Introduction
Major depressive disorder (MDD) is a prevalent mental disorder characterized by impairments in affect, behaviour and cognition. Individuals with MDD persistently suffer from feelings of sadness and worthlessness and experience less enjoyment in social interactions (Belmaker & Agam, Reference Belmaker and Agam2008; Hirschfeld et al., Reference Hirschfeld, Montgomery, Keller, Kasper, Schatzberg, Möller and Versiani2000). Globally, over 300 million people are struggling with depression. It leads to job absenteeism and reduced productivity, which creates a tremendous economic burden in society (Lee et al., Reference Lee, Rosenblat, Lee, Carmona, Subramaniapillai, Shekotikhina and Ho2018; Organization, Reference Organization2017). Thus, understanding the psychological and neurobiological mechanisms underlying depression may help prevent depression and lead to more effective treatments for depression.
Considerable evidence indicates that the anterior cingulate cortex (ACC), a locus of information processing and regulation in the brain, plays an essential role in the pathophysiology of depression. Previous anatomical studies have reported reductions in grey (Wise et al., Reference Wise, Radua, Via, Cardoner, Abe, Adams and Périco2017; Yucel et al., Reference Yucel, McKinnon, Chahal, Taylor, Macdonald, Joffe and MacQueen2008) and white (Ballmaier et al., Reference Ballmaier, Toga, Blanton, Sowell, Lavretsky, Peterson and Kumar2004; Olvet et al., Reference Olvet, Delaparte, Yeh, DeLorenzo, McGrath, Weissman and McInnis2016) matter volume in the ACC in MDD patients, while other studies have indicated that reduced neuronal somal size and increased neuronal density in layer 5 of the ACC may be the key features of MDD (Chana, Landau, Beasley, Everall, & Cotter, Reference Chana, Landau, Beasley, Everall and Cotter2003). Additionally, deep brain stimulation treatment targeting white matter tracts adjacent to the subgenual ACC has been suggested to be effective in relieving depressive symptoms (Johansen-Berg et al., Reference Johansen-Berg, Gutman, Behrens, Matthews, Rushworth, Katz and Mayberg2007; Mayberg, Riva-Posse, & Crowell, Reference Mayberg, Riva-Posse and Crowell2016; Mayberg et al., Reference Mayberg, Lozano, Voon, McNeely, Seminowicz, Hamani and Kennedy2005; Riva-Posse et al., Reference Riva-Posse, Choi, Holtzheimer, Crowell, Garlow, Rajendra and Mayberg2018). More recently, emerging evidence supports the view that depression is characterized by aberrant functional connectivity (FC) across multiple brain regions and dysfunctional interactions among intrinsic functional networks (Kaiser, Andrews-Hanna, Wager, & Pizzagalli, Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015; Peng et al., Reference Peng, Lin, Wu, Gong, Yang and Wang2018; Wang, Hermens, Hickie, & Lagopoulos, Reference Wang, Hermens, Hickie and Lagopoulos2012).
Functional magnetic resonance imaging (fMRI) at rest is a promising technique for evaluating large-scale neural systems that exhibit spontaneous synchronous fluctuations, also known as resting-state functional connectivity (rsFC) (Fox & Raichle, Reference Fox and Raichle2007; Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). Previous studies based on rsFC have indicated widely abnormal connectivity between the ACC and other brain regions (Davey, Harrison, Yücel, & Allen, Reference Davey, Harrison, Yücel and Allen2012; Greicius et al., Reference Greicius, Flores, Menon, Glover, Solvason, Kenna and Schatzberg2007; Rolls et al., Reference Rolls, Cheng, Gong, Qiu, Zhou, Zhang and Cheng2018; Zhu et al., Reference Zhu, Wang, Xiao, Liao, Zhong, Wang and Yao2012). However, morphometric and neuroimaging studies with both human (Öngür, Ferry, & Price, Reference Öngür, Ferry and Price2003; Palomero-Gallagher et al., Reference Palomero-Gallagher, Hoffstaedter, Mohlberg, Eickhoff, Amunts and Zilles2018) and non-human primates (Paus, Reference Paus2001; Schaeffer et al., Reference Schaeffer, Gilbert, Ghahremani, Gati, Menon and Everling2019) have suggested that the ACC is structurally and functionally heterogeneous. Meta-analyses and task-based studies have also demonstrated that subdivisions in the ACC correspond to different functional distinctions (Koski & Paus, Reference Koski, Paus, WX, AM and J2000; Milham & Banich, Reference Milham and Banich2005; Rotge et al., Reference Rotge, Lemogne, Hinfray, Huguet, Grynszpan, Tartour and Fossati2014). More recently, studies on ACC functional segregation using resting-state fMRI have indicated that the ACC could be subdivided into caudal, dorsal, rostral, perigenual and subgenual regions. These subdivisions are thought to be associated with the functional domains of sensorimotor processing, cognitive control, conflict monitoring, social processing and emotional regulation (Kelly et al., Reference Kelly, Di Martino, Uddin, Shehzad, Gee, Reiss and Milham2008; Margulies et al., Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007). Thus, we hypothesized that functional interaction changes in different ACC sub-regions are specific and can be associated with distinct symptoms of depression. However, to our knowledge, no rsFC study has systematically investigated the aberrant FC of ACC sub-regions in patients with MDD.
