Published online by Cambridge University Press: 09 October 2003
This study determined the complete mitochondrial (mt) genome sequence of the canine heartworm, Dirofilaria immitisThe complete nucleotide sequence for the mitochondrial genome of Dirofilaria immitis is available from the EMBL database under the Accession Number AJ537512., and compared its structure, organization and other characteristics with Onchocerca volvulus and other secernentean nematodes. The D. immitis mt genome is 13814 bp in size and contains 36 of the 37 genes typical of metazoan organisms, and lacks the ATP synthetase subunit 8 gene. All of the genes are transcribed in the same direction. For the entire genome, the nucleotide contents are ∼55% (T), ∼19% (each for A and G) and ∼7% (C), which is very similar to those of the protein-coding genes. In the latter genes, most (∼69%) third codon positions have a T, but rarely (∼1–9%) have an A or a C. The C content (8–12%) is higher at the first and second codon positions compared with the third position (∼1%). These nucleotide biases have a significant effect on the codon usage patterns and, thus, on the amino acid composition of the proteins. The mt genome organization of D. immitis is essentially the same as that of O. volvulus, but is distinctly different from other secernentean nematodes sequenced thus far. Irrespective of transpositions of transfer RNA (trn) genes and the non-coding, AT-rich region, there are 4 gene- or gene block-translocations between the mt genome of D. immitis and those of Caenorhabditis elegans, Ascaris suum and the 2 human hookworms, Ancylostoma duodenale and Necator americanus. For D. immitis, the 22 trn genes have secondary structures typical of other secernentean nematodes, and possess a TV-replacement loop instead of a TΨC arm and loop. Like O. volvulus, the mt trnK and trnP of D. immitis use the anticodons CUU and AGG, whereas in other nematodes, UUU and UGG are employed, respectively. Also, the secondary structures of the 2 ribosomal RNA (rrn) genes are similar to the models for other nematodes. Overall, the availability of the complete D. immitis mt genome sequence provides a resource for future studies of the comparative mt genomics and of the population genetics and/or phylogeny of parasitic nematodes.
The majority of metazoan organisms, with few exceptions, possess circular mitochondrial (mt) genomes which usually vary in size from 14 to 19 kb (Boore, 1999; Saccone et al. 1999). Their mt genomes usually comprise genes for a complete set of transfer RNAs (trn), for 2 ribosomal RNAs (rrn) and for 12–13 protein subunits of the enzymes involved in oxidative phosphorylation, namely the subunits I, II and III of cytochrome oxidase (cox1, cox2 and cox3), the cytochrome b (cob), the subunits 6 and/or 8 of the ATPase complex (atp6 and/or atp8) and the subunits 1–6 and 4L of the NADH dehydrogenase (nad1–6 and nad4L) (Wolstenholme, 1992; Boore, 1999). There is also a non-coding, AT-rich (control or D-loop) region, which contains signalling elements for the regulation of replication and transcription (reviewed by Shadel & Clayton, 1997).
Over 200 mt genome sequences have been published for 11 metazoan phyla, but >150 represent chordates. Despite technological advances in mt genome sequencing, there are still major gaps in our knowledge of the mitochondrial genomics for the phylum Nematoda. Complete mt genome sequences have been determined only for 5 secernentean nematodes, including Caenorhabditis elegans (Rhabditida) (Okimoto et al. 1992), Ascaris suum (Ascaridida) (Okimoto et al. 1992), Ancylostoma duodenale, Necator americanus (Strongylida) (Hu, Chilton & Gasser, 2002) and Onchocerca volvulus (Spirurida) (Keddie, Higazi & Unnasch, 1998), and for the adenophorean nematode, Trichinella spiralis (Enoplida) (Lavrov & Brown, 2001). Current information for the class Secernentea reveals that the free-living nematode, C. elegans, and the parasitic nematodes As. suum, An. duodenale and N. americanus all possesses essentially the same mt genome arrangement (with the exception of the position of the AT-rich region) (Okimoto et al. 1992; Hu et al. 2002), whereas the filarioid, O. volvulus, has a distinctly different organization (Keddie et al. 1998).
While some workers have proposed that mt genome arrangements do not usually vary significantly within taxonomic groups (Boore, 1999), recent evidence demonstrates clearly that mt gene rearrangements can occur between species of the same family or even of the same genus (e.g. Le et al. 2000; Rawlings, Colline & Bieler, 2001). Given the paucity of mt genome information, these aspects have not yet been addressed for nematodes. In the present study, we establish the complete mt genome sequence of D. immitis, a parasite of veterinary importance, which belongs to the same order as O. volvulus but a distinct family. These 2 filarioid nematodes differ significantly in their transmission and predilection site in the host, and in the diseases they cause. While the former parasite is transmitted by mosquitoes and causes heartworm disease mainly in canids in subtropical and tropical climatic zones (e.g. Cringoli et al. 2001; Fan et al. 2001), the latter is transmitted by blackflies (of the Simulium species complex) and causes ‘river blindness’ (onchocerciasis) in humans in West Africa (Unnasch, 2002). We also compare the organization (and other characteristics) of the D. immitis mt genome with that of O. volvulus and the 5 other secernentean nematodes for which complete genome information is currently available.
Adult worms of D. immitis were collected at necropsy from the pulmonary artery of a dog from Australia (Victoria), washed in physiological saline and then frozen at −70 °C until use. Total genomic DNA was extracted from (∼2 cm) portions of an individual worm by sodium dodecyl-sulphate/proteinase K treatment (Gasser et al. 1993), column-purified (WizardTM DNA Clean-up; Promega, Madison, WI, USA) and eluted in 40 μl of H2O. The specific identity of the worm was verified using the sequence of the first internal transcribed spacer (ITS-1) of ribosomal DNA, which was compared with that reported recently for D. immitis (Accession number: AF217800) (Mar et al. 2002).
The entire mt genome of D. immitis was amplified by long-PCR (ExpandTM 20 kbPlus kit, Roche) from total genomic DNA from a single worm in 2 overlapping fragments (∼5 kb and ∼9 kb) using the 2 oligonucleotide primer sets COIF-MH4R and MH37F-MH28R, respectively (see Fig. 1). These primers were constructed to mt sequences which are relatively conserved among As. suum, C. elegans and O. volvulus (see Okimoto et al. 1992; Keddie et al. 1998). Primer MH28R was designed to the cox1 gene, while primers MH4R and MH37F were designed to the rrnS gene. Primer COIF was originally constructed from platyhelminths (see Bowles, Blair & McManus, 1992). Long-PCR amplification was performed according to the protocol of Hu et al. (2002): 92 °C for 2 min (initial denaturation); then 92 °C/10 s (denaturation), 50 °C/30 s (annealing), 68 °C or 60 °C/10 min (extension) for 10 cycles; followed by 92 °C/10 s, 50 °C/30 s; 68 °C or 60 °C/10 min for 20 cycles, with a cycle elongation of 10 s for each cycle, and a final extension at 68 °C or 60 °C/7 min. The extension temperatures of 68 °C and 60 °C were used specifically for primer sets MH37F-MH28R and COIF-MH4R, respectively. Amplicons were detected in 1% (w/v) agarose gels after ethidium-bromide staining and ultraviolet transillumination. Products were purified over mini-spin columns (WizardTM PCR-Prep, Promega) and then used as templates in dye-terminator cycle-sequencing reactions according to the supplier's (Perkin Elmer) protocol, employing a primer walking strategy. The primers employed for sequencing and their relative positions in the mt genome are presented in Fig. 1. For some T-rich regions within the D. immitis mt genome, short regions were PCR-amplified and cloned into the plasmid vector pGEM-T™ Easy (Promega) and transformed into Escherichia coli JM109, according to the manufacturer's protocol. Following propagation and plasmid purification over columns (WizardTMPlus SV Minipreps DNA, Promega), inserts were sequenced using the vector primers T7 and SP6 Promega.

