Critical CHD – CHD that is duct dependent or requires surgery in the 1st month of life – occurs in about 170/100,000 live births.Reference Barrington 1 Outcomes are less favourable if diagnosis is delayed and if the child presents with cardiovascular collapse.Reference Brown, Ridout and Hoskote 2 Formalised screening for CHD in the United Kingdom already exists as part of the fetal anomaly screening and newborn and infant physical examination programmes; 3 however, although ultrasound is becoming increasingly effective at making antenatal diagnoses of CHD, it still at best misses half of all cases. 4 , Reference Chew, Halliday and Riley 5 Furthermore, clinical assessment of cyanosis can be challenging,Reference O’Donnell, Kamlin and Davis 6 and consequently newborns with critical CHD can be discharged from the hospital without diagnosis.Reference Wren, Reinhardt and Khawaja 7 Pulse oximetry screening for CHD has gained significant traction over the last decade. A 2013 meta-analysis of 13 studies demonstrated a combined sensitivity of 76.5% (95% confidence interval 67.7–83.5%) and a specificity of 99.9% (95% confidence interval 99.7–99.9%), with a false-positive echocardiography rate of 0.14% (95% confidence interval 0.06–0.33%).Reference Thangaratinam, Brown and Zamora 8 Pulse oximetry screening has also been shown to be cost-effectiveReference Ewer, Furmston and Middleton 9 , Reference Knowles and Hunter 10 and acceptable to mothers.Reference Powell, Pattison and Bhoyar 11
Nevertheless, scepticism still remains: there is no unified protocol in the United Kingdom, and uptake among hospitals both nationally and internationally is sporadic.Reference Kang, Tobin and Kelsall 12 , Reference Singh and Ewer 13 Of particular concern to local hospitals is how to meet the need for timely paediatric cardiology assessment after a positive screening test, as such services are usually only available urgently in regional centres. There is also concern from regional centres that meeting the anticipated extra demand for cardiology services may be challenging. These reservations are echoed by the United Kingdom National Screening Committee newborn pulse oximetry screening pilot 14 , 15 – rolled out in England in summer 2015 – which also raises concerns over the potential burden of false-positive results and the lack of consensus on a unified screening protocol.
This study was designed to determine whether it is practical to implement pulse oximetry screening in a hospital where on-site paediatric echocardiography is not available without placing an unacceptable burden on the neonatal unit or on the demand for transfers to regional centres with echocardiography services; and whether the screening test performance demonstrated in previous research studies involving pulse oximetry can be replicated in routine care within a local non-specialist hospital setting. Record linkage with a national CHD database would allow outcomes of all screened newborns in the study who required a cardiovascular procedure to be tracked.
Materials and methods
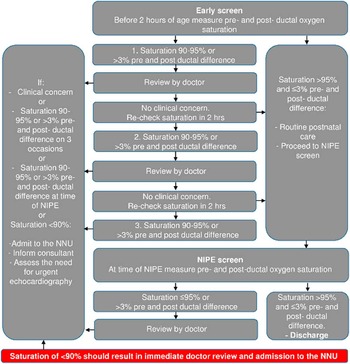
Pulse oximetry screening was introduced in 2011 following two serious untoward incidents where newborns with undiagnosed CHD collapsed on the maternity wards – one with transposition of the great arteries with intact ventricular septum and the other with total anomalous pulmonary venous connection – and did not survive to hospital discharge. In response to these events, a screening protocol was devised (Fig 1).

Figure 1 Pulse oximetry screening protocol. The protocol is applied to any newborn in the maternity wards, and therefore excludes newborns that have been admitted to the neonatal unit (NNU). Newborns with an antenatal diagnosis of critical CHD would be automatically admitted to the NNU after birth. Informing a doctor results in clinical assessment of the child. Following the clinical assessment, the newborn is managed at the discretion of the attending neonatology consultant. NIPE=newborn and infant physical examination.
