Published online by Cambridge University Press: 19 April 2005
The emergence of Lyme borreliosis as a public health burden within the last two decades has stimulated renewed interest in tick-borne infections. This attention towards ticks, coupled with advances in detection technologies, has promoted the recognition of diverse emergent or potentially emerging infections, such as monocytic and granulocytic ehrlichiosis, local variants of spotted fever group rickettsioses, WA-1 babesiosis, or a Lyme disease mimic (Masters' Disease). The distribution of pathogens associated with well-described tick-borne zoonoses such as human babesiosis due to Babesia microti or B. divergens seems wider than previously thought. Bartonellae, previously known to be maintained by fleas, lice or sandflies, have been detected within ticks. Purported ‘new’ agents, mainly identified by sequencing of PCR products and comparison with those sequences present in GenBank, are being increasingly reported from ticks. We briefly review the diversity of these infectious agents, identify aetiological enigmas that remain to be solved, and provide a reminder about ‘old friends’ that should not be forgotten in our pursuit of novelty. We suggest that newly recognised agents or tick/pathogen associations receive careful scrutiny before being declared as potential public health burdens.
The term ‘emerging infection/disease’ is greatly overused today. Although its original use was designed to focus attention on rare or new infectious entities that appeared to be greatly increasing in prevalence, many researchers now designate any previously poorly characterized or recently recognised infection as ‘emerging’, because of the term's sociopolitical and funding implications. At some point, an ‘emerging’ infection should be classified as ‘emergent’. That said, diverse tick-borne infections are emerging or emergent. Modern diagnostic technology, notably polymerase chain reaction and nucleic acid sequencing, has greatly expanded our capacity to identify infectious agents. Human activities continue to change the landscape vastly, altering faunal associations and thereby contact with arthropod vectors, producing circumstances that serve as the basis for the emergence of a vector-borne infection. Few ‘emerging’ tick-borne infections, however, are novel. Many (ehrlichiosis, babesiosis) have long been recognised as veterinary health problems. Some rickettsioses may be due to agents that were once thought to be tick endosymbionts. Others, such as the agents of bartonellosis, may form paratenic or dead-end associations with ticks. Some recently identified agents (deer tick virus, Borrelia lonestari) are ‘in search of an emerging disease’. Emergent epidemiological associations (Masters' Disease) are in search of an agent. Finally, apparently well-characterized tick-borne infections, such as Rocky Mountain spotted fever, tularaemia and tick-borne encephalitis, remain neglected by researchers but retain the potential for resurgence.
The modern approach to identifying and characterizing infectious agents by using nucleic acid amplification and molecular phylogenetic algorithms is very powerful indeed (Relman, 2002). However, two fallacies seem associated with this approach: (1) that data accumulated by older (‘classical’) methods are not as precise and thus not to be trusted; and (2) that if a DNA or RNA sequence is obtained and does not match one that is already present in GenBank or one of the other genetic information databases, then it represents something novel. The corollary of these fallacies is the idea that we really do not know what those species that have been previously described really represent because genetic information is not available. One could, for example, argue that we cannot know for sure that Theobald Smith's Pyrosoma bigeminum (Smith & Kilborne, 1893) is indeed identical to what we see today as Babesia bigemina because his microscope slides no longer exist and we cannot extract DNA from the material for confirmation! To a certain extent, this idea has been formalised by the seemingly nihilistic approach taken by bacteriologists: in 1980, a list of established bacterial taxa was compiled. Names were retained if the reviewing authority thought enough information existed to demonstrate identity. If the name did not appear on the list, the name and therefore the entity was expunged from our knowledge base. The zoologists, whose International Code of Zoological Nomenclature regulates the taxonomy of parasitic protozoa (and helminths) and their animal hosts, continue to (and rightly so) insist that the burden of proof for novelty rests on an exhaustive search for prior descriptions. Viral taxa are evaluated individually as they are identified by appropriate subcommittees within the International Committee on the Taxonomy of Viruses (ICTV) and, more relevant to the field of vector-borne infections, by the American Committee on Arboviruses (ACAV), which maintains a list of registered viral names. Viral taxa that have not been evaluated may be included in the database of registered names but designated as such (in the International Catalogue of Arboviruses), and ‘new’ agents may be compared to such entities before a decision is rendered. Although bacteriologists are tending to weight 16S rDNA sequence as the main criterion of distinction, the zoologists and virologists tend to take a polyphasic approach, examining physiological and ecological factors as well as genetic. The issue of ‘new’ agent as opposed to a previously recognised agent that has now been molecularly characterized takes on much more than an academic historic interest. Much work on the life history or epidemiologic significance may have already been published. Accordingly, every effort needs to be taken to try to match a newly recognised entity with one that may have been identified in the past.
The ehrlichioses, rickettsioses and babesiosis represent the full spectrum of ‘emerging’ tick-borne infections – old, new and rediscovered. With all three, molecular phylogenetic analysis has greatly expanded our understanding of the diversity of the possible aetiologic agents. In the following paragraphs, we attempt wherever possible to relate new findings to historical information and identify research needs. We focus on zoonotic infections, although the principles that serve as the basis for this review certainly apply to emerging tick-borne infections of veterinary importance.
Lyme disease stimulated a renaissance in tick-borne pathogen research in the United States and elsewhere in the world during the 1980s and 1990s. The human ehrlichioses were recognised and emerged as a public health burden during that time in the US. Three clinically similar, acute-onset febrile illnesses comprising headache, myalgia, rigors and malaise are now known to be caused by tick-transmitted monocytotropic (Ehrlichia chaffeensis) and granulocytotropic (Anaplasma phagocytophilum and E. ewingii) rickettsiae. Three epidemiological patterns are apparent in human ehrlichiosis in the US. Human monocytic ehrlichiosis (HME) is due to exposure to Lone Star ticks (Amblyomma americanum) and infection by E. chaffeensis. Human granulocytic ehrlichiosis (HGE) in the northeastern and northern midwestern US is associated with exposure to deer ticks (Ixodes dammini) and A. phagocytophilum or to western blacklegged ticks (I. pacificus) and A. phagocytophilum. A third epidemiological pattern comprises exposure to either dog ticks (Dermacentor variabilis) or Lone Star ticks (A. americanum) and infection by E. ewingii, previously described as the agent of canine granulocytic ehrlichiosis. A fourth pattern may emerge in large areas of the world, due to exposure to brown dog ticks (Rhipicephalus sanguineus) and infection by E. canis; a chronic case of ehrlichiosis due to E. canis was described for a Venezuelan patient (Perez, Rikihisa & Wen, 1996).
Human monocytic ehrlichiosis due to E. chaffeensis was probably first described as Bullis Fever (Anigstein & Anigstein, 1975), a well-characterized clinical entity of presumed rickettsial aetiology that disappeared from the Merck Manual and other medical textbooks by the 1950s. 750 cases of HME, with 8 deaths, were reported by state health departments between 1986 and 1997 (McQuiston et al. 1999). Prospective studies of HME incidence in Army reservists active in Oklahoma and Georgia suggest that it is as common in these States as Rocky Mountain Spotted Fever, roughly 5 cases per 100000 (Fishbein et al. 1989; Eng et al. 1990). The asymptomatic to symptomatic case ratio has not been rigorously described but is likely to be large. Deer serve as reservoirs for E. chaffeensis (Dawson et al. 1994) and the vector is the Lone Star tick: incrimination of the vector and reservoir of the agent of HME has been based upon laboratory transmission experiments (Ewing et al. 1995) complementing field observations of infection in ticks and deer (Lockhart et al. 1995, 1997a,b). Deer serve as hosts for all three active stages of the Lone Star tick (larvae, nymphs, and adults) although the immatures (larvae, nymphs) may also feed on larger birds, rabbits or raccoons. Prevalence of infection in host-seeking ticks appears to be low, of the order of 1% for either nymphal or adult ticks (Anderson et al. 1993; Murphy et al. 1998). All three stages aggressively attack humans; only nymphs and adults may transmit infection. Larvae – known in many places as ‘seed ticks’ – may cause numerous (dozens or hundreds of bites from contact with a larval cluster) granulomatous, pruritic lesions.
Human serological reactivity to E. chaffeensis has been reported from numerous countries, including Argentina, Mexico, Belgium, Italy, the Netherlands, Portugal, Israel, Burkina Faso, Mozambique, Korea and Thailand (Morais et al. 1991; Pierard et al. 1995; Brouqui et al. 1997; Heppner et al. 1997; Nuti et al. 1998; Gongora-Biachi et al. 1999; Keysary et al. 1999; Ripoll et al. 1999; Groen et al. 2002; Heo et al. 2002). Such reports may reflect exposure to a closely related Ehrlichia such as E. muris (Kawahara et al. 1999), the ‘Anan agent’ (Shibata et al. 2000) or the ‘Schotti variant’ (Schouls et al. 1999; probably the same as ‘candidatus Ehrlichia walkerii’, Brouqui et al. 2003) inasmuch as the only known vector, A. americanum, is not present in these countries. On the other hand, a recent report of the detection of E. chaffeensis DNA in Chinese ticks (Cao et al. 2000a) might suggest that HME due to this agent is not restricted to the US or to transmission by A. americanum. Further work seems to be required to determine the geographical distribution of E. chaffeensis and alternative vectors, and on the possible diversity of agents in the Ehrlichia/Cowdria genogroup.
The agent of human granulocytic ehrlichiosis (HGE), A. phagocytophilum, was first studied in the 1930s as the cause of tick-borne fever of sheep and cattle, and a large literature pertains to it as an animal health problem (Woldehiwet & Scott, 1993). The nomenclature of this agent requires some comment. Those researchers who studied tick-borne fever failed to provide a binomial designation for the agent until 1949 when Foggie first applied the name Rickettsia phagocytophila in an abstracted report (indicated in Foggie, 1951). Subsequently, he noted its morphological similarity to an infection of small rodents described by Tyzzer, Cytoecetes microti (Tyzzer, 1938). C. microti was clearly the first description of granulocytic ehrlichiae in North America (Telford et al. 1996). Cytoecetes phagocytophila (Foggie, 1962) was used by most workers for many years despite the assignment of this taxon to Ehrlichia. C. microti and many other validly published ehrlichial taxa were considered as incertae sedis in the 7th edition of Bergey's Manual whereas E. phagocytophila was formally recognised. European workers, however, ignored the official nomenclature, retaining C. phagocytophila in their numerous publications (e.g. Brodie, Holmes & Urquhart, et al. 1988; Juste et al. 1989; Woldehiwet, Carter & Dare, 1991).