Fig. 1. Schematic representation of the circular mitochondrial genome of Dirofilaria immitis. Each transfer RNA gene is identified by the one letter amino acid code on the inner side of the map. All genes are transcribed in the clockwise direction. Black triangles represent the 2 sets of primers employed for long-PCR amplification of 2 portions (shown by large curves) of the mitochondrial genome, whereas the open triangles represent primers used for DNA sequencing. According to the transcriptional direction, F and R in the primer codes indicate ‘forward’ and ‘reverse’, respectively. Regions 1 and 2 each containing 1–2 poly-T tracts (indicated by small arrows) were amplified using primer sets Di19F-Di12R and Di49F-Di48R (indicated by small rectangles), respectively. Sequences of all primers used for amplification or sequencing are given in the box.
Sequences were assembled manually and aligned with the complete mt genome sequence of O. volvulus (Accession number: AF015193; Keddie et al. 1998) using the program Clustal X (Thompson et al. 1997). Also, protein genes (designated according to Le, Blair & McManus, 2000), and translation initiation and termination codons were identified based on the comparison with those reported previously for O. volvulus. The inferred amino acid sequences and codon usage of protein genes were obtained using the computer program MacVector version 7.0 (Oxford Molecular Group). The amino acid sequences inferred for D. immitis were aligned with those of C. elegans, As. suum (Accession numbers: X54252 and X54253; Okimoto et al. 1992), O. volvulus (Keddie et al. 1998), An. duodenale and N. americanus (Accession numbers: AJ417718 and AJ417719; Hu et al. 2002) using Clustal X. Based on the alignment, amino acid sequence identity (%) to homologous genes was calculated. Most trn genes in the mt genome of D. immitis were identified using the tRNAscan program, available at http://www.queensu.ca/micr/faculty/kropinski/online.html (Lowe & Eddy, 1997), whereas others were identified by eye based on their potential trn secondary structures and/or anticodon sequences (Wolstenholme et al. 1987). The 2 rrn genes were identified based on their sequence similarity to those of O. volvulus (see Keddie et al. 1998) and their potential to form rrn-like secondary structures. The secondary structures of the rrn genes were inferred by analogy to previously published structures (see Hu et al. 2002). The stem-loop structures of non-coding regions were inferred using the Mfold program, available at http://www.queensu.ca/micr/faculty/kropinski/online.html (Santalucia, 1998).
The ∼5 kb and ∼9 kb amplicons spanning the entire mt genome of D. immitis (Fig. 1) were subjected to sequencing. Over 90% of the genome could be sequenced directly from these amplicons, except for 2 regions containing 1–2 poly-T sequence tracts (see Fig. 1) located to the genes nad2 (1 with 18Ts) and nad6 (2 with 18–20 Ts), which had to be cloned and then sequenced to obtain readable sequence beyond these tracts. Hence, region 1 (∼0·5 kb) and region 2 (∼1·2 kb) containing the 3 poly-T tracts (Fig. 1) were amplified using primers Di19F-Di12R and Di49F-Di48R, respectively, and then cloned and sequenced, following conventional propagation and purification of the recombinant plasmids.
The circular mt genome of D. immitis is 13814 bp in size (Fig. 2), which is amongst the smallest metazoan mt genomes sequenced to date, such as the platyhelminth Taenia crassiceps (13503 bp) (Le et al. 2002). It is 20–210 bp longer than the mt genomes of C. elegans (see Okimoto et al. 1992), O. volvulus (see Keddie et al. 1998), An. duodenale and N. americanus (see Hu et al. 2002), and 470 bp shorter than that of As. suum (see Okimoto et al. 1992). Other features of the mt genome of D. immitis and its products, including the positions, lengths and start/stop codons of individual genes as well as amino acid sequence lengths of predicted proteins, are shown in Table 1.