Here, we used the rsFC analysis approach to estimate the brain-wide sub-regional ACC connectivity in first-episode medication-naïve patients with MDD compared to healthy controls. In addition, we investigated the relationships between depressive symptom severity and aberrant FC of ACC subdivisions. To verify the reliability of our findings, we conducted a meta-analysis to generate the distributions of MDD-related abnormal regions from previously reported results and compared them to the FC deficits revealed in this study. We aimed to systematically identify specific changes in FC in different ACC subdivisions that manifest in MDD and to further investigate the relationship between these changes and the clinical symptoms of depression.
Methods
Participants
Forty-one first-episode medication-naïve patients with a DSM-IV diagnosis of MDD (16 males; mean age = 33.41 ± 9.13) and 43 demographically matched healthy controls (20 males; mean age = 31.72 ± 8.93) were recruited from the Department of Psychiatry of the Affiliated Xi'an Central Hospital of Xi'an Jiaotong University (see online Supplementary Table S1 for participant demographics and clinical characteristics). The exclusion criteria included the following: history of cortisol medication use or electroconvulsive therapy, history of alcohol or substance misuse, previous head injury with loss of consciousness, age under 18 or over 45, pregnancy, and comorbidity with other psychiatric/neurological illnesses or personality disorders. All participants were right-handed. The Hamilton Depression Scale (HAMD), Hamilton Anxiety Scale (HAMA) and Automatic Thoughts Questionnaire (ATQ) were independently assessed by two clinicians. The two scores were averaged to provide the best estimate. Specifically, the HAMD and HAMA were used to rate the severity of depression and anxiety symptoms in MDD patients, while the ATQ was utilized to measure the occurrence frequency of negative self-statements associated with depression (Hamilton, Reference Hamilton1980; Hollon & Kendall, Reference Hollon and Kendall1980; Maier, Buller, Philipp, & Heuser, Reference Maier, Buller, Philipp and Heuser1988). Written informed consent was obtained from all participants in accordance with the guidelines of the local ethics committee of the Affiliated Xi'an Central Hospital of Xi'an Jiaotong University. Part of this dataset has been previously reported (Peng et al., Reference Peng, Lin, Wu, Gong, Yang and Wang2018).
MRI data acquisition
Resting-state fMRI data were collected on a 1.5-T GE Excite MRI scanner (GE Healthcare, Milwaukee, WI, USA). Functional images were acquired using an echo planar imaging pulse sequence (TR = 2500 ms; TE = 35 ms; flip angle = 90°; FOV = 256 mm × 256 mm; matrix = 64 × 64). All participants had one run of resting-state fMRI (the length of scan for each run was 6.25 min, which contained 150 volumes) and were instructed to lay still and stay awake during the scan. Structural MRI scans were acquired using a 3D fast-spoiled gradient echo sequence (TR = 6.9 ms; TE = 3.2 ms; flip angle = 90°; FOV = 256 mm × 256 mm; matrix = 256 × 256; slice thickness = 1.2 mm; number of slices = 128).