More recently, the rickettsiae have undergone a taxonomic revision based upon 16S rDNA and groEL gene sequencing, with ehrlichiae being reclassified within the Anaplasmataceae, distinct from the Rickettsiaceae (Dumler et al. 2001). The agents of tick-borne fever, bovine ehrlichiosis (formerly E. bovis) and canine cyclic thrombocytopenia (formerly E. platys) are now placed within the genus Anaplasma. Although the new nomenclature greatly confuses the literature and seems to be at odds with aspects of the agents' life cycles (e.g. cell tropisms and pathology), the Bacteriological Code (Sneath, 1992) mandates the use of a taxonomic revision that is validly published in the official organ of the International Society of Systematic Bacteriology. Principle 1 of the Code, however, states that nomenclature should aim to provide stability of names, avoid use of names that foster confusion or error, and avoid useless creation of names. The phylogenetic analysis based on 16S rDNA and groEL gene sequences clearly confirms that there are at least 4 major clades of ehrlichia-like agents (Dumler et al. 2001), a conclusion that was generally accepted based on morphology, life cycle and other criteria. But the aims of Principle 1 may have been accomplished had a more conservative approach been followed when identifying the main clades in this analysis (Fig. 1), retaining Anaplasma and Cytoecetes as distinct entities.

Fig. 1. 16S rDNA phylogenetic tree (neighbour-joining algorithm) of ehrlichia-like taxa showing two schemes for making taxonomic decisions. A, as recommended by Dumler et al. subsuming Cytoecetes with Anaplasma. B, conserving Cytoecetes. One could also retain Cowdria by moving the cut-off farther to the right, and placing the former E. bovis into its own clade; Cowdria's tropism for endothelial cells clearly distinguish it from that of the remainder of the Ehrlichia sensu stricto.
An examination of other phylogenetically informative genetic targets (Inokuma et al. 2001), as well as other described entities such as ‘C. ondiri’ (bovine petechial fever, Scott & Woldehiwet, 1993, ‘C. ovina’ (Uilenberg, 1993), ‘C. kamtchoulii’ (Gretillat, Mattei & Marchand, 1981), and ‘Paranaplasma caudatum’ (Ristic, 1968) may stimulate yet another nomenclatural revision in the future as DNA from these agents is collected and analysed. Each additional taxon provides an independent comparison to assess the strengths of the tree topology generated by the existing phylogenetic algorithms (Tenter et al. 2002). Pending such studies, HGE (the infection) should continue to be called ehrlichiosis, not anaplasmosis.
Nearly 500 HGE cases were reported by US state health departments between 1994–1997 with intensive surveillance in Lyme disease-endemic sites (McQuiston et al. 1999). Three deaths were reported. The distribution of HGE closely parallels that of Lyme disease. Cases of HGE have been identified in virtually all of the eastern US. In addition, cases of HGE have been described from northern California, where the western blacklegged tick (Ixodes pacificus) serves as vector (Richter et al. 1996). HGE cases (identified by seroconversion or PCR detection of A. phagocytophilum) have been reported from Belgium, Denmark, Sweden, Slovenia, Spain, and Italy (Petrovec et al. 1997; Lebech et al. 1998; Nuti et al. 1998; Oteo et al. 2000; Bjoersdorff et al. 2002; Guillaume et al. 2002). Residents of Germany, Norway, United Kingdom, Israel, and Korea, among others, are seroreactive (Sumption et al. 1995; Bakken et al. 1996a; Fingerle et al. 1997; Keysary et al. 1999; Heo et al. 2002). Infected ticks have been reported from virtually all of the countries that have reported human exposure or infection. The agent has also been detected in Siberian rodents (Telford et al. 2002) and in ticks from Russia, China, Finland and Korea (Cao et al. 2000b; Alekseev et al. 2001; Kim et al. 2003; Makinen et al. 2003). It seems likely that the potential distribution of HGE matches that of the I. persulcatus complex of ticks.
The ecology of HGE is relatively well understood in the eastern US because the agent tends to share the same white-footed mouse reservoir and deer tick vector as those of Lyme disease and babesiosis. The reader is referred to the accompanying chapter in this Supplement on Lyme borreliosis by Piesman and Gern for a detailed discussion. A variety of other reservoir hosts may locally contribute to the force of transmission, including mice, voles, chipmunks, deer or sheep (Tyzzer, 1938; Foggie, 1951; Telford et al. 1996; Belongia et al. 1997; Walls et al. 1997). Under experimental conditions, reservoir infectivity appears to be transient, lasting about 2 weeks (Levin & Fish, 2000), in sharp contrast to B. burgdorferi and B. microti, with which reservoirs appear to be infective to the vector for the duration of their life. On the other hand, xenodiagnosis of mice trapped in the fall revealed that a third of them were infectious to ticks (Telford et al. 1996). Enigmatically, the long history of tick-borne fever investigations in the UK poorly explored the role of reservoirs other than sheep or cattle (Woldehiwet & Scott, 1993) and only recently has A. phagocytophilum infection been detected in small rodents there (Ogden et al. 1998; Bown et al. 2003). As with HGE in the US, the ecology of tick-borne fever may be found to parallel that of the agent of Lyme borreliosis. Indeed, evidence is mounting that, as with B. burgdorferi sensu lato, A. phagocytophilum may comprise a group of genospecies. Single nucleotide polymorphisms (SNPs) within the 16S rDNA and groEL genes of A. phagocytophilum seem to associate with strains taken from particular hosts (Massung et al. 2002). European strains appear genetically heterogeneous (Stuen et al. 2002). The infection kinetics of the agent of equine granulocytic ehrlichiosis (‘Ehrlichia equi’) within laboratory mice differs from prototypical HGE, as does its appearance in Giemsa stained blood smears (unpublished observations). The public health significance of members of a putative A. phagocytophilum sensu lato may greatly vary.
Infection due to E. ewingii, the agent of canine granulocytic ehrlichiosis, was originally detected in dogs in Oklahoma (Ewing & Philip, 1966), but its distribution probably includes much of the southern and central US. Several cases of human ehrlichiosis due to E. ewingii have been reported from Tennessee, Missouri and Oklahoma, mostly in immunocompromised patients (Buller et al. 1999; Paddock et al. 2001). The Lone Star tick (A. americanum) was incriminated as the vector by experimental transmission between dogs. E. ewingii DNA has been detected in Rhipicephalus sanguineus and Dermacentor variabilis from Oklahoma, but their competence as vectors remains undescribed (Murphy et al. 1998). Deer have recently been demonstrated to support E. ewingii infection and appear to be frequently infected, probably because they serve as important hosts for all stages of Lone Star ticks (Yabsley et al. 2002). Description of the enzootic cycle of E. ewingii remains to be accomplished, and will be particularly complicated by co-transmission of E. chaffeensis and a putative ehrlichia, ‘white-tailed deer agent’ (Little et al. 1997), known only from DNA sequences.
The spectrum of illness in humans for all three ehrlichioses ranges from completely asymptomatic to fatal, but most individuals will experience an acute febrile illness of a week's duration that may spontaneously resolve. Acute HGE infection has been characterized as ‘spotless Rocky Mountain Spotted Fever’. Fever (>37·5 °C), malaise, rigors, myalgia, sweats and headache are almost universally reported (Bakken et al. 1996b). About a third of reports indicate nausea, anorexia, arthralgia and cough. Confusion, prostration, diarrhea, pneumonia and vertigo are less frequently reported. Rash is rarely present. Chronic infections or sequelae have not yet been described, although weakness or fatigue appear to persist for as long as one month (Bakken et al. 1996b). Tick-borne fever of sheep due to A. phagocytophilum is thought to frequently persist for as long as two years if untreated (Foggie, 1951) and is associated with profound immunosuppression (Woldehiwet & Scott, 1993). Human fatalities due to ehrlichiosis appear to be associated with bacterial or fungal secondary infections (Dumler & Bakken, 1995).
Local variants of spotted fever group rickettsioses are increasingly being recognized. Since 1991, 5 ‘new’ infections have been described including Flinders Island spotted fever, Astrakhan fever, African tickbite fever and Oriental spotted fever (Parola & Raoult, 2000). Thus, there are 11 established rickettsioses (Table 1). Infection due to R. mongolotimonae (Raoult, Brouqui & Roux, 1996) will probably join this list, but to date there has been only one reported case. Rickettsiae that have previously been characterized as ‘harmless’ arthropod endosymbionts have been incriminated as important human pathogens, such as R. slovaca and R. helvetica (Raoult et al. 1996, 1997). But, rickettsiae (or other bacteria) detected within arthropods by molecular methods may never be associated with human infection (Roux & Raoult, 1999; Parola & Raoult, 2000; Shypnov et al. 2001, 2003). Interpreting the public health significance of a newly recognised or better characterized Rickettsia spp. detected within a vector arthropod remains difficult in the absence of objective markers for its capacity to induce human pathology. By necessity, incrimination of an aetiologic agent must await its detection within clinical specimens.