Fig. 2. Linear representation of the mitochondrial genome of Dirofilaria immitis, indicating relevant sequence information. The numbers of nucleotides omitted from within the genome sequence are indicated in parentheses. The sequences of the AT-rich region (double-underlined) and the transfer RNA genes (trn) are shown in full. For each trn gene (underlined), the first and last nucleotides are denoted by ‘[’ and ‘]’, respectively. Trinucleotides representing anticodons (bold-type) are shown directly under each trn gene designation. The beginning and end of each ribosomal and protein-coding gene are marked by ‘|’. Except for the overlap between genes nad1 and trnF, the first and last 6 codons of each protein-coding gene are shown, along with the translated amino acids. The stop codon of each protein-coding gene is denoted by ‘*’. All genes are transcribed from left to right.
All of the 36 genes (12 protein-coding, 2 rrn and 22 trn genes) typically found in secernentean nematodes were identified in the mt genome of D. immitis. They are all transcribed in the same direction (Fig. 1), which is consistent with most other nematodes (Okimoto et al. 1992; Keddie et al. 1998; Hu et al. 2002), flatworms (Le et al. 2002), annelids, and some molluscs, brachiopods and cnidarians (see Boore, 1999). The atp8 gene is absent, consistent with other secernentean nematodes, flatworms (von Nickisch-Rosenegk, Brown & Boore, 2001; Le, Blair & McManus, 2002) and some molluscs (Hoffmann, Boore & Brown, 1992) but in contrast to T. spiralis (see Lavrov & Brown, 2001). Whether the atp8 gene absent from the mt genome of D. immitis is present in the nuclear genome remains to be established.
Consistent with the small size of the D. immitis mt genome, the genes are arranged in an ‘economical fashion’ in that most genes have no bases or only a few nucleotides between their coding regions, and there is evidence that 5 pairs of genes overlap by 1–21 nucleotides (Fig. 2). Specifically, (i) there is a 1-nucleotide overlap between trnE and trnS (AGN). Regarding such an overlap, study of RNA editing in vertebrates has shown that the downstream trn is released in its intact form, whereas the upstream trn is truncated and completed by editing (Yokobori & Pääbo, 1995, 1997); (ii) a 4-nucleotide overlap is present between trnS (AGN) and nad2; (iii) a 1-nucleotide overlap occurs between trnT and nad4; (iv) the last 2 nucleotides of trnY overlap with the first 2 nucleotides of nad1; (v) there are 21 nucleotides shared between nad1 and trnF. An overlap of a similar length is also present in the mt genome of O. volvulus (see Keddie et al. 1998), but not in any other nematode species sequenced to date.
The organization of mt genome of D. immitis is the same as O. volvulus (the only other filarioid nematode for which mt sequence data have been published), but is distinctly different from other secernentean nematodes of different orders which all possess essentially the same genome organization based on the arrangements of protein-coding and rrn genes (and excluding the position of the AT-rich region) (Fig. 3). Discounting the numerous differences in the positions of trn genes and the AT-rich region, a minimum of 4 rearrangements is required to interconvert the mt gene arrangement of D. immitis with those of C. elegans, As. suum, and An. duodenale and N. americanus (Fig. 3). There are 2 gene- (nad2 and nad6) and 2 gene block- (nad4L-rrnS-nad1-atp6 and nad4-cox1) translocations (Fig. 3). Regarding gene rearrangements in animal mt genomes, several models have been proposed to explain the possible mechanisms, including the ‘duplication/random loss model’ (Moritz, Dowling & Brown, 1987; Macey et al. 1997) and the ‘intra-mitochondrial recombination model’ (Lunt & Hyman, 1997; Dowton & Campbell, 2001). Also, some other studies have focussed on the correlation between mt genome rearrangements and biological or molecular phenomena, such as the evolution of parasitism, high mt AT content, accelerated rate of mt genetic divergence as well as adaptive radiation (reviewed by Dowton, Castro & Austin, 2002). Interestingly, the nad2 and nad6 genes of D. immitis both contain relatively long poly-T ([ges ]18) tracts (see Fig. 1) (which originally caused considerable problems when subjected to direct sequencing). Biologically, such tracts may facilitate slipped-strand mispairing because of an increased chance of incidental homology between distant mt genes (Dowton et al. 2002), and may be associated with the translocation of genes. Regarding the 2 gene block translocations, a likely explanation appears to relate to intra-mitochondrial recombination rather than duplication/random loss (reviewed by Dowton et al. 2002).

