compared with low-litter patches, although the magnitude of disease varied with site and year. These results support our initial hypothesis that plant litter can facilitate seed pathogen disease at the soil–litter interface. Facelli et al. (Reference Facelli, Williams, Fricker and Ladd1999) found greater seedling mortality by fungal pathogens in treatments with litter than treatments without litter. In the same study, the researchers found the effects of litter on seedling disease to vary with soil water content (Facelli et al., Reference Facelli, Williams, Fricker and Ladd1999). The subtle differences in water content at the soil–litter interface (Goldberg and Werner, Reference Goldberg and Werner1983) are likely to be of importance in determining the magnitude of disease, which we found to vary among sites and between years. Our findings are correlative in nature and do not indicate which mechanisms (i.e. direct versus indirect effects of litter on disease, or accumulation of seeds) are creating the pattern. However, our results from the proportion of seeds killed in the seed bank showed that high-litter patches had on average a 15% increase in the proportion of seeds killed compared with the low-litter patches. These results suggest that litter facilitation of disease is partially independent of seed density and potentially due to microsite modification by the litter during the seed-infection process.
An important possible mechanism for the litter–disease pattern is that litter may affect the seed–pathogen interaction indirectly by altering seed densities that are fundamental to density-dependent diseases. Pathogen/parasite transmission often shows some level of positive density dependence (Gilbert, Reference Gilbert2002). Beckstead et al. (Reference Beckstead, Meyer, Connolly, Huck and Street2010) found that the high abundance of B. tectorum seed densities increased the incidence of disease by P. semeniperda. In other species litter has been shown to increase seed densities, especially in arid systems (Chambers and MacMahon, Reference Chambers and MacMahon1994; Rotundo and Aguiar, Reference Rotundo and Aguiar2005), and could therefore facilitate indirectly the seed pathogen–Bromus interaction. Supporting the density-dependent disease transmission hypothesis, our results showed that total seed density was two times higher in high-litter patches compared with low-litter patches. This high seed density in high-litter patches could result from litter preventing secondary dispersal of seeds or the build-up of seeds that do not germinate. In addition, the high biomass of plants during the prior season also produces large quantities of seeds and subsequent high amounts of plant litter. Either way, within the high-litter patches there are more hosts for the pathogen to feed on, leading to positive density-dependent frequency for Bromus seed infection (i.e. increasing disease with increasing host seed densities associated with litter accumulation). Our support for this density-dependent disease relationship with litter is based on sampled field data. Future experiments that manipulate seed density are needed to test this hypothesis fully.
Direct litter-to-disease experiment
We found that Bromus litter may directly determine seed disease in the seed bank via transmission of P. semeniperda, both via conidia on the litter surface and mycelium embedded within the plant tissues. Most other studies on spore infection related to plant litter have had systems where the spores are produced within the plant litter itself (e.g. Davidson et al., Reference Davidson, Wickland, Patterson, Falk and Rizzo2005). Distinctly, in our system the conidial spores are produced only on the infected seeds (Beckstead et al., Reference Beckstead, Meyer, Molder and Smith2007), but the spores can adhere to the litter surface and then be a mechanism of disease transmission, as indicated by the data in this paper. Our data do not support Medd's hypothesis that mycelium within plant tissues is an important inoculum source (Medd et al., Reference Medd, Murray and Pickering2003). We found pathogen-caused seed mortality from mycelium infection to be present but rather low from spring-collected plant litter, ranging from 1 to 4%. However, spring is the season when plant litter is most likely to have the greatest contact with the seed bank as a result of winter storms pushing the plant litter from a vertical position to a horizontal position. These results suggest that fungal mycelium within litter does not likely play an important role in the seed death of B. tectorum seed banks. However, if early summer litter infected with fungal mycelium is matted down to the soil surface and seeds are still germinating, then direct disease transmission from fungal mycelium within the litter could occur.