MRI data preprocessing
Preprocessing was carried out using AFNI (https://afni.nimh.nih.gov/), FreeSurfer (http://surfer.nmr.mgh.harvard.edu) and the FSL software library (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) according to the procedures previously described (Peng et al., Reference Peng, Lin, Wu, Gong, Yang and Wang2018) and included the omission of the first four volumes, motion correction by a rigid body registration algorithm, registration to the Montreal Neurological Institute (MNI152) standard template and spatial smoothing with a Gaussian kernel of 6 mm FWHM. In line with the previous meta-analytic work and considering the possible effects of filtering, we applied band-pass filtering within 0.01 Hz < f < 0.08 Hz (Craig, Manktelow, Sahakian, Menon, & Stamatakis, Reference Craig, Manktelow, Sahakian, Menon and Stamatakis2018; Wang et al., Reference Wang, Buckner, Fox, Holt, Holmes, Stoecklein and Li2015; Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). Head motion, whole brain signal, ventricular and white matter signals were then regressed from the filtered data. The preprocessed functional data were aligned to the FreeSurfer fsaverage4 surface template (which consists of 2562 vertices in each hemisphere) through a hybrid surface- and volume-based approach (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). This volume-to-surface procedure included the following: MNI152 structural data were processed using the FreeSurfer ‘recon-all’ (https://surfer.nmr.mgh.harvard.edu/fswiki/recon-all) command to automatically register MNI152 to a common spherical coordinate system. The resting-state fMRI data were then aligned to this common spherical coordinate system via sampling from the middle of the cortical ribbon in a single interpolation step and downsampled to the 4 mm mesh template ‘fsaverage4’. For control analysis, frame-wise displacement (FD) and root-mean-square variance of the temporal derivative of the time series (DVARS) were estimated to identify head motion in the fMRI data. No significant difference in head motion was found between the MDD and HC groups (two sample t tests; FD: t = 1.35, p = 0.18; DVARS: t = 0.30, p = 0.77; see online Supplementary Table S1).
Functional connectivity analysis
According to the previous studies on ACC subdivisions (Kelly et al., Reference Kelly, Di Martino, Uddin, Shehzad, Gee, Reiss and Milham2008; Margulies et al., Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007), five seeds defined for each hemisphere in MNI space divided the ACC into five districts, including the caudal ACC (A1; MNI = ± 5, −10, 37), dorsal ACC (A2; MNI = ± 5, 10, 33), rostral ACC (A3; MNI = ± 5, 27, 21), perigenual ACC (A4; MNI = ± 5, 47, 11) and subgenual ACC (A5; MNI = ± 5, 34, −4) (see Fig. 1). These seeds were located symmetrically in both hemispheres with spheres 6 mm in diameter. Then, we used a volume–surface combined analysis approach to compute the FC maps of each