The pulse oximetry screening test was incorporated into the newborn and infant physical examination, an existing national screening examination performed on all newborns by a doctor or trained midwife before discharge, usually before 24 hours of age. A novel feature of the pulse oximetry screening protocol was the inclusion of an additional “early screen” at 2 hours of age. This early screen was designed to capture CHD as soon as possible to facilitate timely treatment and prevent cardiovascular collapse.
Introducing the screening programme necessitated the purchase of new pulse oximetry equipment – costing ~£1500 – but did not require the recruitment of additional staff. The early screen is performed by the midwife or midwifery assistant attending to the mother, usually while the infant is still in the labour ward. Most staff were already familiar with the use of pulse oximeters, but extra training sessions were also provided as the programme was rolled out. Pulse oximetry training was included in the departmental induction for new medical and midwifery staff.
Following an abnormal early screen, the newborn would be evaluated by a junior doctor and examined, paying attention to the following aspects: temperature, respiratory rate, heart rate, signs of cyanosis or poor perfusion, heart sounds, and femoral pulses. If no other concerns are present, then pulse oximetry would be repeated 2 hours later. An oxygen saturation level of <90% at any time or saturation of 90–95% – or pre-ductal and post-ductal difference of >3% – on three occasions would trigger automatic admission to the neonatal unit, regardless of the clinical condition of the newborn.
The pre-discharge pulse oximetry screen performed at the same time as the newborn and infant physical examination results in admission to the neonatal unit if oxygen saturation level is ⩽95%, or if the pre-ductal and post-ductal difference is >3%. This pulse oximetry screen is performed in the postnatal ward by a midwifery assistant, while the junior doctor completes the newborn and infant physical examination, and therefore adds minimal extra time to the examination process. The hospital also has a midwifery-led birth centre for lower-risk mothers. Newborns in this unit would have the newborn and infant physical examination and pulse oximetry screening performed by a trained midwife. Abnormal pulse oximetry findings would result in a doctor review.
In the neonatal unit, the newborn would be assessed initially by a junior doctor and then by a neonatology consultant. The newborn would be investigated and managed as per any admission to the unit. This might include performing a full blood count, assessment of C-reactive protein levels, blood cultures, blood gas, and chest radiograph; starting empirical antibiotic therapy; and instigating respiratory and cardiovascular support as indicated. The decision to consult with the local paediatric cardiology centre is taken at the discretion of the attending consultant based on the clinical picture. Admission to the unit does not necessarily result in an echocardiogram if the clinical picture fits an alternative diagnosis such as pneumonia and the oxygen saturation levels normalise with treatment.
Of the five neonatology consultants on the neonatal unit, two are trained in echocardiography and can perform “unit” echocardiograms if judged clinically appropriate. Although such echocardiograms could guide management, it is convention to have any abnormal findings confirmed with a repeat scan by a paediatric cardiologist.
To determine how many newborns had been admitted to the neonatal unit due to a screen-positive pulse oximetry screening result, the electronic records of 996 newborns admitted to the neonatal unit from 1 September, 2011 to 31 August, 2013 were retrospectively reviewed. Any newborns >35 weeks corrected gestational age who had been admitted to the neonatal unit from the maternity wards on the basis of a screen-positive result alone – that is, had not been brought to medical attention before screening and had no other documented clinical signs – were recorded. Data were collected from the BadgerNet Standardised Electronic Neonatal Database 16 in which all admissions to the neonatal unit are recorded.
To determine whether any newborns with critical CHD had been missed by the screening programme, the National Health Service numbers of all newborns born within the study period (11,233 live births) were linked with the National Congenital Heart Disease Audit database. The National Congenital Heart Disease Audit routinely collects national data on all paediatric cardiac surgery and interventional procedures for every specialist paediatric cardiac centre in the United Kingdom. 17 Data were obtained on the cardiac diagnoses and procedures of all newborns born from 1 September, 2011 to 31 August, 2013 whose National Health Service numbers matched a newborn at the hospital during this period. The definition of critical CHD adopted for the study was CHD resulting in death or requiring surgical intervention or therapeutic catheterisation within the first 28 days of life.
