Introduction
The bovine ovary contains thousands of preantral follicles and understanding the mechanisms that control ovarian folliculogenesis in vitro can progressively increase the use of oocytes enclosed in preantral follicles in assisted reproductive techniques. The development of an in vitro culture protocol that allows better development of secondary follicles up to the antral stage is of utmost importance to provide information about the follicular needs during ovarian development (Silva et al., Reference Silva, Ferreira, Costa and Figueiredo2002, Reference Silva, van den Hurk and Figueiredo2016). Some in vitro studies have shown that preantral follicles are less susceptible to atresia (Meng et al., Reference Meng, Jan, Hamer, van Pelt, van der Stelt, Keijer and Teerds2018) and, therefore, these have been commonly used to study follicular development in vitro (Almeida et al., Reference Almeida, Magalhães-Padilha, Araújo, Costa, Chaves, Lopes, Donato, Peixoto, Campello, Junior and Figueiredo2015; Paulino et al., Reference Paulino, Cunha, Silva, Souza, Lopes, Donato, Peixoto, Matos-Brito, van den Hurk and Silva2018).
Despite the promising results achieved so far in other species, such as production of embryo after fertilization of in vitro grown oocytes (mice: O’Brien et al., Reference O’Brien, Pendola and Eppig2003; goat: Magalhães et al., Reference Magalhães, Duarte, Araújo, Brito, Soares, Lima, Lopes, Campello, Rodrigues and Figueiredo2011), the complete development and production of embryos from oocytes enclosed in bovine preantral follicles has still not been reported (Araújo et al., Reference Araújo, Gastal, Figueiredo and Gastal2014). According to Beck et al. (Reference Beck, Singh, Dar and Anzar2018), preantral follicles require for their complete development an efficient culture system that approaches their natural conditions, providing nutrients and energy for cells to ensure their development and growth. Therefore, the formulation of specific medium that meets the metabolic characteristics is of paramount importance (Rossetto et al., Reference Rossetto, Saraiva, Dos santos, Da Silva, Faustino, Chaves, Brito, Rodrigues, Lima, Donato, Peixoto and De Figuereido2013).
Fetal bovine serum (FBS) is commonly used as a supplement because of its efficiency in improving cell proliferation, metabolism and differentiation (Cho et al., Reference Cho, Lee and Kim2018). FBS also has the ability to maintain cellular activities, providing essential compounds, such as vitamins, binding factors, and being involved in pH buffering or protease inhibitor (Heger et al., Reference Heger, Froehlich, Pastuscheck, Schmidt, Baer, Mmrowka, Backsch, Schleuβner, Markert and Schmidt2018). However, in addition to biosafety problems, FBS presents in its composition unknown factors that vary biochemically between different lots, therefore interfering in the standardization of culture medium (van der Valk et al., Reference van der Valk, Mellor, Brands, Fischer, Gruber, Gstraunthaler, Hellebrekers, Hyllner, Jonker, Prieto, Thalen and Baumans2004). Therefore, various studies have emphasized the importance of using serum-free medium during culture of follicles (Park et al., Reference Park, Gong, Kim, Kim, Choi, Ahn and Lim2013; Motohashi et al., Reference Motohashi, Taniguchi, Sakamoto, Sankai and Kada2017). As an alternative to FBS, knockout serum replacement (KSR) has a defined formulation and presents numerous positive effects on cells cultured in vitro (Aoshima et al., Reference Aoshima, Baba, Makino and Okada2013). KSR supplement consists of essential substances such as lipid-rich albumin, transferrin, insulin, amino acids, vitamins and antioxidants (Garcia-Gonzalo and Belmonte, Reference Garcia-Gonzalo and Belmonte2008). Recently, Motohashi et al. (Reference Motohashi, Taniguchi, Sakamoto, Sankai and Kada2017) demonstrated that oocyte–granulosa cell complexes from mice preantral follicles cultured in medium with either FBS or KSR were able to be fertilized in vitro and to deliver live pups. Park et al. (Reference Park, Gong, Kim, Kim, Choi, Ahn and Lim2013) also showed that replacement of FBS by KSR did not reduce mice follicle growth or oocyte maturation in vitro. Another protein source commonly added to the medium is bovine serum albumin (BSA), which is the most abundant protein in plasma (Majorek et al., Reference Majorek, Porebski, Dayal, Zimmermman, Jablonska, Stewart, Chruszcz and Minor2012). Therefore, it is hypothesized that KSR can be used as a substitute of other protein sources, like FBS, in culture medium of bovine preantral follicles and consequently improves follicular growth and survival in vitro.