ACC sub-regional region of interest (ROI). Briefly, BOLD signals were extracted and averaged across all voxels included in each ACC sub-regional seed in MNI volume space. This mean signal of the seed derived from the volume space was used to compute FC with all vertices from the cortical surface space for each participant. Finally, Fisher's r-to-z transformed correlation maps were averaged within groups for each seed to generate the mean FC maps.
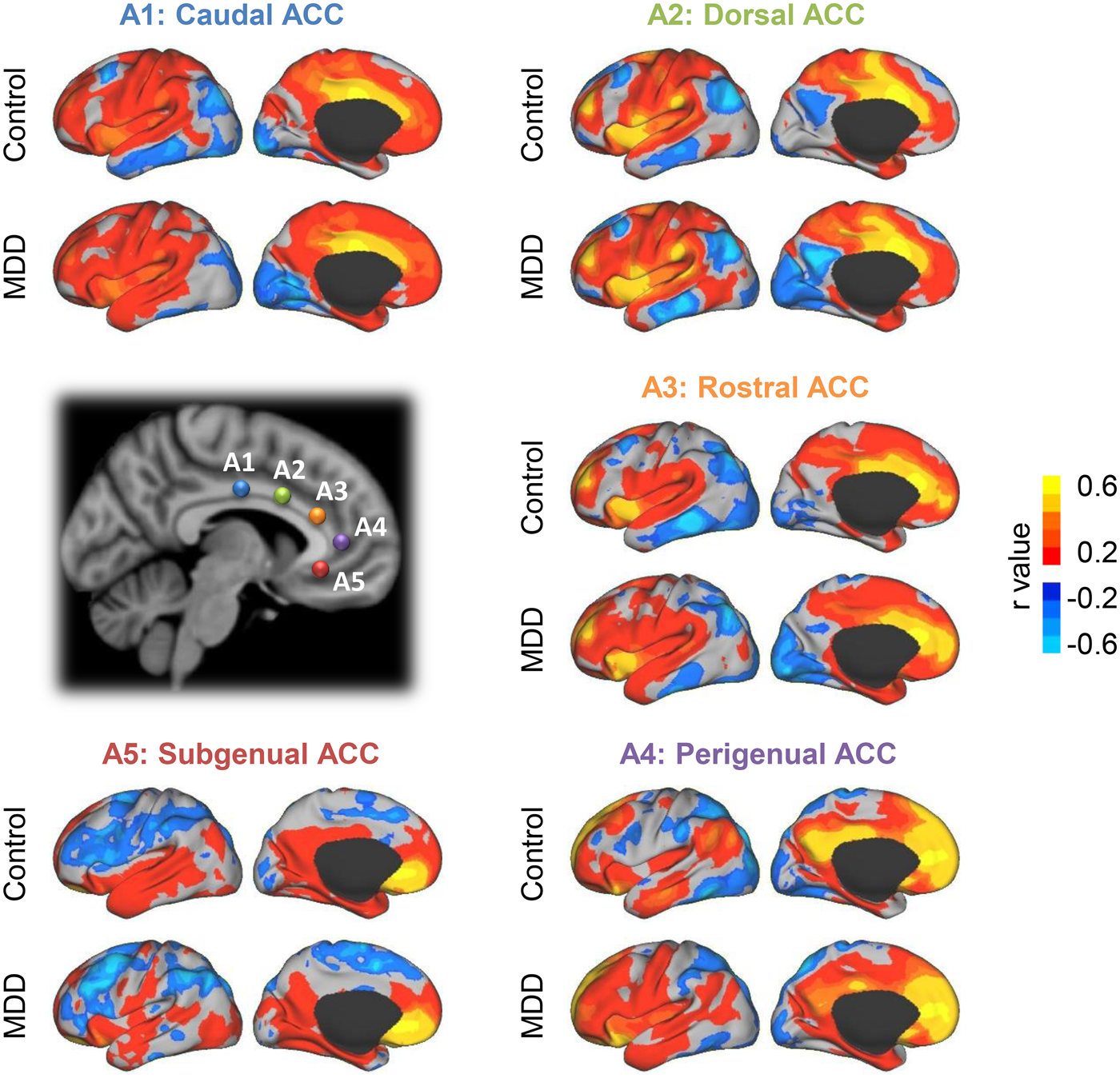
Fig. 1. Functional connectivity maps of five left ACC sub-regions in major depressive disorder (MDD) patients (n = 41) and healthy controls (n = 43). According to previous studies on ACC subdivisions, five seeds were defined for each hemisphere to represent different ACC functional subdivisions, including caudal ACC (L/R-A1, blue, MNI = ± 5, −10, 37), dorsal ACC (L/R-A2, green, MNI = ± 5, 10, 33), rostral ACC (L/R-A3, orange, MNI = ± 5, 27, 21), perigenual ACC (L/R-A4, purple, MNI = ± 5, 47, 11) and subgenual ACC (L/R-A5, red, MNI = ± 5, 34, −4). All these seeds were defined in the Montreal Neurological Institute (MNI) coordinates with the spheres of 6 mm. The positions of seeds on the left hemisphere and their functional connectivity maps derived from the MDD patients and healthy controls are displayed.
Spatial dependent correlation analyses
To test the impact of spatial dependence between adjacent vertices on the estimation of the distribution of FC reconfigurability, we performed repeated (n = 1000) random sampling of 10% of the whole brain vertices. All correlation coefficients were computed and averaged based on the subsets of vertices that passed the Durbin–Watson test for spatial dependence (1.5 < DW < 2.5, p > 0.05).
Statistical analysis
Voxel-wise between-group analyses for each ACC sub-regional seed were performed using a two-sample t test to examine the abnormal FC in the MDD patients. Next, all maps were thresholded (p < 0.001, uncorrected), and 10 000 Monte Carlo simulations were applied to correct for multiple comparisons across surfaces using FreeSurfer (Hagler, Saygin, & Sereno, Reference Hagler, Saygin and & Sereno2006). The Monte Carlo analysis determined the likelihood that the resulting clusters were fully corrected for multiple comparisons across surfaces. The corrected p value for clusters was set to p < 0.01, and the cluster size was set to 200 mm2. To examine the relationship between depression severity and FC, mean FC for a cluster size over 3 vertices within the significant abnormal FC regions was computed and correlated with depressive symptom severity (HAMD, HAMA and ATQ). The correlation results were then corrected for multiple comparisons using the Benjamini–Hochberg procedure to control the false discovery rate (FDR) at the level α = 0.05. In addition, in a control analysis, we also conducted all the above statistical analyses after linear regression with age to control its potential impact on the results.