In order to try and capture newborns that might have died of CHD before any surgical or catheter intervention, information was sought from the child death overview panels covering the two London boroughs served by the hospital. The panels were not able to provide patient-identifiable information but were in a position to confirm whether there were any infant deaths during the study period due to undiagnosed CHD.
To assess screening coverage and ensure programme quality, a review of 429 sets of clinical notes over two discrete time periods was performed to determine adherence to the screening protocol. Case notes were selected from well newborns in the postnatal ward. None of the 429 audited cases required admission to the neonatal unit.
Statistical analysis
Statistical analysis was performed using IBM SPSS for Windows version 22 (IBM Corp., Armonk, New York, United States of America) and Medcalc for Windows version 12.5 (Medcalc, Ostend, Belgium). For comparison of proportions, Fisher’s exact test was applied.
Results
Out of 11,233 live births at the hospital during the study period, 973 newborns were admitted to the neonatal unit before pulse oximetry screening could take place, including newborns born under 35 completed weeks of gestation and newborns who appeared clinically symptomatic after birth and required intensive care.
There were 23 admissions to the neonatal unit due to a screen-positive pulse oximetry result (Fig 2; Table 1). This represents 0.2% (95% confidence interval 0.15–0.34%) of the 10,260 newborns in the screening population. Of these 23 newborns, three (13%) (95% confidence interval 4.5–32.1%) were transferred to a regional cardiology centre, and two were found to have critical CHD. The first newborn had a diagnosis of total anomalous pulmonary venous connection, and the second had transposition of the great arteries with coarctation of the aorta. Both received surgical intervention in the first 28 days of life. Neither newborn was reported to have any abnormal findings on examination. The 23 admissions represent 2.3% (95% confidence interval 1.5–3.4%) of all admissions to the neonatal unit over the study period.

Figure 2 Flow chart illustrating cases of critical CHD identified. DORV=double-outlet right ventricle; NNU=neonatal unit; POS=pulse oximetry screening; PS=pulmonary stenosis; TAPVC=total anomalous pulmonary venous connection; TGA=transposition of the great arteries.
Table 1 All newborns admitted to the neonatal unit on the basis of pulse oximetry screening.

ASD=atrial septal defect; CCHD=critical CHD; CDH=congenital diaphragmatic hernia; CoA=coarctation of the aorta; NIPE=newborn and infant physical examination; PDA=patent arterial duct; POS=pulse oximetry screening: within 2 hours (early) or at the time of the NIPE; T21=trisomy 21; TAPVC=total anomalous pulmonary venous connection; TGA=transposition of the great arteries; TTN=transient tachypnoea of the newborn
The two cases of critical CHD are highlighted in blue. A “Unit” echocardiogram is one performed by a consultant neonatologist on the neonatal unit. An “External” echocardiogram is performed by a paediatric cardiologist in a tertiary referral centre. In some cases, the pulse oximetry value was not recorded by the operator, and was simply documented as “low”
In addition, 16 of the 21 newborns (76.2%, 95% confidence interval 54.9–89.4%) admitted due to a screen-positive test result but who did not have critical CHD received other clinically important diagnoses such as congenital diaphragmatic hernia.
Of the 16 newborns who received an alternative (non-critical CHD) diagnosis, 12 were detected on the early screen (75%, 95% confidence interval 50.5–89.9%). All five admissions with normal transitional circulation – cases 3, 13, 14, 16, and 22 – were admitted as a result of the early screen, meaning that overall 17 of 23 admissions (73.9%, 95% confidence interval 53.5–87.5%) resulted from the early screen. The five cases of normal transitional circulation were all investigated with blood tests and were treated with antibiotics for at least 48 hours.
In total, two cases of trisomy 21 were admitted following pulse oximetry screening. The first (case 5) did not have an antenatal diagnosis and was only noted to have clinical features of trisomy 21 while in the neonatal unit following admission due to pulse oximetry screening. The second (case 17) was diagnosed antenatally but followed the pulse oximetry screening pathway, was admitted following screening at the time of the newborn and infant physical examination, and had an expedited echocardiogram, showing a patent arterial duct with otherwise normal anatomy. The low oxygen saturations in both newborns normalised with time and were attributed to retention of higher than normal pulmonary vascular resistance.Reference Chi 18 Case 5 went on to have an echocardiogram as an outpatient.