Improvement in methods of rickettial isolation (particularly the shell-vial centrifugation method, Vestris et al. 2003) and molecular detection has greatly helped in assigning aetiology in suspected rickettsiosis cases. PCR allows sensitive detection and rapid identification of rickettsiae, perhaps even within a day; previously, cumbersome microimmunofluorescence assays using a panel of species and genotype-specific mAbs were required to identify an isolate, which takes a week or often longer just to propagate. In addition, whereas rickettsial isolation and typing could be performed in only a handful of laboratories with the appropriate biocontainment facilities and experience, PCR may be performed by virtually any laboratory (although there are significant caveats about the reliability of the results depending on experience with the technique, particularly with respect to contamination control). At least two of 5 recently recognised rickettsioses appear to be significant public health burdens, with hundreds of cases having been reported (Astrakhan fever) or potentially being transmitted in areas where reporting is poor (African tick bite fever). Two others (Japanese spotted fever and TIBOLA) may be recognised as more widely distributed given their vector associations.
Astrakhan fever is apparently a new syndrome, not having been reported in the comprehensive volume on rickettsial diseases by Russian workers (Zdrodowski & Golinevich, 1960). It was first recognised in 1970 in patients visiting the Caspian Sea area and who were exposed to Rhipicephalus pumilio ticks. A total of 321 cases was reported during active surveillance from 1983–1989 (Tarasevich et al. 1991). High fevers, headache, myalgias and non-petechial rash are common findings; only a quarter of the patients had an eschar (a characteristic hard plaque covering a dermal ulcer). The illness appears to run a relatively benign course and no fatalities have been reported. The agent is now considered to be a strain of R. conorii (Shypnov et al. 2003) although the original analyses suggested that it was distinct (Ereemeva et al. 1994). The enzootic cycle has not yet been described.
Japanese (Oriental) spotted fever (JSF) also appears to be a new syndrome, although it is possible that it may have been confused with scrub typhus (due to Orientia tsutsugamushi), which was well known by Japanese physicians by the 1920s (Kawamura, 1926). Febrile illnesses with exanthem (a sudden rash) and eschar would have been readily detected. Indeed, JSF was first identified in May–July 1984 in Tokushima prefecture when 3 patients were diagnosed with presumptive scrub typhus. Scrub typhus cases tend to be found during the autumn and winter in Japan, and the summer presentation served to suggest a different aetiology. Serum from these patients failed to react as expected in Weil-Felix tests, showing agglutinins against Proteus OX2, which suggested a spotted fever-like infection. The agent was isolated in 1986, and described as R. japonica (Uchida, 1993). Patients presented with headache, fever, rigors and a rash that became petechial. Virtually all had eschar and about a third recalled a tick bite (Mahara, 1997). From 1984–1995, 144 cases of JSF were reported from southwestern and central Japan, with transmission occuring mainly during the summer; scrub typhus is mainly seen during the fall and winter (Mahara, 1997). Rickettsiae were detected by immunofluorescence and by R. japonica-specific PCR in haemolymph taken from the human-biting ticks Haemaphysalis flava, H. longicornis and Ixodes ovatus, as well as from H. formosensis, H. hystricis and Dermacentor taiwanensis. Vector competence studies have not been performed with these ticks, however, so their relative vectorial capacity remains unknown. The enzootic cycle remains undescribed. H. hystricis and D. taiwanensis occur in large portions of southern China and Indochina; accordingly, evidence of R. japonica infection should be sought wherever these ticks are common.
African tickbite fever (ATBF) was originally described by Pijper in the 1930s from South African patients. Based on guinea pig cross-protection studies, Pijper (1936) suggested the existence of two tick-transmitted rickettsioses there, one now known as Boutonneuse fever due to R. conorii and exposure to R. sanguineus ticks; and a milder febrile illness due to exposure to Amblyomma ticks. The agent was thought to be a variant of R. conorii (Dermacentroxenus rickettsii var. pijperi (Mason & Alexander, 1939). Subsequent work (Gear, 1954) refuted the existence of another rickettsiosis in Southern Africa, and until the 1990s (Kelly et al. 1991), all cases there were attributed to infection by R. conorii and associated with bites of subadult bont ticks (A. hebraeum). In 1992, an isolate made from a Zimbabwean patient was demonstrated to be identical to one previously isolated from Ethiopian Amblyomma spp. (Philip et al. 1966), distinct from R. conorii, and was named R. africae (Kelly et al. 1996). Much is known about the ecology of the bont tick due to its importance as a vector of heartwater and theileriosis (e.g. Norval, 1977a, b). Reproductive hosts are large ungulates such as giraffe, rhinoceros, buffalo and cattle; subadults infest a wide range of hosts including all sizes of mammals, birds and reptiles. It seems likely that R. africae infection is very common inasmuch as A. hebraeum is an important human biter in southern Africa from Zimbabwe southwards. In 1992, 23% of 169 American soldiers who had participated in a 10-day training exercise in Zimbabwe were infected, as demonstrated by seroconversion to antigens of R. africae (Broadhurst et al. 1998). Fever, chills, headache, myalgias, fatigue and lymphadenitis were commonly reported. Nearly all of the infected soldiers had an eschar and a third had more than one eschar, suggesting exposure to multiple bites, probably of subadult ticks. In addition, given the popularity of safaris in southern Africa, ATBF may be a frequent febrile illness of tourists (Brouqui et al. 1997). R. africae has been detected from A. variegatum (Parola et al. 1999), the tropical bont tick, which was introduced from Senegal into the Caribbean island of Guadeloupe in 1828 and spread to much of the West Indies. Thus, ‘African’ tick bite fever might be acquired on a Caribbean vacation. In addition, bites by Hyalomma spp. may transmit R. aeschlimanni (Pretorius & Birtles, 2002) and thus tick-bite fevers in Africa may be due to diverse ticks.
TIBOLA (for tick-borne lymphadenopathy) due to infection by Rickettsia slovaca and associated with the bites of adult Dermacentor marginatus ticks seems likely to become increasingly recognised as a public health burden. Rickettsiae first isolated in 1969 from D. marginatus (Brezina, Rehacek & Majerska, 1969) were recognised as distinct based on microagglutination and complement fixation typing experiments, and the name R. slovaca was applied (Urvolgyi & Brezina, 1976). Although human exposure was suspected given the biting habits of D. marginatus, the role of R. slovaca as the cause of illness was only recently described (Raoult et al. 1997). Due to surveillance for Lyme borreliosis, a series of 86 Hungarian case-patients was described with a distinct syndrome that included a prominent crusting eschar, lymphadenopathy and fever (Lakos & Raoult, 1999). Virtually all cases were bitten by a large tick which attached on the scalp. Two-thirds of the cases were in children younger than 10 years. An eschar, often surrounded by an erythaematous halo, appears to give rise to chronic alopecia at that site. As opposed to Mediterranean spotted fever, a prominent lymphadenopathy (usually occipital or cervical when the bite is on the scalp) accompanies low grade fever, fatigue, headache and myalgias. Of 13 patients from whom skin or lymph node biopsies were analysed by PCR, 10 were confirmed to have been infected by R. slovaca. A similar series of patients was also described from Spain (Oteo & Ibarra, 2002). D. marginatus is a common tick of lowland steppes and grassland. The reproductive hosts include a wide variety of ungulates, carnivores and even hares and hedgehogs; subadults feed on insectivores, rodents, and mustelids (Pomerantsev, 1959). Its geographic distribution is wide, from central Asia, western Siberia, and Crimea into the Balkans, through southern Europe into Spain and Portugal, and including the Mediterranean islands. Previously this tick was considered a main vector of equine piroplasmosis as well as North Asian tick typhus (due to R. sibirica). Given its wide distribution, its generalist feeding habits and propensity to bite humans, D. marginatus-transmitted infections would seem to have a potential for great prevalence.
Flinders Island spotted fever (FISF) due to R. honei poses a zoogeographical enigma. Between 1974–1991, the sole physician on this small island off of the southeastern Australian coast identified 26 cases of a spotted fever-like infection (Stewart, 1991). Patients presented with sudden onset of fever, a maculopapular rash with large individual macules, and arthralgia. Serologic studies confirmed that FISF patients' sera reacted with antigens of the spotted fever group rickettsiae (Graves et al. 1991). Subsequently, isolates were obtained from patients and DNA sequencing of the isolates demonstrated only a distant relationship to R. australis, the agent of Queensland tick typhus, which was expected to be closely related. In fact, the most similar sequences were from TT-118, a Rickettsia sp. recovered from a pool of subadult Ixodes sp. and Rhipicephalus sp. taken from Rattus rattus in Thailand (Robertson & Wisseman, 1973). In 1996, the same agent was detected by PCR in adult Amblyomma cajennense collected from Texas cattle (Billings et al. 1998a). The Flinders Island agent was named Rickettsia honei based on the sequencing of 4 genes as well as serological typing (Stenos et al. 1998). On Flinders Island, the reptile-feeding Aponomma hydrosauri is commonly infected by R. honei and appears to vertically maintain the agent; its lizard hosts may share burrows with mutton birds, a northern migrant (Graves & Stenos, 2003). Apparently, A. hydrosauri will attach to humans and is the vector of FISF (Graves & Stenos, 2003). Although one might attribute the presence of R. honei in Thailand and southeastern Australia to transport of ticks by migratory birds (such as mutton birds) within Australasian flyways, such a mechanism would be unlikely to explain its presence in Texas. Future analyses should explore whether R. honei is a common amblyommine tick ‘endosymbiont’ associated with febrile infections in humans throughout much of the tropics and subtropics.
Human babesiosis once comprised European Babesia divergens infections in splenectomised patients, or northeastern North American B. microti in immune-intact, elderly individuals (Telford & Maguire, 1999). However, isolated cases that were reported in the literature presaged what is increasingly being recognised as a widespread zoonosis due to diverse agents. In 1966, a splenectomised resident of the San Francisco, California area sustained a febrile illness; malaria-like parasites were present in blood smears, many with characteristic babesial ‘maltese-cross’ forms (Scholten et al. 1968). The infecting species was not definitively identified. The authors speculated that because of the frequency of finding Maltese-cross forms in blood smears from B. equi infections, that species may have been the agent, as it was for a similar case involving another splenectomised California resident (Bredt, Weinstein & Cohen, 1981). In 1976, a search for the donor for a transfusion vivax malaria case resulted in identifying an asymptomatic 51 year old Georgia (US) resident with Babesia sp. demonstrable within blood smears (Healy, Walzer & Sulzer, 1976). Serological studies were uninformative and attempts to propagate the parasite by subinoculating hamsters and a rhesus monkey failed. Also in 1976, a serosurvey of Mexican residents heavily exposed to ticks within sites with enzootic canine, equine and bovine Babesia spp. yielded 3 asymptomatic seropositive individuals whose blood gave rise to unidentified parasites when subinoculated into hamsters (Osorno et al. 1976).