Fig. 3. Schematic, linear representation of the mitochondrial genome arrangement of Dirofilaria immitis (showing only protein and ribosomal RNA genes) compared with those published for other secernentean nematodes (see Okimoto et al. 1992; Keddie et al. 1998; Hu et al. 2002).
Twelve protein-coding genes have been identified in the mt genome of D. immitis. Four of them use ATT as a translation initiation codon (Table 1). Four others employ TTG, which is commonly used as an initiation codon in nematodes (see Table 3 in Hu et al. 2002). The codons GTT, GTA, CTT and TAT are inferred to be used for the initiation of the other 4 protein genes, but are rarely used in the nematodes studied to date, including O. volvulus (see Okimoto et al. 1992; Keddie et al. 1998; Lavrov & Brown, 2001; Hu et al. 2002).
Eight of the 12 protein genes (nad2, nad4, cox1, nad6, cox3, nad4L, atp6 and nad5) use a complete translation termination codon, TAG or TAA. The other 4 (cob, nad1, cox2 and nad3) use truncated codons, such as T alone (Table 1). Each of the latter genes is followed by a trn, but in no case do they overlap by 1 or 2 nucleotides with their downstream gene to complete the termination codon. Therefore, in these cases, after transcription and processing, the incomplete stop codon T could be converted to TAA by polyadenylation (Ojala, Montaya & Attardi, 1981).
After the recognition of translation initiation and termination codons, the lengths of all 12 mt protein genes of D. immitis were determined. Table 2 shows the lengths of inferred amino acid sequences and their identities with homologues in other secernentean nematodes. The amino acid sequences inferred for D. immitis are very similar in length ([les ]2 amino acid differences) to those of O. volvulus and are most divergent from those of As. suum ([les ]24 amino acid differences). Proteins COX1, COX3, NAD1, NAD2, NAD4L, NAD5 and NAD6 are 3-24 amino acids longer, and ATP6 and COB are 5-7 amino acids shorter compared with other secernentean nematodes, except O. volvulus. Comparisons also revealed that COX1 is amongst the most conserved proteins, whereas NAD6 and ATP6 are the least conserved (see Table 2).

