Introduction
Genetically engineered crops have been highly successful in contributing to economically valuable levels of pest control (Reed et al., Reference Reed, Jensen, Riebe, Head and Duan2001; Carrière et al., Reference Carrière, Ellers-Kick, Sisterson, Antilla, Whitlow, Dennehy and Tabashnik2003). The incorporation of these bioengineered crops expressing toxins from Bacillus thuringiensis Berlinger into pest management programs has often been compatible with sustainable farming practices, resulting in few discernable differences in populations of non-target natural enemy populations (e.g. Jasinski et al., Reference Jasinski, Eisley, Young, Kovach and Willson2003; Men et al., Reference Men, Ge, Liu and Yardim2003; Sisterson et al., Reference Sisterson, Biggs, Olson, Carrière, Dennehy and Tabashnik2004; Naranjo, Reference Naranjo2005; de la Poza et al., Reference de la Poza, Pons, Farinós, López, Ortego, Eizaguirre, Castañera and Albajes2005). However, areas planted to transgenic crops are continuing to increase (Lawrence, Reference Lawrence2005), giving cause for concern with regard to their impact on the non-target food chain (Wolfenbarger & Phifer, Reference Wolfenbarger and Phifer2000; Groot & Dicke, Reference Groot and Dicke2002; Obrycki et al., Reference Obrycki, Ruberson, Losey, Ehler, Sforza and Matielle2004). This concern exists despite the potential reductions in applications of broad-spectrum insecticide (Cattaneo et al., Reference Cattaneo, Yafuso, Schmidt, Huang, Rahman, Olson, Ellers-Kirk, Orr, Marsh, Antilla, Dutilleul and Carrière2006), translating into advantageous conditions for natural enemies (Gould, Reference Gould1998; Sisterson et al., Reference Sisterson, Biggs, Olson, Carrière, Dennehy and Tabashnik2004). Furthermore, most studies indicate no reduced fitness effects on coleopteran predators exposed to Bt-containing prey (e.g. Lundgren & Wiedenmann, Reference Lundgren and Wiedenmann2002; Ferry et al., Reference Ferry, Mulligan, Stewart, Tabashnik, Port and Gatehouse2006; Harwood et al., Reference Harwood, Samson and Obrycki2006).
The wealth of experiments examining the non-target effects of transgenic crops provide accurate information pertaining to impact assessment towards specific food chains (Romeis et al., Reference Romeis, Meissle and Bigler2006). Despite these studies, relatively few examine the trophic movement of these plant-derived compounds in the field (Harwood et al., Reference Harwood, Wallin and Obrycki2005; Zwahlen & Andow, Reference Zwahlen and Andow2005; Ludy & Lang, Reference Ludy and Lang2006) although low-level uptake of Bt-endotoxins has been identified in a Tetranychus–Orius linkage in the laboratory (Obrist et al., Reference Obrist, Dutton, Albajes and Bigler2006). However, some herbivorous prey have been documented as containing Bt-endotoxins from ingested plant material (Head et al., Reference Head, Brown, Groth and Duan2001; Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001; Harwood et al., Reference Harwood, Wallin and Obrycki2005), thereby exposing predators to elevated Cry1Ab concentrations. These post-mortem gut-content analyses, therefore, allow the accurate detection of target material within predators using antibody or DNA-based technology (Sheppard & Harwood, Reference Sheppard and Harwood2005). Thus, by sampling individuals directly from the field, communities can interact naturally within transgenic agroecosystems; the data are easily interpreted and they enable the post-release recommendations for monitoring (Snow et al., Reference Snow, Andow, Gepts, Hallerman, Power, Tiedje and Wolfenbarger2005) to be incorporated into risk assessments of genetically modified crops.