The aims of the present study were to evaluate the effects of KSR (5% and 10%), FBS (5% and 10%), as well as of BSA (3 mg/ml) on survival and growth of bovine secondary follicles cultured in vitro for 12 days.
Materials and methods
Reagents
The culture medium, as well as other products used in this study, were purchased from Sigma Chemicals (St. Louis, MO, USA).
Ovary collection and follicular isolation
For this study, 20 pairs of ovaries from cows were collected from the local slaughterhouse. After collection, the ovaries were washed (approximately 10 s) in 70% alcohol, and were then transported to the laboratory in saline solution containing antibiotics (100 mg/ml penicillin and 100 mg/ml streptomycin) at 4°C for up to 1 h. In the laboratory, fragments of the ovarian cortex (1–2 mm in thickness) were sectioned using a surgical blade under sterile conditions. These fragments were subsequently placed in fragmentation medium (TCM-199) and then isolation of preantral follicles was performed using a stereoscopic microscope (SMZ 645 Nikon, Tokyo, Japan). Secondary follicles were dissected and isolated from the ovarian cortex using 25 gauge (25G) needles.
In vitro culture of bovine preantral follicles
Follicles with a visible oocyte surrounded by granulosa cells, an intact basement membrane and no antral cavity were individually cultured in 100 μl medium under mineral oil in Petri dishes (60 × 15 mm, Corning, USA). The culture medium, TCM-199+, was supplemented with ITS (1.0 μg/ml, insulin, 0.55 μg/ml transferrin and 0.5 ng/ml selenium), 50 μg/ml ascorbic acid, 3 mM glutamine, 2 mM hypoxanthine, 10 mg/ml penicillin and 10 mg/ml of streptomycin. Secondary follicles were randomly cultured in TCM-199+ alone (culture control) or supplemented with KSR (5% or 10%, Invitrogen, São Paulo, Brazil), FBS (5% or 10%) or BSA (3 mg/ml). The follicles were cultured at 38.5°C with 5% CO2 for 12 days. Medium filtration was performed after adding the supplements. Every 2 days of culture, 60 μl of medium was replaced with fresh medium.
Morphological and viability evaluation of cultured follicles
At days 0, 4, 8 and 12 of culture, morphologically normal follicles with well organized granulosa cells, and intact oocyte and basement membrane were observed. Finally, two perpendicular measurements were performed on normal follicles using an inverted microscope with the software NIS Elements 2.4 (Nikon Instruments Inc., Japan). The presence of an antral cavity in cultured follicles was also evaluated.
To confirm the results of morphological analyzes, follicles that were considered normal were further evaluated for their survival using a more accurate method based on fluorescent probes. After culturing, the follicles (n = 8 per treatment) were stained with 4 mM calcein-AM and 2 mM ethidium homodimer (Molecular Probes, Invitrogen, Karlsruhe, Germany) at 37°C for 15 min. Subsequently, the follicles underwent three washes in TCM-199 and were examined under a fluorescence microscope (Nikon, Eclipse, TS 100, Japan). The oocytes and granulosa cells were considered viable if the cytoplasm was positively stained a green colour (calcein-AM) and were not stained red by the ethidium homodimer (van den Hurk et al., Reference van den Hurk, Spek, Hage, Fair, Ralph and Schotanus1998; Vasconcelos et al., Reference Vasconcelos, Saraiva, Costa, Passos, Silva, Rossi, Portela, Duarte, Magalhães-Padilha, Campelo, Figueiredo, van den Hurk and Silva2013).
Evaluation of follicle morphology by classical histology
Both fresh and 12-day cultured follicles were fixed for 24 h in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) and then, dehydrated in a graded series of alcohol (70, 80, 90 and 100%), diaphanized with xylene and then embedded in paraffin wax. For each group of follicles, sections (5 μm thick) were performed and stained with haematoxylin–eosin. All sections were examined and the follicles were evaluated under a light microscope (Nikon, Japan). To evaluate the follicular morphology, follicles presenting well organized granulosa cells, spherical oocytes with a nucleus, and intact basement membrane were considered to be normal. For each treatment, five follicles were histologically evaluated.