Meta-analysis for MDD-related abnormal regions
To confirm whether regions with significant abnormal FC in our study were identified as abnormal areas across a large number of previous studies, a meta-analysis was conducted using Neurosynth (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, Reference Yarkoni, Poldrack, Nichols, Van Essen and Wager2011). By applying the forward inference, we then restricted the meta-analysis to studies that reported associations between depression (search terms included depression, depressive, major depressive, depressive disorders and disorder MDD) and abnormality (search terms included abnormal and deficit), which included 101 studies. The meta-analysis was conducted in MNI space. Survived abnormal brain regions in MDD patients (FDR q < 0.01) were projected onto the cortical surface for display. Additionally, we also compared the FC group difference maps obtained in this study with these meta-analysis maps by overlapping both binarized abnormal regions on a common cortical surface.
Visualization
All imaging results were projected onto the inflated PALS cortical surface using CARET for the purpose of visualization (Van Essen, Reference Van Essen2005).
Results
ACC sub-regional FC maps in MDD patients and healthy controls
FC of 10 ACC sub-regional ROIs was computed on whole cortical surfaces in the MDD patients and healthy controls. FC maps of each seed were then averaged across each group, and the mean FC maps of left hemisphere ROIs are displayed in Fig. 1 (see FC maps of all 10 ACC sub-regional seeds in online Supplementary Fig. S1). The ACC sub-regional seeds located in the left and right hemispheres showed a similar pattern of FC maps (HC group A1: r = 0.893, A2: r = 0.960, A3: r = 0.967, A4: r = 0.938, A5: r = 0.943; MDD group A1: r = 0.929, A2: r = 0.955, A3: r = 0.950, A4: r = 0.953, A5: r = 0.940; all p < 0.0001). Specifically, caudal ACC seed A1 was mainly connected with the cingulo-opercular network, sensorimotor cortex and superior parietal lobe (SPL). Dorsal ACC seed A2 displayed FC with the anterior and posterior insula, dorsal lateral anterior prefrontal cortex (dlPFC) and supplementary motor cortex, while rostral ACC seed A3 connected with the frontal pole, anterior insula and dlPFC. In addition, perigenual seed A4 was associated with regions including the posterior cingulate cortex (PCC), ventral medial prefrontal cortex and inferior parietal lobe (IPL), while subgenual ACC seed A5 correlated with limbic areas, orbitofrontal cortex and default network (DN)-related regions. These FC distributions of ACC sub-regions, in line with prior studies, had similar patterns in both the MDD patients and healthy controls (Kelly et al., Reference Kelly, Di Martino, Uddin, Shehzad, Gee, Reiss and Milham2008; Margulies et al., Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007).
FC changes in MDD patients relate to depressive symptom severity
Group comparisons for FC analysis were applied to explore the abnormal FC changes in the MDD patients compared with the healthy controls. To improve the statistical power for the multiple comparison results, surface-based cluster-wise correction was performed at the significance threshold of p < 0.01 and the cluster size threshold of 200 mm2 (see results for group comparison in Table 1). In the MDD patients, significantly reduced FC was obtained between the L-A1 seed and right SPL (Fig. 2a; p < 0.001), which negatively correlated with HAMA scores (r = −0.48, p = 0.001). In addition, the results from the MDD patients also demonstrated decreased FC between the L-A4 seed and left PCC (Fig. 2b; p < 0.001) and was negatively associated with ATQ scores (r = −0.41, p = 0.008). Notably, all the correlational analyses in this study were FDR corrected for multiple comparisons using the Benjamini–Hochberg procedure at the level α = 0.05. The corrected threshold is p = 0.0119. Although there was no significant difference in age between the two groups (p = 0.39), to exclude the potential impact of age on the results, these analyses were also performed after linear regression with age. The results derived from the age-corrected and age-uncorrected calculations are consistent (online Supplementary Fig. S2). Compared to the seven functional networks (Fig. 2e) derived from prior work on cortical parcellation (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011), the caudal ACC demonstrated a reduced connection with the dorsal attention network (dATN), while the perigenual ACC showed decreased FC with the DN.