In this study, we specifically examined temporal variability in the uptake of Bt-endotoxins by exotic and native coccinellids in North America and discuss the potential linkages within these food webs before, during and after anthesis. Given that some coccinellids readily feed on pollen (Lundgren et al., Reference Lundgren, Huber and Wiedenmann2005), it is further predicted that significant increases in the uptake of Bt-endotoxins will occur immediately following anthesis. We also examine the persistence of Cry1Ab-endotoxins in Coleomegilla maculata (De Geer) (Coleoptera: Coccinellidae) by collecting adults entering overwintering sites around the bases of trees adjacent to Bt-corn fields at the research site. C. maculata routinely overwinter as adults in aggregated communities on the ground surface under leaf litter (Benton & Crump, Reference Benton and Crump1979). Therefore, if Bt-endotoxins persist in the gut of these predators or they are exposed to these proteins through the food chain post-harvest, individuals entering overwintering sites would be predicted to contain detectable quantities within their guts.
Materials and methods
Field sampling protocols
During late May–early September 2005, a transgenic corn agroecosystem (Bt-hybrid N79-L3, Bt-11 event, Syngenta Seeds, Golden Valley, MN, USA) at the University of Kentucky Spindletop Research Station, Lexington, KY, USA (Universal Trans-Mercator Grid References: 4224676 Northing, 689850 Easting, Zone 16) was surveyed weekly for adults of four species of coccinellid (Harmonia axyridis (Pallas), Coccinella septempunctata L., Cycloneda munda (Say) and C. maculata). The Bt-corn field was surrounded by mixed agriculture, dominated by alfalfa Medicago sativa L. (Fabaceae), uncultivated plots and non-Bt-crops. These coccinellids were individually collected by aspirator, transferred into 1.5-ml microcentrifuge tubes on ice and frozen in a portable Engel MT15 freezer (Engel USA, Jupiter, FL, USA) within 1 h of collection. Samples were transferred into a −20°C freezer in the laboratory until assayed by ELISA for Bt-endotoxins.
In addition to the collection of samples from fields of corn, C. maculata were collected on November 14, 2005 from overwintering sites at the base of four silver maple trees, Acer saccharinum L. (Aceraceae), within 10 m of Bt-corn fields. Prior sampling in non-Bt-fields at this research station revealed no evidence for Cry1Ab-endotoxins in the guts of non-target herbivores or higher-order predators (Harwood et al., Reference Harwood, Wallin and Obrycki2005).
ELISA-screening protocols
The presence of Bt-endotoxins was determined using a sandwich enzyme-linked immunosorbent assay (Abraxis L.L.C., Warminster, PA, USA) following protocols optimized for endotoxin detection in non-target natural enemies. Prior to screening, each coccinellid was allowed to thaw at room temperature and the foregut extracted by teasing apart the thorax and abdomen. The foregut was weighed, diluted on a weight:volume ratio in extraction buffer to a working concentration of 1:100 (mg μl−1) and homogenised using disposable Kontes™ Pellet Pestles (Fisher Scientific Company L.L.C., Pittsburgh, PA, USA). Occasionally, gut samples contained little material or were empty, necessitating a working dilution of 1:500 (mg μl−1). In such instances, the calculation of Bt-endotoxin concentrations were modified to factor out variable dilution rates. The homogenate was dispersed for 20 s on a vortex mixer, centrifuged at 5000 g for 5 min and the supernatants added into wells of an Abraxis L.L.C. Cry1Ab/Cry1Ac antibody-coated ELISA plate, at 100 μl per well.