Statistical analysis
For analysis of follicular growth, the data were expressed in mean (± standard error of the mean, SEM) and submitted to analysis of variance (ANOVA); means were compared using the Kruskal–Wallis test using Stat View 5.0 (SAS Institute Inc., Cary, NY, USA). After 12 days of culture, percentages of follicular survival were analyzed using the chi-squared test. The differences were considered significant when P-values were < 0.05.
Results
Effects of different protein sources on follicular growth and viability
In general, a progressive increase in follicular diameter was observed in follicles cultured in vitro. After 8 days, follicles cultured in TCM-199+ alone or supplemented with 5% and 10% KSR, as well as 10% FBS had higher diameters than those follicles at day 0. After 12 days, follicles cultured in all media had higher diameters when compared with follicles at day 0 (Table 1). However, when comparisons among media were performed after 4, 8 or 12 days of culture, the presence of KSF (5 or 10%), FBS (5 or 10%) or BSA did not influence follicular growth in vitro. Antrum formation was rarely observed during culture.
Table 1. Effects of different protein sources on the diameters (µm) of bovine secondary follicles during 12 days of culture
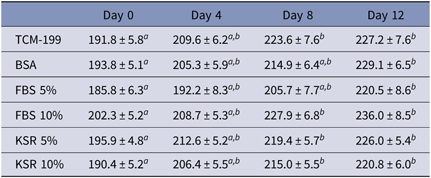
a,bMeans difference between days of culture within each treatment.
After 12 days, follicles cultured with 5% or 10% KSR had higher rates of viability (P < 0.05) when compared with those cultured in control medium alone or supplemented with FBS or BSA (Fig. 1). Conversely, follicles cultured in the presence of BSA and 10% FBS showed a similar rate of follicular survival when compared with those cultured in control medium. In addition, follicles cultured in medium with 5% FBS had the lowest viability rate among treatments.
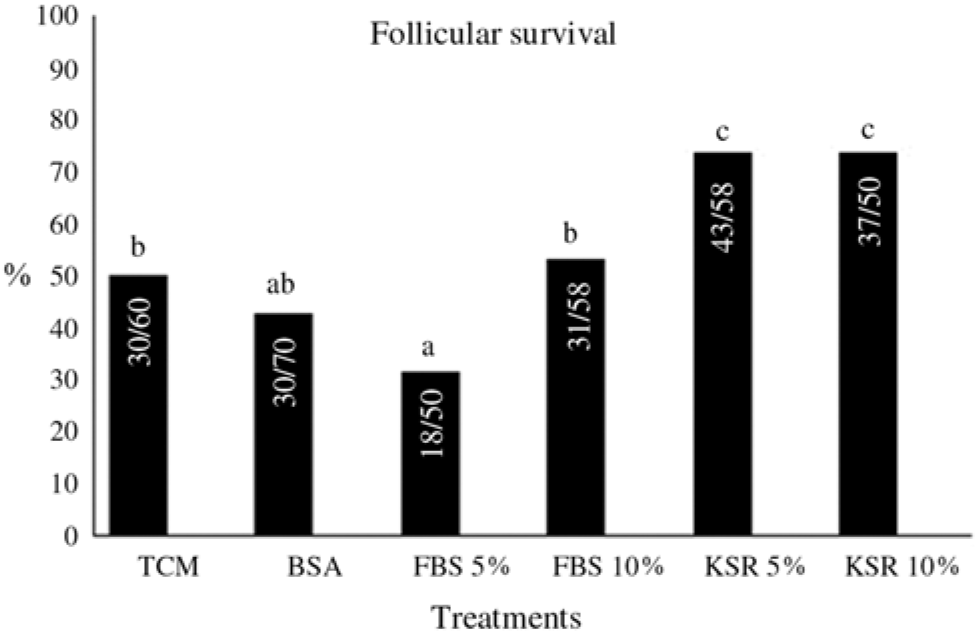
Figure 1. Survival rate of bovine secondary follicles cultured in TCM-199+ alone or supplemented with BSA, FBS (5% or 10%) KSR (5% or 10%) after 12 days of culture in vitro. a–cLowercase letters show significant differences between treatments.