The nucleotide compositions of the entire mt genome sequence of D. immitis, and its coding and non-coding regions are compared in Table 3. The whole genome has a T content of 54·9%, the A or G content is 19·3%, and the C content is 6·6%. These percentages are similar to those for O. volvulus, but distinct from those of other secernentean nematodes in that the percentage of A (22–28%) is higher and the percentages for T (44–49%) and G (15–20%) lower (see Table 1 in Hu et al. 2002). In protein-coding genes, the third codon position has a higher AT content (78%) than the first (70·1%) and second (69·9%) codon positions (Table 3). The increased AT content at the third position relates mainly to the high percentage (68·9%) of T, whereas the percentages of A and C at this position are low (9·1% and 1·1%, respectively). The greatly reduced frequencies of A and C at the third codon position appear to reflect the mutational pattern in the mt genome, as the nucleotides at this position are under the least ‘selective pressure’ (Sharp & Matassi, 1994). Interestingly, the frequency of C (7·7–12%) at the first and second codon positions is higher than at the third (1·1%) (Table 3). These findings suggest that, although the mt genome of D. immitis favours T against C, there is some selection for a higher C content at the former 2 positions which is critical for the maintenance of certain, key amino acids. These amino acids (relating to codons containing a C at the second position) are alanine (2·1%), proline (2·1%), serine (5·2%) and threonine (2·7%) (Table 4). For instance, alanine and proline are non-polar, hydrophobic amino acids which are membrane-bound and, thus, are considered integral in the maintenance of mitochondrial structure and function (Asakawa et al. 1991). Of all 20 amino acids, leucine, phenylalanine and serine are the most commonly used by D. immitis, whereas arginine, glutamine and histidine are least employed (Tables 4 and 5).