In parallel to coating with field samples, 100 μl of Bt-standards with concentrations of 0.25, 0.5, 1.0, 2.0 and 4.0 ng ml−1 Cry1Ab, a negative control (0 ng ml−1 Cry1Ab) and a positive control (1.5 ng ml−1 Cry1Ab) were all added to the ELISA plate. After rotation of the plate to ensure mixing within individual wells, taking care not to contaminate material between wells, the plates were covered with an acetate sheet and allowed to incubate at room temperature for 30 min. After incubation, all material was ejected from the plate and the wells were washed three times with Abraxis L.L.C. Wash Buffer. The Cry1Ab/Cry1Ac-endotoxin specific rabbit polyclonal antiserum was added, at 100 μl per well, and the plate carefully rotated for 20 s to ensure mixing within wells prior to a further 30 min incubation period at room temperature. As above, all material was ejected following incubation, wells were washed and 100 μl of horseradish peroxidase-labelled goat anti-rabbit enzyme conjugate (100×dilution) was added to all wells and incubated at room temperature for 30 min. After further washing of the wells, 100 μl of color-solution consisting of 3,3′, 5,5′-tetramethyl benzidine in an organic base was added to all wells for 20 min, after which 50 μl of dilute acid stopping solution was added to terminate the reaction. Absorbance was recorded at 450 nm using a Thermo Labsystems Multiskan Plus® spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA).
Data analysis
The classification of field-collected samples as screening positive for target Cry1Ab proteins was given to individuals with an absorbance value greater than the mean +2.5 SD of the level recorded by negative control specimens. The concentration of Cry1Ab-endotoxins within coccinellid guts was calculated using the OD450 value for each sample and extrapolating the concentration from the calibration regression for Bt-standards coated on each plate. To convert from micrograms to nanograms of endotoxin per gram fresh weight, each value was multiplied by the dilution factor (×100 or, occasionally, ×500) and subsequently divided by 1000.
The proportion of C. maculata containing significant quantities of Bt-endotoxins was split into four time periods based on the phenology of the corn: before anthesis (30 May–27 June), during anthesis (4 July–18 July), after anthesis (25 July–8 August) and late-season (15 August–5 September), which was at least six weeks after the first documented incidence of anthesis in the field. These frequencies testing positive for Cry1Ab-endotoxins were compared using χ2 analysis. The sample sizes for H. axyridis, C. munda and C. septempunctata were too small to allow statistical comparisons among sample dates.
Results
Gut-content analysis of 1126 adult coccinellids from fields of Bt-corn indicated small, but significant, numbers screened positive for Cry1Ab-endotoxins (table 1). Whilst few H. axyridis, C. septempunctata and C. munda screened above this positive threshold, 12.8% of C. maculata elicited absorbance readings signifying detectable quantities of Cry1Ab-endotoxin within their guts at a mean concentration of 0.225±0.065 μg g−1 Cry1Ab-endotoxin.
Table 1. Number and proportion of adult coccinellids collected from within a Bt-corn agroecosystem screening positive for Cry1Ab-endotoxins. Concentration of Cry1Ab-endotoxins represents the mean values (±SE) for those individuals testing positive by ELISA.
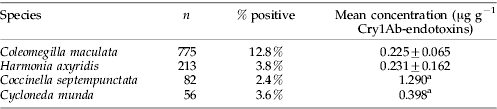
a SE not presented (n=2 positive samples).
The proportion of adults containing significant levels of Cry1Ab-endotoxins varied throughout the season (fig. 1), although all four species contained specimens screening positive prior to anthesis and before access to Bt-pollen. Furthermore, large numbers of H. axyridis and C. maculata, the two most abundant coccinellids in Bt-corn, screened strongly positive for Bt-endotoxins up to ten weeks after anthesis occurred in the field (fig. 1), peaking around 4–5 weeks after pollen was shed when approximately 40% of individuals contained Bt-endotoxins. In C. maculata, this temporal variation was significant (χ2=28.84, df=3, P<0.001) with more adults testing positive 2–3 weeks after anthesis compared to pre-anthesis, during anthesis and late in the season (fig. 1a).

Fig. 1. Temporal variation in percentage of (a) Coleomegilla maculata and (b) Harmonia axyridis screening positive for Cry1Ab-endotoxins. Arrow represents the start of anthesis.
In November, 107 adult C. maculata were collected from overwintering sites in close proximity to Bt-corn fields. There was no evidence for persistence within the coccinellid food chain with no adults entering overwintering screening positive for Cry1Ab-endotoxins using this ELISA.