Fig. 2. Functional connectivity decreased in the MDD patients and was negatively associated with depression severity. Regions of significant functional connectivity differences (cluster-wise correction for multiple comparisons at the significance threshold of p < 0.01 and the cluster size threshold of 200 mm2) are plotted on the cortical surface. (a) MDD patients demonstrate decreased connectivity between the L-A1 seed and right SPL (p < 0.001). Their connectivity is negatively associated with the Hamilton Anxiety Scale scores (r = −0.48, p = 0.001). (b) Functional connectivity between the L-A4 seed and left PCC is decreased in the MDD patients (p < 0.001) and negatively associated with the Automatic Thoughts Questionnaire scores (ATQ; r = −0.41, p = 0.008). (c) MDD patients show decreased connectivity between the L-A5 seed and posterior regions of the default network, including the inferior parietal lobe (IPL; p = 0.003) and posterior cingulate cortex (PCC; p < 0.001). Functional connectivity between L-A5 and pIPL is negatively associated with ATQ scores (IPL: r = −0.40, p = 0.009). (d) R-A5 seeds demonstrate similar reductions in functional connectivity with the IPL (p = 0.004) and PCC (p < 0.001) and are negatively correlated with ATQ scores (IPL: r = −0.52, p < 0.001; PCC: r = −0.45, p = 0.003). Black lines on the cortical surface represent the boundaries of seven functional networks (e) derived from a cortical parcellation approach, including the visual (Vis), sensorimotor (Mot), dorsal and ventral attention (dATN and vATN), limbic (LMB), frontoparietal control (FPN) and default (DN) networks.
Table 1. Brain regions showing a significant difference between MDD and HC

MDD, major depressive disorder; HC, healthy control; SPL, superior parietal lobe; PCC, posterior cingulate cortex; pIPL, posterior inferior parietal lobe; dmPFC, dorsal medial prefrontal cortex.
Vertex index refers to the index of vertex with max t value on fsaverage4 cortical template.
Clusterwise corrected: p < 0.01, cluster size >200 mm2.
Additionally, both the bilateral subgenual ACC regions demonstrated reduced FC with the posterior areas of the DN (Fig. 2c and d). Specifically, significant decreases in FC were found between L-A5 and the left posterior inferior parietal lobule (pIPL; p = 0.003), L-A5 and the left PCC (p < 0.001), R-A5 and the left pIPL (p = 0.004), and R-A5 and the PCC (p < 0.001). Abnormal FC regions derived from A5 seeds in both hemispheres were consistent in size and position. FC between the subgenual ACC and these posterior DN regions also exhibited negative associations with ATQ scores (L-A5–pIPL: r = −0.40, p = 0.009; R-A5–pIPL: r = −0.52, p < 0.001; R-A5–PCC: r = −0.45, p = 0.003).
Furthermore, significantly increased FC was observed between the rostral ACC and dorsal medial prefrontal cortex (dmPFC; p = 0.002), which belongs to the anterior part of the DN (Fig. 3).

Fig. 3. The rostral ACC shows increased functional connectivity with the anterior regions of the default network. There is increased functional connectivity between the L-A3 seed and dorsal medial prefrontal cortex (dmPFC; p = 0.002), which is known as an anterior part of the default network.
Decreased FC regions in the posterior default network are highly consistent with previous studies
To determine if the currently identified abnormal FC regions overlapped with abnormal areas reported in previous studies, we performed a meta-analysis using Neurosynth (for inclusion criteria, see Methods). A total of 101 studies were retrieved from over 14 000 publications. Surviving abnormal brain regions in patients with MDD were displayed on the cortical surface (Fig. 4a; FDR q < 0.01), including the anterior insula, dorsal ACC and regions related to the DN (e.g. ACC/PCC and IPL). Posterior areas of the DN, which demonstrated decreased FC with the subgenual ACC in the present study, highly overlap with the abnormal regions derived from the meta-analysis (Fig. 4b).