The bias in the nucleotide composition of the mt genome of D. immitis is reflected in its codon usage (Table 5). Of 64 possible codons, 62 are used. Codons ACC and CGC are not employed, whereas T-rich codons (with T at 2 of the 3 codon positions), such as TTT (18·9%), TTA (3·4%), TTG (8·6%), ATT (5·7%), GTT (7·2%), TAT (6·6%) and TGT (3·0%), are used more frequently than other codons, except CTT and TTC (<0·1%). These findings reveal that this genome is strongly biased against C. When each codon family is examined, the usage of synonymous codons in the proteins representing the D. immitis mt genome follows the same pattern as the nucleotide frequency (i.e. T>G>A>C). This bias is evident for both the 4-fold and 2-fold degenerate codon families, suggesting that the third codon position usually reflects the mutational bias.
The 22 trns encoded in the mt genome of D. immitis vary in size from 52 to 66 nucleotides. While their locations in this genome are the same as for O. volvulus, they are distinctly different from those in other nematodes. Secondary structures predicted for the 22 trns of D. immitis (Fig. 4) are similar to those of all other secernentean nematodes examined to date (Okimoto et al. 1992; Keddie et al. 1998; Hu et al. 2002). Twenty of them possess a TV-replacement loop, typical for nematodes (Wolstenholme et al. 1987). The DHU arms of the 2 serine trns are replaced by a DHU-replacement loop which is not necessarily exclusive to nematodes. Whilst all of these trns lack a T or D arm (considered crucial for the delivery of the amino-acyl trn to the ribosome; Wolstenholme, Okimoto & Macfarlane, 1994), recent studies of As. suum have identified 2 translation elongation factors Tu (EF-Tu1 and EF-Tu2) which recognize and subsequently deliver the amino-acyl trns to the mitochondrial ribosomes (Ohtsuki et al. 2001, 2002). Clearly, this finding could have important implications for studying translational processes in the mitochondria of other parasitic nematodes.

Fig. 4. Predicted secondary structures of the 22 transfer RNA genes in the mitochondrial genome of Dirofilaria immitis.
Each trn in the D. immitis mt genome is predicted to have an amino-acyl acceptor stem of 7 nucleotides, an anticodon stem of 5 nucleotides and an anticodon loop of 7 nucleotides (Fig. 4). However, 1–3 mismatches appear in the amino-acyl stem of 16 trns. Only 6 of the 22 trns have perfect base-pairing in this stem, allowing for G-U pairing. Like O. volvulus, the most notable mismatches are present in trnY (see Keddie et al. 1998). While mismatches in the amino-acyl stem are common for metazoan mt trn, it is suggested that they are corrected effectively by RNA-editing (Yokobori & Pääbo, 1995, 1997; Lavrov, Brown & Boore, 2000).
Twenty of the D. immitis mt trns have a 4 nucleotide DHU stem, and 5–15 nucleotides in the DHU loop (Fig. 4). Their TV-replacement loop varies in size from 4–8 nucleotides. The first of the 2 nucleotides separating the amino-acyl stem from the DHU arm is a U in 16 of the trns; the second is an A in 19 of them. The nucleotide separating the DHU arm from the anticodon stem is an A for trnA, trnN, trnH, trnL (CUN), trnL (UUR), trnM, trnF, trnW and trnY, a G for trnR and trnC, and a U for trnD, trnQ, trnE, trnG, trnI, trnK, trnP, trnT and trnV. The two nucleotides immediately preceding the anticodon are always U, except for trnK and trnS (AGN). The nucleotide after the anticodon is always a purine, except for trnI and trnL where it is a U.
Twenty of the 22 anticodons are the same as those of the other secernentean nematodes studied to date. The 2 other anticodons are identical to those of O. volvulus (see Keddie et al. 1998). These are CUU in trnK rather than UUU, and AGG in trnP rather than UGG (Fig. 4). Anticodon CUU is also present in the trnK of platyhelminths (Le, Blair & McManus, 2001), hemichordates (Castresana, Feldmaier-Fuchs & Pääbo, 1998), echinoderms (Giorgi, 1996) and some arthropods (Black & Roehrdanz, 1998; Campbell & Barker, 1999).
The rrnS and rrnL genes are separated by nad1, atp6, cox2 and 5 trns (Fig. 1). The lengths of these rrn genes are 687 bp and 968 bp, respectively, which is similar to those of other nematodes (Okimoto et al. 1992; Keddie et al. 1998; Lavrov & Brown, 2001; Hu et al. 2002) but shorter than for most other Metazoa (cfWolstenholme, 1992, Table VI). The sequence identities in the rrnS and rrnL genes between D. immitis and O. volvulus are 89·4% and 83·1%, respectively. Interestingly, the secondary structure predicted for the rrnS gene is similar to that of the hookworms An. duodenale and N. americanus, in that most stems are conserved, except for stems 22 and 37 (Fig. 5) which are absent. Also, the rrnL gene structure is similar to that of both hookworms, and the nucleotides predicted to be associated with amino-acyl binding and peptidyl-transfer appear to be conserved (Fig. 6).