Discussion
Risk assessment of transgenic crops often involves field surveys of population densities or laboratory ‘worst-case’ scenarios examining the effects of non-target species feeding on Bt-containing food. Although some field studies have reported reductions in natural enemy populations (Daly & Buntin, Reference Daly and Buntin2005), probably as a result of lower lepidopteran densities, most have documented no negative consequences resulting from the planting of transgenic crops (e.g. Jasinski et al., Reference Jasinski, Eisley, Young, Kovach and Willson2003; Sisterson et al., Reference Sisterson, Biggs, Olson, Carrière, Dennehy and Tabashnik2004; Dively, Reference Dively2005; Pilcher et al., Reference Pilcher, Rice and Obrycki2005). Similarly, laboratory studies tend to report no adverse effects following exposure, although some have revealed negative interactions with the non-target food chain (Obrycki et al., Reference Obrycki, Ruberson, Losey, Ehler, Sforza and Matielle2004; Lövei & Arpaia, Reference Lövei and Arpaia2005). As a consequence of these studies, non-target effects have been well characterized and decision mechanisms established for their risk assessment towards insect natural enemies (Romeis et al., Reference Romeis, Meissle and Bigler2006).
Despite the application of molecular methods for studying ecological interactions in the field, rarely have tri-trophic movements of endotoxins been examined (Lundgren & Wiedenmann, Reference Lundgren and Wiedenmann2005; Harwood et al., Reference Harwood, Samson and Obrycki2006; Obrist et al., Reference Obrist, Dutton, Albajes and Bigler2006). Furthermore, two of these studies reported no detectable quantities of Bt-endotoxins in higher order natural enemies following consumption of herbivores exposed to Bt-containing plants (Harwood et al., Reference Harwood, Wallin and Obrycki2005; Lundgren & Wiedenmann, Reference Lundgren and Wiedenmann2005), whilst the other (Obrist et al., Reference Obrist, Dutton, Albajes and Bigler2006) found low levels in Orius majusculus (Reuter) (Hemiptera: Anthocoridae) after consumption of Tetranychus, and these endotoxins persisted for a very short period of time. However, mechanisms are clearly operating whereby trophic linkages allow generalist predators to take up Bt-endotoxins through specific connections in their complex food web.
Pollen is an important food resource for some coccinellids (Lundgren et al., Reference Lundgren, Huber and Wiedenmann2005); but other tri-trophic interactions facilitate the movement of endotoxins along this food chain, especially in those species, such as adult H. axyridis, which are not pollinivorous (Lundgren et al., Reference Lundgren, Razzak and Wiedenmann2004). Large numbers of C. maculata screened positive for Cry1Ab-endotoxins before (n=221, 14.9% positive) and after (n=106, 25.5% positive) anthesis, confirming the movements of toxins through other, non-pollen, pathways. It is unlikely that the Bt-corn→aphid→coccinellid linkage facilitates significant flow of endotoxins to the second trophic level given that aphids tend not to take up Bt-endotoxins (Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001; Dutton et al., Reference Dutton, Klein, Romeis and Bigler2002), although other herbivores have been found in fields of transgenic corn with significant quantities of Bt-endotoxin in their guts (Harwood et al., Reference Harwood, Wallin and Obrycki2005). For example, Obrist et al. (Reference Obrist, Dutton, Albajes and Bigler2006) documented the transfer of endotoxins along the Tetranychus→Orius pathway, and these spider mites contained increased concentrations of Bt-endotoxins after anthesis. Given the abundance of nymphal nabids and Orius within this Bt-corn agroecosystem, it is possible that the corn→nabid→coccinellid and corn→Orius→coccinellid pathways were responsible for some tri-trophic transfer of endotoxins. However, laboratory studies would be required to confirm this prediction. Similarly, other plant→herbivore→coccinellid and plant→omnivore→coccinellid interactions may be possible, but the specific mechanisms for endotoxin transfer are unclear. C. maculata also feeds on fungal spores; it is possible that microbial action on pollen and decaying plant material on the ground could, in part, be responsible for this movement if endotoxins are transferred into fungal spores.