Case 15 was the only admission to the neonatal unit where the pulse oximetry values fell in the 90–95% range. This newborn was admitted immediately following doctor review without repeat pulse oximetry.
After the introduction of pulse oximetry screening in September, 2011, 329 newborn case notes were audited. In the 1st month of the programme, 56.5% of newborns did not receive an early pulse oximetry screen and 11.6% of newborns did not receive pulse oximetry screening at the time of the newborn and infant physical examination. Overall, 93.3% of newborns were screened with pulse oximetry at least once before discharge. To enhance screening coverage, quality improvement measures were introduced, including ward checklists, improved training, and an allocated section in the clinical notes for recording the pulse oximetry screening results. A re-audit of 100 newborn case notes, performed between October and December, 2013, showed a marked improvement, with only 3% of newborns missing the early screen and 2% of newborns missing the screen at the time of the newborn and infant physical examination. Overall, 100% of newborns were screened with pulse oximetry at least once before discharge.
Audit data show that of those newborns that received an early screen, 9.1% required second or third measurements before the oxygen saturation levels normalised. The median time for performance of the early screen was 80.5 minutes, and the median time for the screen at the newborn and infant physical examination was 24 hours.
The two cases of critical CHD, which were detected relatively soon after the introduction of the screening programme, did not receive early pulse oximetry screening. This was also true of case 2. The three other cases admitted following abnormal pulse oximetry results at the time of the newborn and infant physical examination (cases 15, 17, and 18) had normal pulse oximetry results reported when the early screen was performed.
The two newborns with critical CHD identified by pulse oximetry screening represented half of all the newborns born at the hospital with critical CHD during the study period (Table 2). The two other cases were admitted to the neonatal unit before pulse oximetry screening could take place, and thus are not included in test accuracy calculations; however, these cases of critical CHD were detected by other screening modalities.
Table 2 All newborns diagnosed with critical CHD born during the study period.

AS=aortic stenosis; ASD=atrial septal defect; CoA=coarctation of the aorta; DORV=double-outlet right ventricle; NIPE=newborn and infant physical examination; NNU=neonatal unit; PA=pulmonary artery; PDA=patent arterial duct; PFO=patent oval foramen; POS=pulse oximetry screening; PS=pulmonary stenosis; TAPVC=total anomalous pulmonary venous connection; TGA=transposition of the great arteries; VSD=ventricular septal defect
Data from the National Congenital Heart Disease Audit and from the local child death overview panels confirmed that no newborns with normal screening results, using antenatal ultrasound, newborn and infant physical examination, and pulse oximetry screening, went on to have surgical or catheter intervention in the first 28 days of life or died of undiagnosed CHD.
Sensitivity of pulse oximetry screening in this context was 100% (95% confidence interval 15.8–100%), and the specificity was 99.8% (95% confidence interval 99.7–99.9%) (Tables 3 and 4). Disease prevalence, including the two cases diagnosed antenatally, was 0.04% (95% confidence interval 0.02–0.1%). Severe cases of critical CHD diagnosed antenatally are often transferred to a regional hospital for delivery. During the study period, there were five known cases of complex CHD transferred antenatally to other centres. This would account for why the prevalence of critical CHD in this study was lower than expected. Antenatally diagnosed cases considered to have cardiac anatomy not requiring immediate intervention do not require antenatal transfer.
Table 3 Cross tabulation of pulse oximetry screening results.

NNU=neonatal unit
Table 4 Test accuracy data with 95% confidence intervals.

Discussion
There are uncertainties surrounding pulse oximetry screening for CHD, which the current United Kingdom National Screening Committee pilot is aiming to address. In particular, concerns exist over the extra demands pulse oximetry screening might place on the National Health Service resources. This includes the potential burden of false positives and the demand on paediatric echocardiography services. Furthermore, there is no consensus on the optimum screening protocol or how cases of CHD missed by pulse oximetry screening should be tracked.