The seminal event that clearly demonstrated that human babesiosis may comprise diverse organisms, and that stimulated a renewed interest in infection due to these sporozoans occurred in 1992. An agent designated WA-1, closely related to B. gibsoni, a parasite of dogs, infected a Washington state resident (Quick et al. 1993). A transfusion-related case has since been described (Herwaldt et al. 1997). The index WA-1 case was a 41 year old man who was not immunocompromised. His malaria-like illness was originally thought to be due to B. microti but his serum failed to react with antigens of that agent. The parasite was propagated by subinoculating hamsters which died within 10 days. B. microti rarely kills hamsters, and subsequent sequencing of the 18S rDNA clearly distinguished it from B. microti and B. divergens (Quick et al. 1993). Intensive retrospective and prospective case-finding on the West Coast of the US for WA-1 cases identified archived blood samples from 4 babesiosis cases (one fatality) involving splenectomised California residents which were reanalysed by PCR sequencing (Persing et al. 1995). The causative agents' 18S rDNA differed from that from WA-1 and B. microti. Recent phylogenetic analyses incorporating larger numbers of taxa (Kjemtrup et al. 2000; Fig. 2) suggest that the CA-type babesia appear to be related to newly recognised infections of bighorn sheep (Thomford et al. 1993). Thus, within a decade two distinctive babesial infections were identified from the western US. The vectors and reservoirs of WA-1 and CA-type parasites remain undescribed despite an intensive search. It seems likely that the previously reported Californian babesiosis cases attributed to B. equi were actually CA-1 or WA-1 infections.

Fig. 2. Phylogeny of Babesia spp. based on 18S rDNA (neighbour-joining algorithm), indicating relatedness of known human pathogens (in capital letters).
Although B. divergens, the causative agent of bovine redwater in Europe, is currently considered to be solely endemic in Eurasia (and indeed, is legally excluded by the US Department of Agriculture from US importation in any form), the first autochthonously acquired American case of B. divergens babesiosis terminated fatally in a Missouri resident in 1995. Parasites identified on the blood smear were indistinguishable from B. divergens and the 200 bp piece of 18S rDNA was also identical to that of B. divergens. Although all evidence pointed to the diagnosis of B. divergens, since B. divergens was by definition not in North America, the agent was designated new and named MO-1 (Herwaldt et al. 1996). In 2001, another B. divergens case occurred in a Kentucky resident. This patient was diagnosed by blood smear with typical B. divergens morphology (accole forms, paired pyriforms) and recovered with quinine and clindamycin treatment. Sequencing of the entire 18S rDNA demonstrated its close identity (>99% sequence similarity in the 18S rDNA gene) with the European cattle agent (Beattie, Michelson & Holman, 2002). Recent work on Nantucket Island, Massachusetts has described the maintenance of this same agent (100% identical 18S rDNA sequence) among cottontail rabbits (Goethert & Telford, 2003a). Rabbit sera reacts with B. divergens (Purnell strain) antigen, more so than to the sympatric and closely related B. odocoilei. B. divergens is known to be transmitted transovarially by Ixodes ricinus in Europe (Joyner, Davies & Kendall, 1963; Donnelly & Pierce, 1975), and on Nantucket it is similarly maintained by a closely related tick, the rabbit-infesting Ixodes dentatus. Five years of field observations reveal that B. divergens is endemic on the island (Goethert & Telford, 2003a). However, together with the cases from the midwest, these data suggest that B. divergens is not a recent import (due to incidental transport of ticks by errant migratory birds, for example) and has been in North America for some time. The public health risk remains to be described. To date, only cases of babesiosis due to B. microti have been identified on Nantucket Island, and the enzootic cycle in the midwest has not been investigated.
The dogma that babesiosis in splenectomised Europeans has been due to B. divergens infection has recently been challenged (Herwaldt et al. 2003). Two cases in splenectomised individuals residing in Italy were attributed to a Babesia sp. that is morphologically similar to B. divergens but upon sequencing of the entire 18S rDNA were demonstrated to be most closely related to B. odocoilei which infects American cervids. It is likely that this parasite, dubbed ‘EU-1’ with implied novelty, is actually B. capreoli (Enigk & Friedhoff, 1962) based upon morphologic and expected host attributes (both B. capreoli and B. odocoilei infect deer). Although the suggestion is made that we do not really know which species was the infecting agent for the majority of the 22 reported clinical cases of babesiosis in Europe (Herwaldt et al. 2003), many of these had been confirmed by subinoculation of cattle or jirds or review by experienced microscopists. The ‘new’ agents of babesiosis (MO-1, EU-1) serve as examples of the molecular fallacies alluded to at the beginning of this review. Although much new information has been derived from DNA sequencing, agents that appear novel may represent those that were previously described. On the other hand, our recent work on a rabbit piroplasm that appears to have zoonotic potential (Goethert & Telford, 2003a) may represent too conservative a view: available data suggest identity with B. divergens, and it will be referred to that taxon until further work demonstrates otherwise regardless of the unusual lagomorph host and presence in the New World. Cattle inoculation experiments or sequencing of additional genes may eventually demonstrate that it is not B. divergens. Clearly, the field of emerging tick-borne infections will benefit from a balance of the old with the new where careful life cycle and morphological studies complement DNA data.
Even human babesiosis due to Babesia microti appears to be more complicated than previously appreciated. Hundreds of cases have been reported from immunocompetent persons from coastal New England and upper Midwestern US sites, with a 5% case fatality rate (Telford & Maguire, 1999). Although B. microti is considered to have a Holarctic distribution, few cases outside the US have been reported (van Peenen et al. 1977; Shih et al. 1997; Tsuji et al. 2001). The lack of cases in Europe is particularly enigmatic because competent reservoir rodents have frequently been found to be infected and are sympatric with a competent vector, Ixodes ricinus (Franca, 1910; Krampitz & Baumler, 1978; Healing, 1981; Telford & Spielman, 1993; Bajer et al. 2001; Gray et al. 2002). Serological evidence of exposure to B. microti has been detected in tick-exposed people (Foppa et al. 2002; Hunfeld et al. 2002) making the absence of reports of clinical illness even more puzzling. B. microti has always been assumed to be a homogeneous species throughout its distribution, but historically most studies relied solely on parasite morphology for identification.
A recent genetic analysis of 18S rDNA and β-tubulin genes of B. microti (identified by morphology) sampled throughout the United States and Eurasia demonstrates that this organism is a genetically diverse species-complex (Fig. 2). At least three distinct groups of organisms have been identified: two in rodents and one in medium-sized mammals (Goethert & Telford, 2003b). The samples from areas where human babesiosis is regularly reported (tick, sigmodontine rodent or human isolates from Massachusetts, Connecticut and Wisconsin), as well as some areas where it is rare or absent, such as Maine, Russia and Switzerland (microtine rodent or tick samples) comprised a B. microti sensu stricto group. A second distinct group was found in microtine rodents from such divergent areas as Alaska, Maine and Montana, all areas in which no human cases have been described. A third group of B. microti-like parasites were all from carnivores; this group includes the recently described ‘Theileria annae’ that causes fulminating disease in Spanish dogs (Zahler et al. 2000; Camacho et al. 2001). This parasite is only distantly related to true Theileria spp. such as T. parva. No evidence was presented (Zahler et al. 2000) for pre-erythrocytic, lymphocyte-infecting stages and thus describing this agent as a Theileria seems premature. Parasites from a fox and raccoon from Massachusetts were also included within this group, and we suspect that an American canine death from ‘B. gibsoni’ (Anderson et al. 1979) was also due to this group of B. microti-like parasites.
Given the genetic diversity of B. microti that is apparent from analysis of a limited number of strains, it may be that particular genotypes are less likely to cause human disease. Indeed, evidence for such a hypothesis was recently demonstrated by the first report of B. microti babesiosis in Japan: the parasites recovered from the transfused patient were typical of those found only in rodents collected from Awaji Island, whereas a different 18S rDNA genotype was commonly found virtually everywhere else in Japan (Tsuji et al. 2001). Interestingly, B. microti babesiosis first emerged on Nantucket Island although this protozoan was endemic elsewhere in coastal New England. Perhaps island populations select for intensely transmissible strains.
‘Seek and ye shall find’ (Matthew 7) was once invoked in an early paper on the epidemiology of human babesiosis (Hoare, 1980) given the wide distribution and diversity of Babesia spp. Their role as a confounder for chloroquine resistant malaria (babesias are not susceptible to chloroquine but are treatable with quinine) in tropical countries was suggested inasmuch as morphological discrimination from the plasmodia within blood smears may be difficult (Young & Morzaria, 1986). HIV infection, now hyperendemic in many tropical areas, may render humans more susceptible to infection by, and disease due to, diverse Babesia spp., as do other forms of immune suppression. Piroplasms should routinely be sought as an aetiology for febrile illnesses wherever humans are intensely exposed to ticks.
Perhaps the greatest epidemiological puzzle in tick-borne disease research in the USA is the presence of a Lyme disease mimic in the southern-central states. Another epidemiological entity, which urgently needs intensive study, is the potential for tick-borne rickettsiae to cause cardiac pathology.