The evidence of high mycelial infection from litter collected in early summer and mid-summer does indicate that the fungus can live systemically within plant tissues as the plant is senescing. Likewise, P. semeniperda has been isolated from 3-week-old leaf tissues of B. tectorum seedlings infected with P. semeniperda (L. Poston and J. Beckstead, unpublished data) and also from reproductive B. tectorum individuals collected from the field (G. Newcomb, unpublished data). Studies have shown that some pathogens can be commensal endophytes under certain conditions and pathogens under others (Moricca and Ragazzi, Reference Moricca and Ragazzi2008). Whether or not P. semeniperda behaves as a pathogen while in B. tectorum leaf or stem tissue, or whether its role is endophytic, will require further investigation.
Indirect litter-to-disease experiment
Our data provide evidence for positive indirect litter effects on seed disease caused by P. semeniperda. Specifically, we found pathogen-killed seeds to be four times higher in high-litter treatments compared with low-litter treatments in our artificially created seed-bank experiment. When seed density and inoculum loads are controlled, the indirect effects are most likely related to seed-bank microenvironments. Likewise, our results from the field seed-bank experiment, which examined the effects of litter on seed disease independent of seed density, also pointed towards indirect effects involving seed-bank microsite modification by litter. Evans and Young (Reference Evans and Young1970) found that the accumulation of litter at the soil–litter interface in arid systems with Bromus spp. increased moisture retention and reduced temperature fluctuations. Although most studies have focused on the indirect litter effects of seed germination and seedling survival (Hamrick and Lee, Reference Hamrick and Lee1987; Facelli and Peickett, 1991), it is likely that these same altered microenvironments are at play in the disease–host–environment triangle of the P. semeniperda–B. tectorum seed interaction. Growth of P. semeniperda is sensitive to moisture, temperature and light (Campbell et al., Reference Campbell, Medd and Brown2003), thus indicating that litter could definitely influence disease by this organism. Although we found positive, small, indirect effects of litter on seed disease, our findings were not consistent across the two repeated trials of the indirect litter-to-disease experiment (no disease was monitored in the second trial; see Methods for description). It is likely that the subtle localized changes in seed-bank microsite created by litter could be overwhelmed by the larger scale microsite and weather conditions. For example, our second trial was located adjacent to a large tree, which could have shielded the pots from adequate rainfall. Or the timing of dew events and rain events could have held the pathogen infection at a latent mycelial stage in the second trial, such that disease, evidenced by stromata formation, was not observed. Future studies should examine directly how litter modifies the microenvironment to complement the data presented here.
Alternatively, the positive indirect effect of litter on seed disease may be the result of leachate from litter stimulating fungal growth and seed infection. If litter provides a chemical cue needed for pathogen conidial germination, then pots with higher litter additions will experience greater disease than plots with lower litter additions. Unfortunately, there is little plant litter data supporting this potential mechanism. For example, Bosy and Reader (Reference Bosy and Reader1995) found that litter leachate in a prairie system reduced seedling emergence instead of enhancing emergence. Likewise, litter leachate effects on fungi are commonly negative (Nilsen et al., Reference Nilsen, Lei, Clinton, Semones, Walker and Miller1999; Piotrowski et al., Reference Piotrowski, Rillig and Morford2008) not positive as hypothesized.
In conclusion, our findings indicate that plant litter is an important factor affecting pathogen-caused seed mortality in seed banks at the soil–litter interface. We have shown that indirect effects of litter can operate with density-dependent disease relations and also that significant, but small, indirect effects of litter on disease can occur through alteration of the seed microenvironment. In general, seed banks are dynamic, both temporally and spatially. Seed pathogens have the potential to reduce seed banks dramatically, such that an understanding of factors that affect the seed pathogen–seed bank interaction will help us to better understand this early life-stage interaction.
Acknowledgements
We thank Hanford Reach National Monument, Hugh Lefcort and the Bureau of Land Management Salt Lake City District for access to field study sites; Susan Meyer for input on experimental design and supplying fungal inoculum for experiments; Trevor Davis, Chris Watson, Lindsay Poston, Kristina Bair, Sallie Cataldo, Katie Horelick, Ingrid Velez, Katie Merrill, Heather Finch and Susan Meyer for their help with data collection of experiments and litter collections in the field. Funding was provided by the National Research Initiative of USDA-CSREES (2008-35320-18677; J.B.), the Joint Fire Science Program (2007-1-3-10; J.B.) and the Howard Hughes Medical Institute through the Undergraduate Science Education Program awarded to Gonzaga University.