This study serves to address some of these uncertainties. At our hospital, pulse oximetry screening was introduced as part of the newborn and infant physical examination, and consequently the workload of performing the screening itself was minimised. The number of external echocardiograms required over the 2-year period was manageable, as were the number of false-positive admissions to the neonatal unit, the majority of which had important alternative diagnoses.
This was a relatively small, retrospective, observational study with only two cases of critical CHD detected by pulse oximetry screening, and consequently the confidence intervals for the sensitivity value of 100% are wide. Nevertheless, the test performance in the study replicates the success demonstrated in larger prospective trials.
The study allowed for detailed scrutiny of medical records to determine with a high degree of accuracy all asymptomatic screen-positive newborns admitted to the neonatal unit over the 2-year study period. Crucially, if any newborn admitted to the unit was initially brought to medical attention due to concerns about respiratory distress, poor feeding, or any other clinical sign or symptom, and subsequently had pulse oximetry screening performed, they were excluded from the study. In total, there were 23 asymptomatic newborns admitted following screening, 21 of whom did not have critical CHD. The specificity level of 99.8% and false-positive rate of 0.2% are reliable indicators of how many extra admissions, or the additional workload, a neonatal unit that introduces pulse oximetry screening can expect. The false-positive rate is consistent with the false-positive rate of 0.14% in the meta-analysis by Thangaratinam et al.
The low number of false-positive admissions from a screening population of >10,000 newborns is encouraging and may be due in part to the degree of tolerance that is built into the screening protocol. If a newborn has oxygen saturations of 90–95%, then, provided that a medical assessment has taken place, a re-assessment of the oxygen saturations after 2 hours takes place with the expectation that in some cases the saturations will normalise as the fetal circulation transitions. None of the newborns collapsed while awaiting re-assessment.
Of the 23 admissions, only three of the newborns were referred urgently to a paediatric cardiology centre for formal echocardiography, and two of them had critical CHD. This reflects what can pragmatically be achieved at a local hospital where echocardiography is not available. In previous large studies, all newborns with a screen-positive result had an echocardiogram. In our practice, if an alternative diagnosis presented itself and/or the oxygen saturations normalised with time or treatment, an echocardiogram was not performed urgently. This means that the outcome of a screen-positive result is dependent on the clinical decision making of the attending consultant, and the management pathway following a screen-positive result is not standardised; however, such an approach in the local hospital setting allows screening to be instituted without excessive pressure on staff or resources. If a newborn has pneumonia, and clinical suspicion of critical CHD is low, then urgent referral and transfer to a cardiology centre is not practical or desirable.
In some cases, an echocardiogram was performed in the neonatal unit by the attending consultant neonatologist. In our practice, abnormal findings still require further assessment by a paediatric cardiologist, but clearly having the extra information an echocardiogram provides significantly aids decision making; however, for most admissions, a “unit” echocardiogram was not performed and it was still possible to rule out critical CHD based on other clinical criteria.
Moreover, 16 of the 21 admissions that did not have critical CHD had other clinically significant pathology and were not noted to be symptomatic at the time of pulse oximetry. It is not the purpose of the screening programme to detect illness that is not CHD; nevertheless, the conditions detected in this study are not trivial, including cases of pneumonia and one case of congenital diaphragmatic hernia. These cases may well have come to medical attention eventually, but earlier identification and treatment is potentially beneficial by preventing clinical deterioration.
No newborns with critical CHD were missed by our screening programme. The sources of routine data used to identify false-negative screening results in the study are well established for reporting, are of high quality, and have good coverage. The National Congenital Heart Disease Audit data are robust for newborns who require surgical or catheter intervention in the first 28 days of life; however, it does not include newborns that died with CHD before an intervention could take place or those only diagnosed with CHD post-mortem. Attempts were not made to identify newborns whose procedure was after 28 days, as they were considered not to have “critical” forms of CHD. Local data from the child death overview panels for the two London boroughs served by the hospital show that there were no newborns who died with CHD after a negative screening test. It cannot be discounted that a newborn died with CHD at a hospital outside of the two boroughs. The record linkage technique used in the study could be used to monitor outcomes should pulse oximetry screening be expanded nationally in the United Kingdom.