The issue of Lyme disease in the southern and central US has been greatly controversial. Erythema migrans rashes were noted on patients in these areas since the late 1980s (many reported by Dr Edwin Masters in Cape Girardeau, MO) (Weder et al. 1989; Masters & Donnell, 1995; Masters et al. 1998; Roberts et al. 1999), and indeed Borrelia burgdorferi was detected in Ixodes ticks in North Carolina and Alabama as early as 1983 (Pegram et al. 1983). Because of the CDC surveillance case definition (at the time) requiring the presence of a known vector, viz., I. dammini (deer ticks), great skepticism accompanied such reports because deer ticks only infested sites in the northern US. I. dammini is now considered by most workers to be conspecific with I. scapularis, the blacklegged tick (Oliver et al. 1993). However, blacklegged ticks in southern US sites rarely feed on humans as nymphs, representing a major epidemiological difference that accounts for low risk even in sites where Lyme disease spirochetes are enzootic (Telford, 1998). In fact, the central and southern US EM rashes are associated with A. americanum, the Lone Star tick, bites (Masters et al. 1998).
Cases of Southern EM rashes are referred to as Masters' Disease or STARI (southern tick associated rash illness) to ensure its distinction from bona fide Lyme borreliosis. Other than the rash, the illness is nonspecific, relatively mild and is said to resolve spontaneously without sequelae; on the other hand, stoic Missouri farmers who rarely go to physicians for any reason will seek treatment for this illness (Dr Edwin Masters, personal communication). Residents of an endemic site in Maryland reported myalgias, fever and arthralgia (Armstrong et al. 2001). Of 98 residents who reported a rash, 53% recalled that the tick was still attached when the rash developed, in sharp contrast to the erythema migrans of Lyme borreliosis, which appears a week after exposure (Nadelman, Herman & Wormser, 1997). Of 1556 ticks that were saved and submitted by residents who had been bitten in that site, 95% were A. americanum, mainly nymphs.
The aetiology of Masters' Disease remains undescribed, although a Borrelia related to B. theileri (the agent of bovine borreliosis, described at the turn of the 20th century), detected within 1–5% of host-seeking A. americanum, and designated ‘B. barbouri’ (Rich et al. 2001) or ‘B. lonestari’ (Barbour, 1996) may eventually be incriminated as the agent. To date, samples from such patients have generally failed to provide evidence of spirochetal aetiology: BSK cultures of skin biopsies are negative, PCR of such samples in multiple laboratories have been negative (except in the instance of one case; James et al. 2001), and seroconversion to borrelial antigens ambiguous. At the very least, some reactivity with spirochetal antigens (B. burgdorferi s.l., B. hermsi) would be expected inasmuch as the borreliae are widely crossreactive. Specific reactivity to B. ‘lonestari’ can not yet be tested because the agent fails to propagate in vitro from ticks or patient samples. No association with other known tick-borne pathogens (such as E. chaffeensis, R. rickettsii, Francisella tularensis) seems apparent, but other agents within Lone Star ticks that might cause such a rash are spiroplasmas (unpublished), Trypanosoma cervi and Rickettsia amblyommi. Although Masters' Disease patients' sera generally fail to react with antigens of Rickettsia rickettsii, no attempt has been made to determine whether they may specifically recognise R. amblyommi antigens. Recent molecular phylogenetic analyses suggest that R. amblyommi may differ enough from R. rickettsii, and thus sufficient antigenic differences may exist to render serological assays for SFG reactivity insensitive enough to fail to confirm rickettsial infection in Masters' Disease patients. Assigning aetiology is made even more complex in some sites because both I. dammini and A. americanum may co-occur, such as in much of New Jersey, and individuals may not accurately identify ticks. A. americanum bites may also provoke a cutaneous reaction that may be misidentified as erythema migrans (Goldman, Rockwell & Richfield, 1952).
R. helvetica was first described from Swiss I. ricinus (Burgdorfer et al. 1979; Beati et al. 1993) as a non-pathogenic member of the spotted fever group rickettsiae. Subsequently this agent was detected in many other I. ricinus populations, with prevalence as great as 22% (Nilsson et al. 1999a). In 1999, rickettsia-like organisms were detected by light microscopy in sections of heart tissue taken at autopsy of two young Swedish hockey players. The aetiology of sudden cardiac death was being intensively scrutinised due to an epidemic of this syndrome among orienteers, athletes in the prime of their life (Wesslen, 2001). Chronic perimyocarditis reminiscent of scrub typhus was detected at autopsy and a search for rickettsiae was initiated. Because R. helvetica was known to infect Swedish I. ricinus ticks, this agent was the primary candidate as the cause of these putative rickettsial lesions. R. helvetica was detected within myocardial tissue by means of PCR sequencing of the citrate synthase gene (Nilsson, Lindquist & Pahlson, 1999b). Rickettsiae were visualized in perimyocardial tissues by immunohistochemistry using anti-Proteus OX (the Weil-Felix reagent, which crossreacts with rickettsial lipopolysaccharide) used because the investigators lacked specific anti-R. helvetica sera. Transmission electron microscopy confirmed the presence of rickettsiae within myocardial endothelium. Sera from these cases were reactive against antigens of R. helvetica. Although two cases do not make an epidemic, more study is urgently required to determine the frequency with which R. helvetica (or other tick-borne ‘endosymbiotic’ rickettsiae) may cause myocarditis and sudden cardiac death given its wide distribution and prevalence in an aggressive human-biting vector. This agent has also been associated with sarcoidosis (Nilsson et al. 2001). Fever cases in sites where I. ricinus are present should always consider R. helvetica infection as part of the differential diagnosis; a recent report indicated that about 9% of sera from an Alsace study population were seroreactive to R. helvetica antigens (Fournier et al. 2000). Recently, R. helvetica was detected in Japanese Ixodes persulcatus (Fournier et al. 2002), and it may be that this agent may be found wherever these and related ticks (the main vectors of Lyme borreliosis, granulocytic ehrlichiosis and tick-borne encephalitis) are present.
Because of shared life cycles, ticks may contain multiple agents. ‘Coinfection’, based largely on observations of concurrent babesial, spirochetal and ehrlichial infection in I. dammini ticks or mouse reservoirs, has emerged as a paradigm for explaining some variations in the clinical spectrum of Lyme borreliosis (Krause et al. 1996). However, the frequency of concurrent infection in humans, as opposed to sequential infection, and the attributable effects thereof, remains poorly defined.
Early work demonstrating that I. dammini nymphs are frequently concurrently infected by B. burgdorferi and B. microti (Piesman et al. 1986) used the Feulgen reaction for microscopically detecting salivary infections of babesial sporozoites. Nearly 20% of host-seeking nymphal deer ticks were concurrently infected. At that time only B. microti was known to have infected I. dammini in New England. We now know that B. odocoilei, commonly detected in deer throughout the eastern US, is present in the northeastern US (Armstrong et al. 1998) and thus the morphology-based estimates for B. microti prevalence may have been inflated. A simple restriction analysis of a babesia-specific PCR product can now discriminate between B. microti and B. odocoilei and thus clarify the prevalence of each agent in coastal New England sites.
On the other hand, PCR analyses for B. microti in unfed ticks tend to underestimate prevalence (Telford & Persing, unpublished observations) because of a small number of babesial genomes (sporoblasts or kinetes) within an unfed tick Dormant sporoblasts reactivate during feeding to produce thousands of sporozoites, thereby increasing the sensitivity of any detection method when ticks are allowed to ‘pre-feed’ prior to analysis. Using a combination of pre-feeding, microscopy and PCR with stringent contamination control, we detected only 0·2% of 427 (95% confidence interval 0–0·7%) host-seeking nymphal I. dammini that was triply infected (Lyme, HGE and babesia) in four coastal New England sites (unpublished observations), although Lyme/babesia (1·9%, 0·6–3·2) and Lyme/HGE (1·6%, 0·4–2·8) infection was more common. Concurrent infection in host-seeking ticks, however, seems to be less common than expected. Further observations are required to determine whether the prevalence of concurrent infection reflects an interaction at the level of reservoir immunity (in the vertebrate or in the vector) or that reservoir host species are more diverse and locally determined than the literature suggests.
Increased scrutiny of ticks, often by the use of eubacterial PCR assays, has suggested that there may be diverse inquilinic (‘resident in’, without implying a commensal, symbiotic or parasitic association) microbes. In particular, a common commensal of rodents, the bartonellae, have been suggested as another tick-borne infection. Bartonella spp. are the aetiologic agents of cat scratch fever (B. henselae), trench fever (B. quintana) and oroya fever (B. bacilliformis); their vectors (identified by careful experimental and natural history studies) are fleas, lice and sandflies, respectively. During early investigations of the causes of oroya fever, Noguchi (1926) demonstrated that B. bacilliformis could be experimentally transmitted between monkeys by the bites of D. andersoni ticks; to our knowledge this is the only reported demonstration that ticks may serve as vectors of bartonellae.
Because bartonellae are common in the rodents that most commonly serve as hosts for sub-adult ticks in the I. persulcatus species complex (the main vectors of Lyme borreliosis), it should not be surprising to detect evidence of these bacteria within ticks. Trans-stadial survival does not imply vector competence. Even ungulates appear to be infected by specific bartonellae and indeed I. ricinus removed from deer contained Bartonella DNA (Schouls et al. 1999). Such evidence may simply represent the presence of these bacteria within the bloodmeal or non-blood fluids ingested from the dermis as does the recent report of B. henselae within ticks removed from humans (Parola et al. 2003). Evidence of frequent bartonella infection has been detected in host-seeking I. pacificus (Chang et al. 2001, 2002) but the low annealing temperature used for the PCR assay (42 °C) would appear to invite non-specificity regardless of the design of the primers. Sequencing of the amplicons was not routinely performed and thus the identity of the agents represented by these DNA fragments remains unclear. A more convincing case for an association of bartonellae with ticks was reported for a Lyme borreliosis case in New Jersey from whom B. henselae was cultivated. Host-seeking I. dammini from the patient's yard yielded B. henselae amplicons that were confirmed by sequencing (Eskow, Rao & Mordechai, 2001). The ability of deer ticks to transmit B. henselae remains unproven. Evidence of B. henselae infection in I. ricinus that had been removed from asymptomatic cat scratch disease (seropositive) humans has also been recently reported (Sanogo et al. 2003).