Surprisingly, no increase in Bt-endotoxin concentration was observed around anthesis, but concentrations in H. axyridis and C. maculata increased many weeks after pollen was shed (fig. 1). Whilst this trend may be different in larval coccinellids, Bt-containing pollen was not directly responsible for all the uptake of Bt-endotoxins by adults. The post-anthesis peak in C. maculata testing positive for Bt-endotoxins could be due to the consumption of pollen shed a few weeks earlier (and therefore partially degraded, thus releasing detectable endotoxins) or the consumption of pollen-feeding prey, which themselves consumed the freshly-shed pollen. In the case of H. axyridis, adults readily consume other less competitive coccinellids. Therefore, C. maculata larvae would be likely to have consumed Bt-pollen and their tri-trophic interaction with H. axyridis could be responsible for endotoxin movements through these food chains. Exposure of predators to Bt-endotoxins could also occur for extended periods of time given their persistence in tissue samples after harvest (Zwahlen et al., Reference Zwahlen, Hilbeck, Gugerli and Nentwig2003) and in the soil (Baumgarte & Tebbe, Reference Baumgarte and Tebbe2005). Therefore, should extended persistence of Bt-endotoxin in the gut of coccinellids occur or food webs continue to be exposed to these proteins post-harvest, early overwintering populations would be expected to contain detectable concentrations of Cry1Ab-endotoxins. No evidence was gathered to suggest extended exposure or persistence in these food webs, with all C. maculata entering overwintering as adults containing no detectable Bt-endotoxins. However, the high mobility of C. maculata could result in overwintering adults originating from non-Bt-corn fields, thereby limiting their exposure to Bt-endotoxins earlier in the year.
The field studies reported here present the first evidence for temporal variability in Bt-endotoxin uptake in coccinellid food chains and the lack of a direct correlation between anthesis and Bt-endotoxin concentrations in predator guts. Although the mobility of adult coccinellids increases the likelihood of collecting samples moving between non-Bt- and Bt-crops, during periods of peak endotoxin concentration in predators, approximately 40% of C. maculata and H. axyridis screened positive; but this occurred four to five weeks after anthesis and was not directly correlated with the consumption of pollen. These data provide clear evidence for the need for future risk assessment of transgenic crops to non-target food chains in the field, specifically identifying trophic linkages through which endotoxins are most likely to flow and the retention time of Bt-endotoxins following the consumption of Bt-containing food items. Given that B. thuringiensis var. kurstaki is an ubiquitous and widely distributed bacterium found in the soil (Martin & Travers, Reference Martin and Travers1989), it is possible that some detectable Cry1Ab-endotoxins were transferred from native Bt in the soil or from plants. However, the absence of Bt-proteins in natural enemies and non-target herbivores from non-transgenic habitats (Harwood et al., Reference Harwood, Wallin and Obrycki2005) and overwintering sites (this study) makes this scenario unlikely. Ultimately the incorporation of laboratory exposure experiments, field population surveys and quantitative assessments of Bt-endotoxin movements through non-target food webs can provide accurate information upon which the safety of bioengineered crops can be assessed.
Acknowledgements
This research was supported by USDA-CSREES Biotechnology Risk Assessment Grant #2006-39454-17446 and Kentucky Science and Engineering Foundation Agreement #KSEF-148-502-04-121 with the Kentucky Science and Technology Corporation. JDH and JJO are further supported by the Kentucky Agricultural Experiment Station Project KY099004. We are very grateful to two anonymous reviewers for their valuable comments. This is publication number 06-08-016 of the University of Kentucky Agricultural Experiment Station.