Fig. 4. Functional connectivity decreases in the posterior regions of the default network are highly consistent with the abnormal areas in MDD patients derived from a meta-analysis. (a) Abnormal brain regions in MDD patients were computed from a meta-analysis that included 101 studies using Neurosynth (FDR q < 0.01). These abnormal areas are mainly located in the anterior insula, dorsal anterior cingulate cortex and regions related to the default network, including the anterior and posterior cingulate cortex and inferior parietal lobe. (b) Decreased functional connectivity derived from bilateral A5 seeds (red regions) and abnormal brain regions derived from a meta-analysis (green regions) were binarized and projected onto a common cortical surface. The results highly overlap in the posterior areas of the default network (yellow regions).
Discussion
In this study, ACC sub-regional FC changes in the patients with MDD were quantified. Specifically, the subgenual and perigenual ACC demonstrated decreased FC with the posterior regions of the DN, including the pIPL and PCC. FC of these regions was negatively associated with ATQ scores and largely overlapped with the abnormal regions reported in the previous studies from our meta-analysis. In addition, we found reduced FC between the caudal ACC and SPL, which was negatively correlated with HAMA scores. Furthermore, we also found FC increases between the rostral ACC and dmPFC.
The DN, which anatomically consists of anterior and posterior midline regions, the lateral parietal cortex, the prefrontal cortex and the medial temporal lobe (MTL), usually becomes engaged during passive task states (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner2010; Buckner, Andrews-Hanna, & Schacter, Reference Buckner, Andrews-Hanna and Schacter2008). Task-based studies have indicated that the DN participates in a cognitive activity related to an individual's internal mentation, including theory of mind, self-referential processing and affective decision making (D'Argembeau et al., Reference D'Argembeau, Stawarczyk, Majerus, Collette, Van der Linden, Feyers and Salmon2010; Spreng, Mar, & Kim, Reference Spreng, Mar and Kim2009). More recently, Vatansever and co-workers indicated that the DN can also integrate memory-based information and generate top-down associative predictions for automated, fast and efficient decision making and plays a potential role in adaptive human cognition (Vatansever, Manktelow, Sahakian, Menon, & Stamatakis, Reference Vatansever, Manktelow, Sahakian, Menon and Stamatakis2016; Vatansever, Menon, & Stamatakis, Reference Vatansever, Menon and Stamatakis2017). These features implicate the DN in the symptoms of depression, such as rumination and the recurrent reflective focus on negative self-relevant information (Grimm et al., Reference Grimm, Ernst, Boesiger, Schuepbach, Hell, Boeker and Northoff2009; Hamilton, Farmer, Fogelman, & Gotlib, Reference Hamilton, Farmer, Fogelman and Gotlib2015; Nolen-Hoeksema, Reference Nolen-Hoeksema2000). Emerging evidence has indicated that abnormalities in DN FC play an essential role in the physiopathology of depression (Sheline et al., Reference Sheline, Barch, Price, Rundle, Vaishnavi, Snyder and Raichle2009; Yokoyama et al., Reference Yokoyama, Okamoto, Takagaki, Okada, Takamura, Mori and Yamawaki2018).
Our current findings demonstrated reductions in FC between the subgenual/perigenual ACC and PCC/precuneus in the MDD patients. The subgenual ACC is a prominent node of the limbic system subserving emotional responsiveness and regulation (Taylor et al., Reference Taylor, Martis, Fitzgerald, Welsh, Abelson, Liberzon and Gehring2006), while the perigenual ACC is centrally implicated in social cognitive functions such as mentalizing and self-reflection (Amodio & Frith, Reference Amodio and Frith2006). The DN also extends into these two regions since they are spatially close to the anterior medial prefrontal cortex (amPFC) (Connolly et al., Reference Connolly, Wu, Ho, Hoeft, Wolkowitz, Eisendrath and Paulus2013; Menon, Reference Menon2011; Smith et al., Reference Smith, Fox, Miller, Glahn, Fox, Mackay and Laird2009). According to previous studies, the PCC/precuneus and amPFC are common midline cores of the DN that relate to people's self-relevant, affective decision making (Andrews-Hanna et al., Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner2010; Buckner et al., Reference Buckner, Andrews-Hanna and Schacter2008). Decreased FC between the subgenual/perigenual ACC and PCC may induce communication interruptions and lead to emotional regulation deficits in MDD. In addition, we found that FC between the subgenual/perigenual ACC and PCC was negatively associated with ATQ scores in the MDD patients. The ATQ is used to measure the frequency of automatic negative self-statements in depression (Hollon & Kendall, Reference Hollon and Kendall1980). A higher ATQ score indicates greater negative automatic thoughts and more severe depressive symptoms. Combined with our previously described results, this finding suggested that disrupted connectivity between the subgenual/perigenual ACC and posterior midline core of the DN (PCC/precuneus) may lead to a patient's lack of ability to regulate their negative self-relevant emotions and persistently high levels of depressive affections. These observations are in line with several previous studies. Connolly et al. found decreased FC between the subgenual ACC and the precuneus to be significantly associated with higher levels of depression in adolescent MDD individuals (Connolly et al., Reference Connolly, Wu, Ho, Hoeft, Wolkowitz, Eisendrath and Paulus2013). Brown et al. showed that weaker levels of subgenual ACC FC with the PCC, angular gyrus and dorsal prefrontal cortex were correlated with higher depressive symptoms (Strikwerda-Brown et al., Reference Strikwerda-Brown, Davey, Whittle, Allen, Byrne, Schwartz and Harrison2014).