Statistical associations have also been utilized to suggest that bartonellae are tick-transmitted. The common bartonellae infecting P. leucopus mice, Grahamella peromysci Tyzzer (Tyzzer, 1942) was detected during surveys of Wisconsin and Minnesota sites for the agent of HGE using the ‘specific’ Ehr545/742 primers (Hofmeister et al. 1998). These authors argued that because evidence of Bartonella infection was statistically more likely to be found in association with Lyme or babesia infection, they must share a common vector. Evidence of flea infestation was not sought in that study, nor association with Trypanosoma microti, which is maintained by the common mouse flea, Orchopeas leucopus. Bartonella infection of P. leucopus, associated with O. leucopus, is found virtually anywhere, including urban Boston or northern New Hampshire where ticks are not present (Telford, unpublished observations). Subsequently, this bacterium was detected in autopsy material from a Montana farmer who had died of endocarditis and was given the subspecific epithet B. vinsonii arupensis (Welch et al. 1999). Identity with G. peromysci is suggested because the 2198 strain of this agent, as sequenced in the Wisconsin/Minnesota study, derived from P. leucopus collected from the type locality, Martha's Vineyard. Another study that used statistical arguments for the role of ticks as vectors of bartonellae examined canine co-infections with several Ehrlichia spp. and babesia (Kordick et al. 1999); dogs that were infected by B. vinsonii were statistically more likely to also be infected by babesia and ehrlichiae. Such statistical arguments fail to consider confounding by general ectoparasite infestation, that is, those dogs with babesial or ehrlichial infection have ticks because they belong to owners who are lax about treating for ectoparasites; concurrent flea infestation would be likely. Failure to detect fleas on such hosts during cursory examinations may relate to their smaller size and agility compared to ticks.
Viability, let alone vector competence should not be inferred from the detection of DNA; indeed, DNA from dead organisms may amplify as well as that from live ones. Although it remains premature to conclude that ticks might transmit bartonellae to humans (or between animals), it is also premature to conclude otherwise. Careful experimental observations such as those demonstrating the role of fleas as the vectors of rodent grahamellae (e.g. Krampitz & Kleinschmidt, 1960) seem crucial to complement molecular epidemiological observations.
A case of hepatitis C infection was recently reported to be transmitted by I. dammini (Wurzel, Cable & Leiby, 2002). A blood donor in Connecticut was recorded as seropositive for Babesia microti in July 1999 but negative for all other markers of infectious diseases. Follow-up serum samples were tested as part of a B. microti natural history study and, during September 1999, the donor reported having signs and symptoms of hepatitis. Hepatitis C virus infection (HCV) was diagnosed based on serology and RT-PCR. The donor denied classical risk factors for acquiring HCV, including occupational exposure to blood (even though the individual was employed as a medical technologist). The authors of the report argued that because B. microti-specific IgM was present in July, this could be interpreted as the patient acquiring an infectious tick bite during June. The demonstration of HCVviraemia between July and August was consistent with acquisition during June as well. Furthermore, the relatively small risks of acquiring B. microti or HCV, as estimated from the blood donor population, would argue that the ‘simultaneous discovery of these two infective events … would be highly unusual’ (Wurzel et al. 2002). The authors suggested co-transmission of B. microti and HCV inasmuch as ticks serve as vectors for other flaviviruses (tick-borne encephalitis). Although the unrelated hepatitis E virus is commonly found in rodents (Favorov et al. 2000) and could theoretically be transmitted by ticks, HCV is known only from hominoid primates (Lemon & Brown, 1995); it would be unusual for I. dammini to feed to repletion on a human and survive to moult. Thus, this report is most likely explained by unrecognised occupational exposure to HCV and is unlikely to represent a new tick–microbe association.
PCR and nucleic acid sequencing provides us with numerous entities for which matching GenBank accessions are not identifiable. Whether truly newly recognised or simply rediscovered, many such agents are ‘in search of an emerging disease’. As responsible public health professionals, we should take all due care to explore aetiologic roles following well-established criteria prior to proclaiming to have discovered a ‘new’ emerging disease. Koch's postulates, the microbiological standard by which an aetiologic agent is incriminated, traditionally require that (1) the agent is always associated with the illness, and under circumstances that account for pathology and clinical signs or symptoms of the illness; (2) that it does not occur in healthy individuals; and (3) that the signs and symptoms of the illness may be reproduced by exposure to the pure, in vitro cultivated agent (Evans, 1976). These postulates have been significantly modified through the years to reflect the ever-evolving modes of discovery, culminating in the routine use of the polymerase chain reaction (Fredricks & Relman, 1996). Kochs postulates and variants thereof for use at the population (public health) level remain a useful framework for incriminating an aetiologic agent – or evaluating the burgeoning literature of emerging diseases.
Many examples of prospecting for ‘agents in search of emerging diseases’ may be found in the recent literature (e.g. Billings et al. 1998b; Shpynov et al. 2001; Simser et al. 2002). Indeed, the use of broad range eubacterial 16S rDNA primers has identified a diverse flora associated with ticks (Martin & Schmidtmann, 1998; Schabereiter-Gurtner, Lubitz & Rolleke, 2003), which may represent soil contaminants of the waxy cuticular surface as well as those that truly infect ticks. We focus on one such report as an example of the questions that are raised when such prospecting is successful. We also call attention to a greatly neglected group of potentially pathogenic microbes, the spiroplasmas, which to date have been routinely ignored when they are detected within ticks.
During a search for the biological basis for human serological reactivity to antigens of E. chaffeensis, A. phagocytophilum and the spotted fever group rickettsiae in Southeast Asia (Parola et al. 2003), two Ehrlichia spp. were detected by PCR sequencing in Haemaphysalis hystricis ticks from Vietnam. One of the amplicons was 99·4% identical to E. chaffeensis in the large portion of the 16S rDNA that was sequenced. Because H. hystricis is known to feed on humans, human exposure to the agents represented by these amplicons might confound epidemiological surveys for evidence of infection by known Ehrlichia spp. Serological cross-reactivity is well-known among Ehrlichia spp. In the same study, two Rickettsia spp. were identified by the same methods from Dermacentor auratus and pools of Dermacentor larvae from Thailand. Other than their placement in the spotted fever group and R. bellii clades, no other information is available regarding these microbes. Isolates were not made. How, then, does one designate the organism represented by the DNA sequence? How different is different when it comes to sequence information? When does one consider a sequence to represent a unique taxon, taxon'-like' or virtually identical? Are such findings to be considered conservatively as representative of endosymbiotic microbes? Or, because there is human seroreactivity to known human pathogens whose DNA sequences are very similar to those detected in human biting ticks, is a logical hypothesis to test that they do indeed represent agents in search of an emerging disease? In light of the HIV pandemic and the susceptibility of HIV patients to opportunistic pathogens, one organism's endosymbionts may well become another's pathogen.
The spiroplasmas (Mycoplasmatales) are commonly found within ticks during darkfield microscopy for the detection of borreliae, appearing as small (2–4 microns in length) spirochete- or filament-like organisms with varying degrees of motility. Mycoplasma are the smallest free-living microorganisms, with reproductive units as small as 125 angstroms. They lack a cell wall and are thus highly pleomorphic. Although the vast majority appear to be commensal, mycoplasma are known to cause a number of veterinary syndromes such as contagious bovine pleuropneumonia, chronic respiratory disease of fowl and infectious catarrh of rodents (Hayflick, 1969; Anonymous, 1972). Their role in causing human disease is controversial, other than that of M. pneumoniae and M. genitalium (Ureaplasma urealyticum), the agents of primary atypical pneumonia and non-gonococcal urethritis, respectively. They have been implicated as aetiologic agents in erythema multiforme (Ludham, Bridges & Benn, 1964). However, the difficulty with which they are detected within clinical samples and the potential for confusion with common commensal or environmental mycoplasma ensures difficulty in describing their role in the aetiology of an illness.
Spiroplasma are well-known as endosymbiotic agents of arthropods and seem to be transmitted by insects to plants, sometimes causing pathology (e.g. citrus canker). Spiroplasma ixodetis was described from host-seeking I. pacificus ticks collected in Oregon (Tully et al. 1995). S. mirum was isolated from Haemaphysalis leporispalustris (Tully et al. 1983). The relevance of these agents to human health is unknown. Spiroplasmas have been identified by fluorescent antibody in about a third of a sample of German I. ricinus (Tenckhoff et al. 1994), but to our knowledge no other published reports exist on their prevalence within ticks. Given the diverse dermatological manifestations associated with tick bites, the role of spiroplasmas should be explored, particularly with lesions that appear similar to erythema multiforme.
In the current research climate that focuses on ‘new’ emergent infections, there is a risk that ‘old’ and well-established public health burdens due to ticks have become neglected. Three such infections for which ‘auld acquaintance’ should not be forgotten are Rocky Mountain spotted fever, tularemia and tick-borne encephalitis.
Rocky Mountain Spotted Fever (RMSF) was once the most important of tick-borne infections in the US (Harden, 1990) but now appears to be about a tenth as commonly reported as Lyme disease. Before antibiotic treatment was available, the case fatality rate was 20% or greater and even today it is 5% (Walker, 1998). From 1981–1995, 9223 RMSF cases were reported in the US (Dalton et al. 1995); as a comparison, over 100000 cases of Lyme disease were reported from 1990–1999 (http://www.cdc.gov/ncidod/dvbid/lyme/ldcases90-99.htm). The agent is currently classified as a Category C Select (bioterrorism) Agent by the US government due to its capacity to cause severe morbidity and mortality, as well as potential for being disseminated by aerosol. First described in the Rocky Mountain region by Wood (1896), the role of ticks as vectors was confirmed by Ricketts (1906) during his investigation of the virulent epidemic in the Bitterroot Valley of Montana, where nearly 100 cases had occurred during 1895–1902 with 70% mortality. The rickettsial aetiology of RMSF was definitively demonstrated by Wolbach (1919). Interestingly, although hundreds of RMSF cases continue to be reported in the US, most such cases are from southcentral (Oklahoma, Arkansas, Texas and Missouri) and middle Atlantic states (North Carolina, Virginia and Maryland). Relatively few (about 2%) are reported from the Western US (Dalton et al. 1995). Whether the seeming shift in the endemic areas represents human activity, tick density, vagaries of reporting or a true change in the ecology of R. rickettsii remains undescribed.