To confirm follicle viability, the fluorescence analysis showed that normal follicles after 12 days of culture had homogeneous granulosa cells and oocytes that were positively stained for calcein (green). In some follicles, especially those cultured in TCM-199 alone or supplemented with BSA or 10% KSR had stromal/thecal cells around the follicles stained positively with ethidium homodimer (red) (Fig. 2).
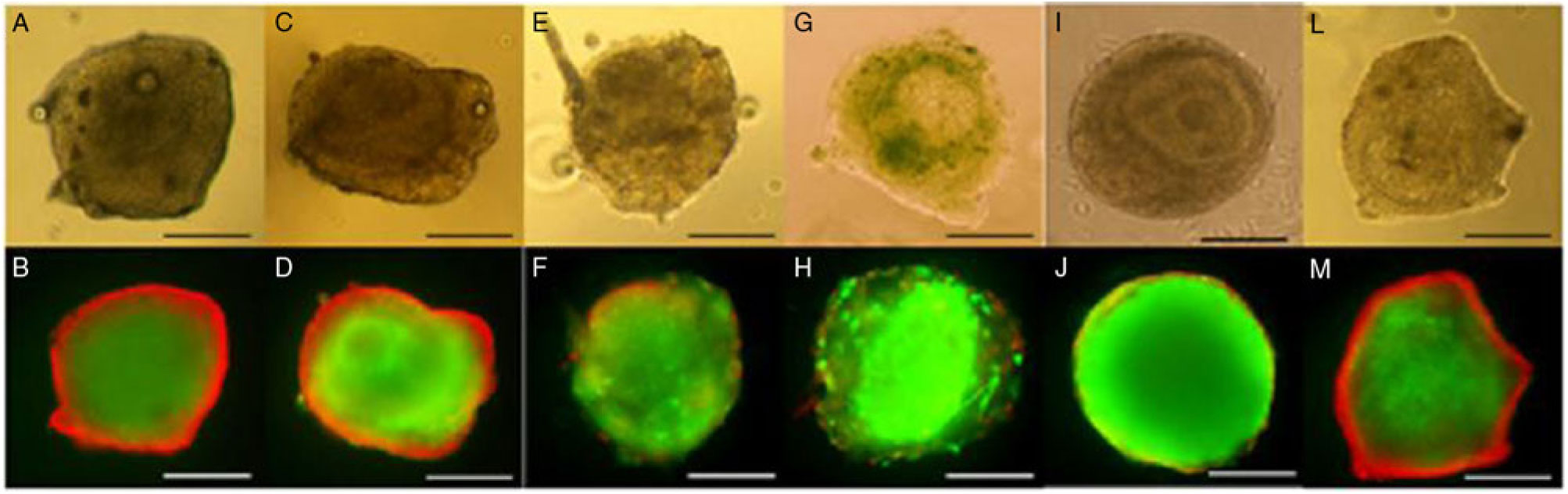
Figure 2. Secondary follicles cultured in TCM-199+ alone (A, B) or supplemented with BSA (C, D), 5% FBS (E, F), 10% FBS (G, H), 5% KSR (I, J) or 10% KSR (L, M) for 12 days and evaluated by light microscopy and after staining with calcein-AM (green) and ethidium homodimer-1 (red). Considering that only surrounding stromal cells were stained with ethidium homodimer-1 in some follicles, all of these were considered viable. Scale bars represent 100 µm.
Histological evaluation of secondary follicles before and after 12 days of culture
Figure 3 shows that secondary follicles within uncultured ovarian tissue had centralized oocyte various layers of well organized granulosa cells (Fig. 3A). Figure 3B shows that, after isolation, secondary follicles keep their follicular structure with intact oocyte, granulosa cells and basement membrane. After 12 days of culture in the presence of 5% KSR, secondary follicles increased their diameter and maintained the morphology of their oocyte, granulosa and theca cells (Fig. 3C). Figure 3D shows the morphology of degenerated follicles with rupture of basal membrane, follicle retraction and disorganized granulosa cells, in addition to absence or oocyte degeneration.