Fig. 5. Predicted secondary structure for the small ribosomal RNA gene subunit (rrnS) in the Dirofilaria immitis mitochondrial genome. Canonical base pairs C[ratio ]G and U[ratio ]A are indicated by lines. G[ratio ]U pairs are denoted by small dots, and other non-canonical pairings by large dots. Proposed tertiary interactions are represented by long, straight lines. Bold numbers (1–48) identify secondary structure elements considered to be conserved.

Fig. 6. Predicted secondary structure of the large ribosomal RNA gene subunit (rrnL) in the Dirofilaria immitis mitochondrial genome. Canonical base pairs C[ratio ]G and U[ratio ]A are indicated by lines, G[ratio ]U pairs by dots, and other non-canonical pairings by large dots. Proposed tertiary interactions are represented by long, straight lines. Sites of amino-acyl binding (A), peptidyl-transfer (P) or both (AP) are indicated in bold-type.
The longest non-coding region (362 bp) in the mt genome of D. immitis, located between cox3 and trnA (Fig. 1), is 85·9% AT-rich. It is proposed to represent the control (AT-rich) region, since there is no other region of a similar length with such a high AT content. The presence of pairs of inverted repeat sequences in the structure of the AT-rich region (Fig. 7), common also to other nematodes (Hu et al. 2002), suggests functional significance. While the AT-rich region in D. immitis does not contain any tandem-repeat motifs (such as the CR1-CR6 in C. elegans or the AT dinucleotide repeats in As. suum; see Okimoto et al. 1992), it does contain a poly-A region typical of other secernentean nematodes (Hu et al. 2002).

Fig. 7. Predicted secondary structure for the non-coding, AT-rich region in the mitochondrial genome of Dirofilaria immitis.
Overall, the mt genome of D. immitis is compact, with only a small number of short non-coding regions between coding genes (Fig. 2). The longest such region (between the genes trnW and nad6) is 56 bp, being similar in size to a trn gene. However, a secondary structure could not be inferred for this region, which indicates that it is neither a trn gene nor a duplicated trn pseudogene (Beagley, Okimoto & Wolstenholme, 1999). Also, it does not have a stem-and-loop structure, which suggests that it is not involved in the synthesis of the second strand of the mt genome (Boore & Brown, 1994).
The mt genome of the canine heartworm, D. immitis, exhibits several features, such as size, gene content and secondary structures of the trn and rrn genes, which are typical of other secernentean nematodes. However, this genome possesses specific characteristics shared solely by that of O. volvulus, the only other filarioid nematode whose complete mt genome sequence has been determined. While the organization of the D. immitis mt genome is very similar to that of O. volvulus, it is distinctly different from that of all other secernentean nematodes studied to date. Also, this genome is particularly T-rich, and some regions contain poly-T sequence tracts, and 2 trns use distinct anticodons compared with non-filarioid nematodes. In conclusion, the present study describes the first mt genome sequence and structure for any filarioid nematode of veterinary importance. This information adds significantly to the knowledge of mt genomics of parasitic nematodes, provides a resource for the design of primers for mt genome sequencing projects (via comparison among all mt genome sequences for nematodes) and for future comparative mt genome analyses and phylogenetic studies of nematodes, and may yield genetic markers for molecular epidemiological and population genetics investigations into filarioid parasites.
The authors would like to thank Ian Beveridge for collecting the D. immitis used in this study. Min Hu is the recipient of a postgraduate scholarship from The University of Melbourne.