The main vectors of RMSF, D. andersoni in the western US and D. variabilis in the eastern and central US, are widespread and ecologically successful three-host ticks. The reproductive hosts are generally medium-sized mammals such as skunks, raccoons and foxes; sub-adult ticks tend to feed on rodents such as voles and deer mice. The peridomestic predilection of the reproductive hosts suggests that RMSF should be as great or greater a public health hazard as is Lyme borreliosis in and around suburban sites. Indeed, a cluster of cases has been reported from around a Manhattan park (Salgo et al. 1988), a distinctly urban site.
Then too, the public health burden of the tropical American RMSF variants such as Sao Paolo typhus and Mexican Fiebre Manchada remain poorly explored. Medline from 1966–2002 lists only 7 references for tick-transmitted rickettsiosis in Brazil, and 3 for Mexico. In Brazil, a particularly virulent form of RMSF, with a high case fatality rate, has been described associated with A. cajennense (Monteiro, Fonseca & Prado, 1931). In Mexico, RMSF-like infection was described in the 1940s (Bustamante, Varela & Ortiz-Mariotte, 1946) and recently an analysis of a dengue outbreak in the Yucatan suggested that some of the cases may have been due to spotted fever or a similar rickettsiosis (Zavala-Velazquez et al. 1999). Describing the burden of tick-transmitted rickettsiosis in tropical countries would seem a fruitful line of inquiry.
A mechanism for explaining the distribution of R. rickettsii-infected ticks (Burgdorfer, Hayes & Mavros, 1981a) involving competitive displacement by endosymbiotic rickettsiae (‘East side agent’, now known as R. peacocki; Niebylski et al. 1997b) remains one of the most innovative ecological theories ever devised for explaining the regulation of natural microbial populations. Similar hypotheses should be tested for other tick-borne infections in other parts of the world. A better understanding of the transmission dynamics and the nature of such competitive displacement may allow an evaluation of the capacity for RMSF to become resurgent in sites, such as the Rocky Mountain states; or its potential to emerge as a public health burden within the heavily populated and suburbanized northeastern US.
Tularemia has received renewed interest given its placement within Category A – those most likely to be used for bioterrorism – of the US Select Agent list (Dennis et al. 2001). Studies on Dermacentor andersoni in the Bitteroot Valley spotted fever epidemic, isolated F. tularensis from these ticks (Parker, Spencer & Francis, 1924) and thereby implicated them as vectors. Tularemia in North America appears to be maintained between ticks and rabbits (Jellison & Parker, 1944), and greater than 90% of all American cases appear to be related to rabbit exposure (Gill & Cunha, 1997). In contrast to the main epidemiological features in North America, tularemia in Eurasia seems more of an environmental infection acquired from agricultural activities such as hay threshing, from water contaminated by muskrats or water voles, during the processing of animal products (Pavlovsky, 1966) or, interestingly, by mosquito bites (probably representing contaminative transmission, Hopla, 1974). These differences served for an epidemiological basis of classification in which two distinct types of F. tularensis were thought to exist, differing with respect to distribution, reservoirs and virulence (Olsufiev, Emelyanova & Dunayeva, 1959). Type A organisms (also known as F. tularensis biovar tularensis or F. tularensis nearctica) are prevalent in North America but not in Eurasia, maintained in cottontail rabbits, are frequently transmitted by ticks and may cause severe disease (Gill & Cunha, 1997). Type B (F. tularensis biovar palaearctica or F. tularensis holarctica) causes episodic outbreaks (epizootics) in beavers, muskrats and arvicoline rodents in either North America or Eurasia, may be isolated from water or soil and causes a milder disease.
Molecular phylogenetic analyses have demonstrated that the arthropod endosymbionts known as ‘Wolbachia’ comprise diverse unrelated groups (O'Neill et al. 1992). ‘Wolbachia persica’, originally described by Suitor & Weiss (1961) from Argas spp. have recently been placed within the genus Francisella based upon 16S rDNA sequencing (Niebylski et al. 1997a; Noda, Munderloh & Kurtti, 1997; O'Neill et al. 1992; Sun et al. 2000). Tissues from ticks infected by these agents, interestingly, appear to be moderately infectious for vertebrates (Suitor & Weiss, 1961). Their roles as potential human pathogens remains to be explored.
The enzootic cycle and vector–pathogen relationship of F. tularensis, particularly Type A in North America, remains poorly explored. Rabbits (and rodents) are relatively poor reservoir hosts because they quickly succumb to infection, often within days. The rabbit tick (H. leporispalustris) is thought to be a main interepizootic reservoir (Jellison, 1974), but alternative enzootic associations such as D. variabilis and its hosts have not been carefully investigated. Elucidation of the actual mode of transmission requires work: the few experimental transmission studies that have been done do not definitively identify salivary transmission as opposed to contamination by bacteria-laden faeces deposited during feeding. The one report that would suggest that F. tularensis may be introduced by salivation into the dermis during feeding reported on the reduction in tularemia mortality in tick-bite sensitized guinea pigs (Bell, Stewart & Wikel, 1979) fed upon by infected ticks. The role of transovarial transmission as a mode of perpetuation (Hopla, 1974) needs clarification, as well.
Infection of host-seeking I. ricinus by F. tularensis has been reported from Slovakia and Austria, in addition to the more commonly infected D. reticulatus (Vyrostekova et al. 2002). Such findings would confirm that Palearctic tularemia may be acquired by tick bite, although it is said that most infections there are associated with exposure to rodent-contaminated hay, muskrats or even mosquito bites (Tarnvik, Sandstrom & Sjostedt, 1996). In North America D. variabilis and A. americanum are the only human-biting ticks that have been implicated as zoonotic vectors. More than 1000 adult I. dammini in coastal New England have been screened for arboviral infections by mouse inoculation of tick tissues, with no identification of F. tularensis infection (Telford, unpublished observations), and it seems unlikely that these ticks serve as vectors. Although tick-transmitted tularemia is said to be accompanied by an eschar and lymphadenopathy (‘ulceroglandular’), it may be that such a presentation varies locally. A recent Nantucket case, for example, presented as community-acquired pneumonia and was diagnosed as pneumonic tularemia. An intensive physical examination discovered a healing eschar without lymphadenopathy, suggesting acquisition by tick or other arthropod bite (Shapiro & Mark, 2000) and thus not representing primary (inhalational) pneumonic tularemia. At least 3 of 21 cases in the ongoing Martha's Vineyard ‘pneumonic’ tularemia outbreak (Feldman et al. 2003) were likely to have been due to tick bite. Tick-borne tularemia is probably more common and with a more variable presentation than textbooks would suggest.
Tick-borne encephalitis (TBE) was, until recently, the most prevalent tick-borne disease affecting humans. This dubious honour has now been assumed by Lyme borreliosis. However, in many parts of the world (such as vast areas of Russia), TBE is considered the most burdensome vector-borne infection because of its morbidity and mortality. TBE viruses (TBEV) are members of the family Flaviviridae, which includes the causative agents of West Nile, yellow fever, dengue and Japanese B encephalitis. Molecular phylogenetic analyses demonstrate that three major clades comprise TBEV: TBEV sensu lato, Tyuleniy and Powassan corresponding to previous classification schemes based upon serological typing. Eight subtypes of TBEV sensu lato are recognised: Far Eastern or Russian Spring Summer Encephalitis (RSSE); Central European Encephalitis (CEE); Louping Ill (LI); Turkish Sheep Encephalitis (TSE); Omsk Hemorrhagic Fever (OHF); Kyasanur Forest Disease (KFD); and Langat (Zanotto et al. 1995). All of these, other than TSE and Langat, are known human pathogens. The Tyuleniy clade seems associated with seabirds and their ticks, and the potential for human infection is unknown although serosurveys suggest human exposure (Chastel, 1980). Powassan virus has caused two dozen cases of a devastating meningoencephalitis in residents of Eastern Canada and the northeastern US (Anonymous, 1995a). The ecology and epidemiology of KFD and OHF have been extensively studied by Indian and Russian workers, respectively, and the reader is referred to comprehensive reviews (Banerjee, 1986; Lvov, 1986) for information on these haemorrhagic fevers.
Because infection may occur by inhalation and the case fatality rate can be great for RSSE, OHF and KFD, Biosafety Level 4 (BSL-4) facilities, practices, and procedures are recommended for working with these pathogens within the United States and elsewhere (Richmond & McKinney, 1993). BSL-3 practices and procedures are suggested for work with all the other TBE group viruses. The TBE viruses are considered Category C Select Agents. These recommendations have tended to limit the number of investigators who may effectively continue to pose questions about their biology. Thus, the placement of TBE in the ‘auld lang syne’ category may not reflect neglect due to mistaken satisfaction with the state of our knowledge, but rather a perceived logistical inability to initiate new studies.
TBE is associated with Ixodes persulcatus and I. ricinus (Gresikova & Calisher, 1989), the Eurasian vectors of Lyme borreliosis and granulocytic ehrlichiosis. Although other ticks such as Dermacentor spp. and Haemaphysalis spp. are experimentally susceptible and occasionally found to be infected in nature, the vectorial capacity of I. persulcatus and I. ricinus seems much greater. Small mammal reservoirs such as the woodmouse, Apodemus spp., or the bank vole, Clethrionomys glareolus, serve to amplify the number of infected vector ticks within circumscribed, longstanding ‘natural foci’ of transmission (Kozuch et al. 1967; Gresikova & Calisher, 1989). Nymphal I. persulcatus or I. ricinus attach and complete feeding upon a non-immune rodent host. Within a week after the infecting tick feeds, a viraemia develops in the circulating blood that, when imbibed, is sufficient to infect either larval or nymphal ticks. The resulting nymph or adult may attach to and infect humans or other animals. Transovarial transmission of TBEV serves to complement maintenance by the rodent–tick cycle (Rehacek, 1962). In addition, a non-viraemic mode of perpetuation exists wherein uninfected ticks become infected by ‘co-feeding’ in proximity to the mouthparts of an infected tick (Labuda et al. 1993). In either case (co-feeding or amplification by viraemia in a reservoir), the transmission cycle depends on the nearly simultaneous temporal appearance of larvae and nymphs (Randolph et al. 1999; Randolph, 2002).