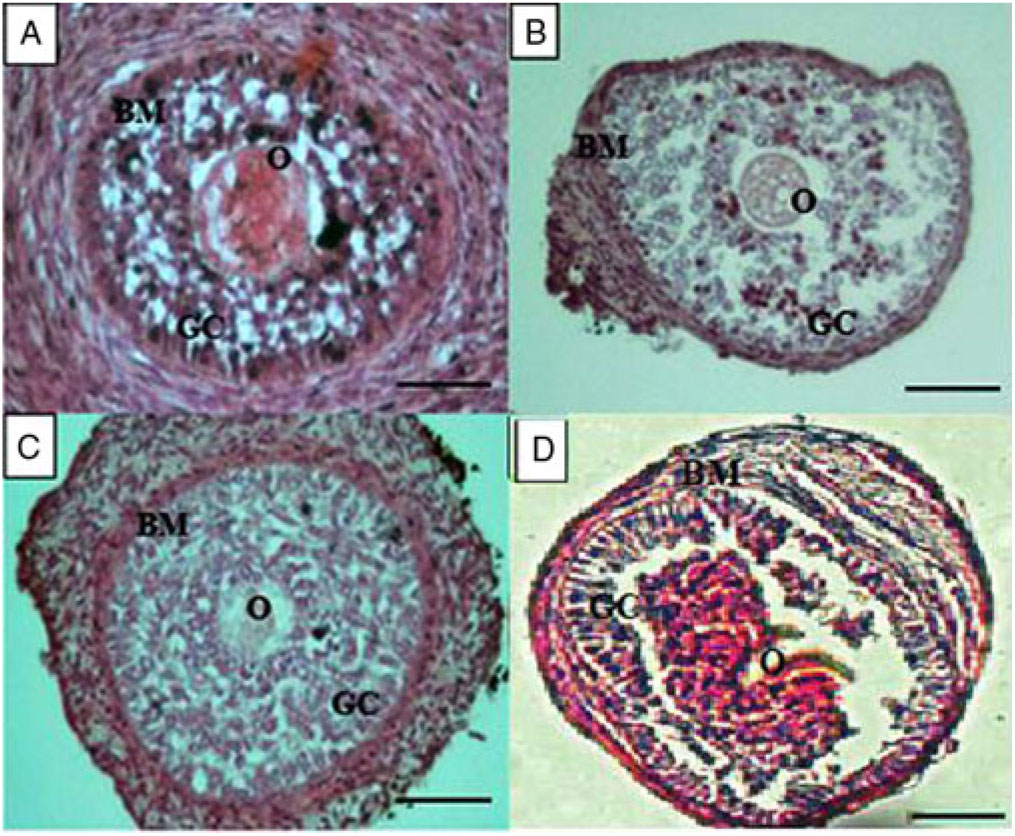
Figure 3. Characteristics of secondary follicles within ovarian tissue (A), after being isolated (B), and cultured in vitro for 12 days in the presence of 5% KSR (C). A degenerated secondary follicle after 12 days of culture in vitro is shown in (D). Basal membrane (BM), oocyte (O) and granulosa cells (GC). Scale bars represent 50 µm.
Discussion
This study shows that supplementation of culture medium with 5% or 10% KSR increases the survival of bovine secondary follicles when compared with follicles cultured in the presence of FBS or BSA after 12 days of culture in vitro. Previously, Fujihara et al. (Reference Fujihara, Comizzoli, Wildt and Songsasen2012) reported that feline preantral follicles cultured in medium supplemented with 10% KSR had higher viability than those cultured in medium with added FBS. In mice, oocyte–granulosa cell complexes from preantral follicles cultured in medium with either FBS or KSR achieved fertilization in vitro and post-implantation development (Motohashi et al., Reference Motohashi, Taniguchi, Sakamoto, Sankai and Kada2017). Park et al. (Reference Park, Gong, Kim, Kim, Choi, Ahn and Lim2013) also showed that replacement of FBS by KSR did not reduce mice preantral follicle growth or oocyte maturation in vitro. Comparing the effects of BSA and FBS during culture of bovine preantral follicles, Bernuci et al. (Reference Bernuci, Rosa-e-Silva, Batista, Garcia, Campos and Sá2013) reported a greater percentage of viable follicles, antrum formation, and of retrieved healthy cumulus–oocyte complexes in follicles cultured with BSA. Supplementation of culture medium with 5% KSR also improved the rates of porcine oocyte maturation and blastocyst formation after in vitro fertilization (Jin et al., Reference Jin, Lee, Setyawan, Taweechaipaisankul, Kim, Han, Ahn and Lee2018). In other studies, the supplementation of culture medium with 5% KSR enhanced the in vitro viability of porcine blastocysts (Sakurai et al., Reference Sakurai, Suzuki and Yoshioka2015). KSR has a well defined and standardized composition and can be widely used as a direct substitute of FBS or BSA in some existing protocols, as KSR presents a mixture of small organic molecules (amino acids, vitamins and antioxidants), trace elements and three proteins, namely insulin, transferrin and lipid-rich albumin in its composition, which can help to maintain follicular viability (Garcia-Gonzalo and Belmonte, Reference Garcia-Gonzalo and Belmonte2008; Price et al., Reference Price, Goldsborough and Tilkins1998).