Fig. 1. Schematic representation of the circular mitochondrial genome of Dirofilaria immitis. Each transfer RNA gene is identified by the one letter amino acid code on the inner side of the map. All genes are transcribed in the clockwise direction. Black triangles represent the 2 sets of primers employed for long-PCR amplification of 2 portions (shown by large curves) of the mitochondrial genome, whereas the open triangles represent primers used for DNA sequencing. According to the transcriptional direction, F and R in the primer codes indicate ‘forward’ and ‘reverse’, respectively. Regions 1 and 2 each containing 1–2 poly-T tracts (indicated by small arrows) were amplified using primer sets Di19F-Di12R and Di49F-Di48R (indicated by small rectangles), respectively. Sequences of all primers used for amplification or sequencing are given in the box.

Fig. 2. Linear representation of the mitochondrial genome of Dirofilaria immitis, indicating relevant sequence information. The numbers of nucleotides omitted from within the genome sequence are indicated in parentheses. The sequences of the AT-rich region (double-underlined) and the transfer RNA genes (trn) are shown in full. For each trn gene (underlined), the first and last nucleotides are denoted by ‘[’ and ‘]’, respectively. Trinucleotides representing anticodons (bold-type) are shown directly under each trn gene designation. The beginning and end of each ribosomal and protein-coding gene are marked by ‘|’. Except for the overlap between genes nad1 and trnF, the first and last 6 codons of each protein-coding gene are shown, along with the translated amino acids. The stop codon of each protein-coding gene is denoted by ‘*’. All genes are transcribed from left to right.

Table 1. Positions and nucleotide (nt) sequence lengths of individual mitochondrial genes of Dirofilaria immitis, and start and stop codons for protein-coding genes as well as the lengths of their predicted amino acid (aa) sequences

Fig. 3. Schematic, linear representation of the mitochondrial genome arrangement of Dirofilaria immitis (showing only protein and ribosomal RNA genes) compared with those published for other secernentean nematodes (see Okimoto et al. 1992; Keddie et al. 1998; Hu et al. 2002).

Table 2. Predicted amino acid sequence lengths and identity of individual mitochondrial proteins of Dirofilaria immitis (Di) with those of the other secernentean nematodes, Onchocerca volvulus (Ov), Ancylostoma duodenale (Ad), Necator americanus (Na), Ascaris suum (As) and the free-living nematode Caenorhabditis elegans (Ce)

Table 3. Nucleotide compositions (%) for the entire or regions of the mitochondrial genome of Dirofilaria immitis

Table 4. Amino acid composition of proteins inferred from the mitochondrial genome sequence of Dirofilaria immitis

Table 5. Nucleotide codon usage for 12 protein-coding genes of the mitochondrial genome of Dirofilaria immitis (nt: nucleotide; aa: amino acid; nc: numbers of codons; *: stop codon; total no. of codons is 3463)

Fig. 4. Predicted secondary structures of the 22 transfer RNA genes in the mitochondrial genome of Dirofilaria immitis.
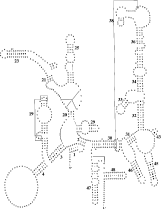
Fig. 5. Predicted secondary structure for the small ribosomal RNA gene subunit (rrnS) in the Dirofilaria immitis mitochondrial genome. Canonical base pairs C[ratio ]G and U[ratio ]A are indicated by lines. G[ratio ]U pairs are denoted by small dots, and other non-canonical pairings by large dots. Proposed tertiary interactions are represented by long, straight lines. Bold numbers (1–48) identify secondary structure elements considered to be conserved.
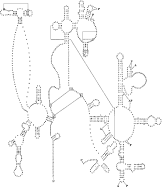
Fig. 6. Predicted secondary structure of the large ribosomal RNA gene subunit (rrnL) in the Dirofilaria immitis mitochondrial genome. Canonical base pairs C[ratio ]G and U[ratio ]A are indicated by lines, G[ratio ]U pairs by dots, and other non-canonical pairings by large dots. Proposed tertiary interactions are represented by long, straight lines. Sites of amino-acyl binding (A), peptidyl-transfer (P) or both (AP) are indicated in bold-type.
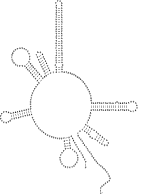
Fig. 7. Predicted secondary structure for the non-coding, AT-rich region in the mitochondrial genome of Dirofilaria immitis.