TBE incidence fluctuates from year to year, with local incidence peaks often seen in two to four year intervals (Kunz, 1992). These fluctuations are likely to reflect fluctuation in tick and feeding host populations as well as environmental factors, such as temperature and humidity, which may directly affect virus activity. The extent to which global climate change may influence the prevalence of tick-borne infections in general, and TBE in particular, remains to be defined. Predictive risk mapping suggests that TBE foci may disappear from many areas if hot and dry summers become more common as a result of climate change (Randolph & Rogers, 2000). Alteration of vector seasonal activity, however, may be counterbalanced by effects mediated by the extrinsic incubation temperature of the pathogens. More research is needed on the relative importance of these and other factors in enzootic cycles.
TBE incidence has increased within the past decade or so in many European nations. In Latvia, 100 to 300 cases were reported during 1984 to 1992, but a median of 791 cases was reported from 1993–1995 (Antykova, 1989; Anonymous, 1995b). In southwestern Germany, a total of 78 cases of TBE was reported in the years 1978–1984 whereas in 1994 alone, there were 234 (Ackermann, Kruger & Roggendorf, 1986; Kaiser, 1996). Similar trends have been reported elsewhere in Europe. Furthermore, new foci of zoonotic TBE transmission are emerging (Treib, 1994; de Marval, 1995). It is not clear whether this apparent emergence corresponds to an increase in the distribution of TBE microfoci, to increased contact between humans and infected ticks, to local weather changes or to a combination of these factors. The fact that those new zoonotic transmission foci tend to appear at the fringes of established transmission areas suggests that this phenomenon may not be caused by increased human exposure alone (Wellmer & Jusatz, 1981; Randolph, 2002).
Due to the overlap of enzootic transmission cycles of different tick-borne pathogens, co-infection with at least one additional agent is relatively common and should always be considered as a possibility. Concurrent Lyme borreliosis, for example, has been shown to be associated with particularly severe TBE manifestations (Oksi et al. 1993). Whether TBEV coinfection with piroplasms or ehrlichiae may modify disease in the human host is not known. Louping ill virus infection in sheep is exacerbated by that of tick-borne fever due to A. phagocytophilum (Reid, 1986) and thus some effects are anticipated for human coinfection.
The effects of enzootic overlap between closely related pathogens remains poorly explored. Powassan virus (POW) is maintained by I. cookei, I. texanus or other ticks that focus their feeding on woodchucks, skunks and other medium-sized mammals. Such ticks only occasionally bite humans, accounting for the relative scarcity of cases for a virus which is geographically widespread and intensively transmitted: 23–64% of woodchucks were seropositive for POW in New York and Ontario (Artsob, 1989). In addition, the deer tick, I. dammini, the aggressive main vector for Lyme borreliosis in the northeastern US, is experimentally vector competent for Powassan virus (Costero & Grayson, 1996). This tick naturally maintains a Powassan subtype (‘deer tick virus’ or DTV, Telford et al. 1997) for which human infection remains to be described. Should deer ticks begin to ‘bridge’ virulent Powassan virus from the I. cookei-woodchuck cycle, it may be that Powassan fever will become more prevalent. On the other hand, DTV may competitively exclude POW within a site, thereby preventing the more severe infection from more commonly becoming a public health hazard. It may also be that deer tick virus causes an FUO-like illness (fever of unknown origin) that resolves without sequelae, and that transmission might intensify as has that of Lyme disease over the last decade or two. These issues pose important public health questions as well as some for basic disease ecology.
Pathogens, vectors and reservoir hosts exist in predictable ecological assemblages (Pavlovsky, 1966). An extension of this is the idea that microbes exist in guilds, which are the basic units of community structure and represent groups of unrelated taxa that share a common resource (Root, 1967). Fleas, mites, lice and ticks infesting a mouse would comprise a guild of ectoparasites; babesia, trypanosomes, hemogregarines and grahamellae comprise a guild of rodent haematozoa. Such host–pathogen assemblages usually have analogues (ecological equivalents) in each biogeographic region and may be maintained in small enzootic (natural) foci without implying human exposure. Human risk, or the ‘zoonotic’ condition, denotes overflow or bridging from the enzootic cycle (Spielman & Rossignol, 1984). Emergence of a zoonosis thus requires unusual circumstances of great vector and reservoir density, promoting overflow, intrusion of humans into enzootic foci or the introduction of a man-biting bridge vector. And, because of microbial guilds, rarely does just one agent ‘emerge’. An old poster from the Rocky Mountain laboratories stresses the multiple nature of hazards from tickbite (Fig. 3), calling the wood tick a ‘pandora's box'.

Fig. 3. The Rocky Mountain wood tick as Pandora's box. From the Rocky Mountain Laboratories, Hamilton, MT.
Based upon the guild concept, one could attempt to predict the presence of microbial agents within local tick taxa. For example, for I. dammini in North America, we would expect to detect A. phagocytophilum, a tick-borne encephalitis-like flavivirus, and an orbivirus in ticks or rodents from sites where Lyme disease spirochetes, B. microti, and the other haemoparasites are present because these agents comprise the guild in European I. ricinus. In 1994, the index case-series for human granulocytic ehrlichiosis (HGE) was reported from the vicinity of Duluth, MN (Chen et al. 1994), an area that is thought to be a relict, long-standing site of transmission for deer tick agents (Telford et al. 1993). Rapidly thereafter, using the guild concept, the index case of HGE for New England (Telford et al. 1995) was identified by a specific search for the rickettsial agent in blood samples from I. dammini-exposed patients. The success of this approach stimulated a search for a flavivirus, inasmuch as tick-borne encephalitis virus is a prominent member of the microbial guild associated with I. persulcatus-like ticks in Eurasia (Telford & Foppa, 2000). Deer tick virus (DTV) was subsequently identified (Telford et al. 1997); molecular and serological characterisation now suggests that it is a subtype of Powassan virus (Ebel, Spielman & Telford, 2001). Human infection due to DTV remains undescribed, although prototypic Powassan virus is well-known as a cause of a devastating meningoencephalitis (Artsob, 1989). We have, in fact, argued that DTV may be a less pathogenic subtype inasmuch as prevalence of infection in deer ticks approaches that for HGE or B. microti, but severe meningoencephalitis cases have not been frequently reported from our study populations.
Other tick-maintained microbial guilds seem apparent in the northeastern US (Table 2) awaiting attention by researchers. Although diverse ixodid ticks may be found there, we present as an example three that are ecologically successful (widely distributed, abundant) and with some degree of anthropophily. Dog ticks (D. variabilis) have been intensively studied due to their main role as vector of Rocky Mountain Spotted Fever. They are widely distributed in the eastern and central US, with isolated populations occurring through the Western states. Lone Star ticks (A. americanum), a major pest species through the south and central states, appears to be expanding its distribution into New England. Finally, rabbit ticks (Ixodes dentatus) may be found wherever cottontail rabbits have been introduced; furthermore, these ticks are readily transported by passerine birds, suggesting the possibility for rapid and extensive dispersal of any agent that it transmits. I. dentatus appears to bite humans more frequently than previously thought (Armstrong et al. 2001).

Similar tables can and should be compiled for common human-biting ticks throughout the world, such as R. sanguineus, D. marginatus and A. cajennense. Ecological theory provides us with a prospective approach to identifying emergent tick-borne pathogens. Such hypothesis-based, prospective analyses of tick–pathogen–reservoir systems may provide us with an ever expanding list of aetiological agents ‘in search of an emerging illness’. Emergent clinical entities, in turn, may be determined to be due to well-known or poorly characterized tick-borne microbes by focusing our attention on locally abundant ticks and their likely microbial guilds. Rare tick-borne infections may emerge as public health burdens due to changes in tick density and environmental disturbance. In all such situations, the laboratory findings enabled by the powerful molecular techniques must be evaluated within an epidemiological and ecological context to determine the actual risk to human health.
Our work has been funded by grants from the National Institutes of Health (AI 37993, 39002) and the Centers for Disease Control and Prevention (U50/CCU 119560).

Fig. 1. 16S rDNA phylogenetic tree (neighbour-joining algorithm) of ehrlichia-like taxa showing two schemes for making taxonomic decisions. A, as recommended by Dumler et al. subsuming Cytoecetes with Anaplasma. B, conserving Cytoecetes. One could also retain Cowdria by moving the cut-off farther to the right, and placing the former E. bovis into its own clade; Cowdria's tropism for endothelial cells clearly distinguish it from that of the remainder of the Ehrlichia sensu stricto.

Table 1. Old and new rickettsioses. Established clinical entities (with more than a couple of described cases) for which rickettsial aetiology is established. Date when first recognised refers to first published clinical report for the infection; the agent may have been incriminated much later in time. Question marks refer to probable but not yet demonstrated
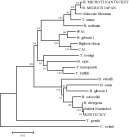
Fig. 2. Phylogeny of Babesia spp. based on 18S rDNA (neighbour-joining algorithm), indicating relatedness of known human pathogens (in capital letters).

Fig. 3. The Rocky Mountain wood tick as Pandora's box. From the Rocky Mountain Laboratories, Hamilton, MT.

Table 2. Microbial guilds presumed associated with ixodid ticks common in New England. Question marks indicate that the agent has not been looked for but is likely to be detected; asterisks denote an agent in search of an emerging infection