Retrospective analyses of pulse oximetry screening programmes have been published by other groups. Prudhoe et alReference Prudhoe, Abu-Harb and Richmond 19 in Sunderland, United Kingdom, assessed the outcome of routine pulse oximetry in detecting CHD by gathering data from a local database of cardiac anomalies. From a screening population of 29,925, they found five cases of critical cardiac disease detected by pulse oximetry screening; however, the burden of false-positive admissions was not quantified, and the difficulties in obtaining timely echocardiograms were not discussed. Our experience provides a more comprehensive overview of how pulse oximetry screening might impact upon the workings of a local neonatal unit.
Singh et alReference Singh, Rasiah and Ewer 20 performed a retrospective review of their pulse oximetry screening programme in Birmingham, United Kingdom, over a 40-month period. They found that 5.9% (208/3552) of all admissions resulted from pulse oximetry screening, compared with 2.3% (23/996) in our study. This could be explained by the differences in the screening protocol – in our study, an abnormal result in the context of a well newborn was allowed two further checks before being admitted, whereas the Birmingham group allowed one. In all, 29% (61/208) of their screen-positive newborns had an echocardiogram, compared with 13% (3/23) in our study. This perhaps represents a difference in the threshold for performing echocardiography influenced by the relative availability of the test. The Birmingham group performed their analysis at a regional neonatal unit with potentially easier access to echocardiography. Our lower threshold for performing an echocardiogram could also explain why 100% of the echocardiograms performed detected CHD (two cases of critical CHD), compared with 28% in the Birmingham study.
We in effect performed two separate screenings – a “hypoxia” screen at 2 hours of age and a congenital CHD screen with the newborn infant physical examination. The early screen brings with it the risk of picking up and admitting newborns with normal transitional circulation, and indeed the five newborns admitted with transitional circulation were all detected following the early screen and they received potentially unnecessary treatment. Despite these drawbacks, we have not changed our hospital protocol, and we consider the early screen a vital part of the screening protocol. It is beneficial that cases of complex CHD should be picked up as early as possible – the two newborns who died after collapsing on the maternity ward at our hospital would not have been helped by receiving pulse oximetry screening at the time of the newborn and infant physical examination. In addition, other important pathology can be detected earlier, and performing testing at least twice might increase the chances of picking up CHD that can be missed by pulse oximetry screening, such as coarctation of the aorta.
Implementing our screening programme required a small financial outlay for pulse oximetry equipment but did not require extra staff recruitment. Performing the screening test at the same time as the newborn and infant physical examination minimised the extra time needed to perform the test. Our audit data demonstrate that ensuring good screening coverage at the start of the programme can be challenging, and efforts should be focussed on staff training and measures such as ward checklists to ensure no newborns are missed. Our protocol entails an additional early screen, allows repeat testing of clinically well newborns, and is not rigid about referring newborns for echocardiography. Such a pulse oximetry screening programme, once embedded in routine practice, can complement established screening methods by contributing additional diagnoses of critical CHD, as well as other important causes of hypoxia, without the burden and expense of excessive false-positive admissions and referrals.
Acknowledgements
The authors thank all the midwives, midwifery assistants, and junior doctors at Northwick Park Hospital for their commitment to the pulse oximetry screening programme. The authors also thank Pam Mellor at Northwick Park Hospital for her help in acquiring maternity data, Dr David Cunningham at the National Institute for Cardiovascular Outcomes Research for his help with interrogating the National Congenital Heart Disease Audit database, and Dr Ruby Schwartz and Dr Arlene Boroda for providing access to local child death overview panel data. Authors’ Contributions: A.J., E.M.-A., and R.N. contributed to the conception and planning of the work. A.J. and C.H. contributed to the acquisition and analysis of data. A.J. drafted the paper. A.J., R.K., C.H., R.N., and E.M.-A. critically revised the paper. A.J., C.H., R.N., E.M.-A., and R.K. approved the final version.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.