Differently from the results obtained with the presence of 5% KSR, the supplementation of culture medium with BSA or FBS did not improve secondary follicles viability in vitro. Consistent with the present results, Hulshof et al. (Reference Hulshof, Figueiredo, Beckers, Bevers, van der Donk and van den Hurk1995) found that the FBS did not influence follicular viability, but decreased oocyte development and caused loss of basement membrane integrity in cultured follicles (Thomas et al., Reference Thomas, Leask, Srsen, Riley, Spears and Telfer2001). The addition of 10% FBS to medium used for cryopreservation of zebrafish ovarian follicles did not allow the achievement of successful cryopreservation indicating a compromised metabolic status (Zampolla et al., Reference Zampolla, Rawson and Zhang2012). Although FBS is commonly used in the in vitro production of bovine embryos (Del Collado et al., Reference Del Collado, Saraiva, Lopes, Cruz, Gaspar, Oliveira, Perecin and Garcia2014, Reference Del Collado, Saraiva, Lopes, Gaspar, Padilha, Costa, Rossi, Vantini and Garcia2015), the presence of FBS in culture medium may cause a divergence in results during in vitro production of embryos (Brunner et al., Reference Brunner, Frank, Appl, Schoffl, Pfaller and Gstraunthaler2010). During in vitro oocyte maturation, the addition of BSA failed to support cumulus expansion for bovine or hamster cumulus–oocyte complexes and did not support cumulus expansion or completion of meiosis I in bovine COCs (Leibfried-Rutledge et al., Reference Leibfried-Rutledge, Critser and First1986).
This study shows that follicles cultured with 5% KSR have oocyte, granulosa cells and basement membrane histologically well preserved. Cadoret et al. (Reference Cadoret, Frapsauce, Jarrier, Maillard, Bonnet, Locatelli, Royère, Monniaux, Guérif and Monget2017) reported the importance of preservation of follicular structure after culture in vitro and that the maintenance of integrity of basement membrane is essential to increase the follicle diameter, as well as the number of follicular cells. Moreover, they also noticed that some of the cultured follicles did not survive until the end of the culture period, mainly due to rupture of the basement membrane and opening of the follicular structure.
In this study, the protein sources tested (KSR, FBS or BSA) did not improve preantral follicle growth and antrum formation was rarely observed after 12 days of culture. Bernuci et al. (Reference Bernuci, Rosa-e-Silva, Batista, Garcia, Campos and Sá2013) also did not detect differences in diameters of preantral follicles cultured in the presence of either BSA or FBS, but they showed that follicles cultured for 21 days with BSA had a higher rate of antrum formation than follicles cultured in the presence of FBS. These data emphasize that antrum formation in vitro depends on longer culture periods, as preantral follicles need around 70 days to grow up to antral stages (Gougeon, Reference Gougeon2010). In addition, the replacement of FBS by KSR did not influence the number of follicles that formed a pseudo-antrum cavity after in vitro culture (Park et al., Reference Park, Gong, Kim, Kim, Choi, Ahn and Lim2013).
In conclusion, after 12 days of in vitro culture, the presence of 5% or 10% KSR increases the survival and maintained the morphology of oocyte, granulosa and theca cells compared with follicles cultured in the presence of FBS or BSA. However, the presence of these supplements in culture medium was not able to increase follicular diameter.
Financial support
J.R.V. Silva is a researcher at the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Brazil (grant number 310821/2015-0).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical standards
The authors declare that all procedures were performed according to national and institutional guides on the care and use of animals.