Additionally, we found that the bilateral subgenual ACC had decreased FC with the pIPL and was negatively correlated with ATQ scores in the patients with MDD. The pIPL belongs to the MTL subsystem of the DN and is preferentially active when people make episodic decisions about their future (Andrews-Hanna et al., Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner2010). Medial line cores of the DN share functional properties of the MTL subsystem when constructing mental scenes or imagining the future and depend on similar neural machinery required for memory (Schacter, Addis, & Buckner, Reference Schacter, Addis and Buckner2007). Disrupted connectivity between the bilateral subgenual ACC and posterior parts of the MTL subsystem may lead to difficulty in mnemonic imagery-based processes and result in negative expectations for the future. In addition, a recent study by Vatansever et al. indicated that the DN can operate in an ‘autopilot mode’ to integrate memory-based information and to generate predictions under stable environmental contexts for automated and fast decision making (Vatansever et al., Reference Vatansever, Menon and Stamatakis2017). However, this efficient ‘autopilot mode’ may break down in MDD due to deceased connections between anterior regions (subgenual/perigenual ACC-related amPFC) and posterior regions (PCC and pIPL) of the DN and further lead to deficits in social interactions and a conscious sense-of-self. A dissociation of the anterior and posterior nodes of the DN has repeatedly been reported in MDD (Gotlib & Joormann, Reference Gotlib and Joormann2010), including a decrease in structural connectivity (Korgaonkar, Fornito, Williams, & Grieve, Reference Korgaonkar, Fornito, Williams and Grieve2014) and FC (Connolly et al., Reference Connolly, Wu, Ho, Hoeft, Wolkowitz, Eisendrath and Paulus2013; Sadaghiani & D'Esposito, Reference Sadaghiani and D'Esposito2014) between the subgenual ACC and posterior DN and decreased FC in the angular gyrus and IPL (Strikwerda-Brown et al., Reference Strikwerda-Brown, Davey, Whittle, Allen, Byrne, Schwartz and Harrison2014; Zhu et al., Reference Zhu, Wang, Xiao, Liao, Zhong, Wang and Yao2012). We also note that these posterior areas of the DN (PCC and pIPL), which have altered connections with the subgenual/perigenual ACC, highly overlap with abnormal regions previously reported in patients with MDD derived from our meta-analysis (Fig. 4). This result confirms the considerable role of the posterior areas of the DN in the physiopathology of depression.
In contrast to the decreased FC between the anterior and posterior parts of the DN, our results demonstrated increased FC within the anterior part of the dmPFC subsystem of the DN, specifically between the rostral ACC and dmPFC. These regions are related to self-referential decision making concerning the individual's present situation or mental state (Andrews-Hanna et al., Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner2010; Saxe, Moran, Scholz, & Gabrieli, Reference Saxe, Moran, Scholz and Gabrieli2006) and to inferring the mental states of other people (Amodio & Frith, Reference Amodio and Frith2006; Lombardo et al., Reference Lombardo, Chakrabarti, Bullmore, Wheelwright, Sadek, Suckling and Baron-Cohen2010). Since the dmPFC subsystem participates in making personal judgements by comparing with other people (D'argembeau et al., Reference D'argembeau, Collette, Van der Linden, Laureys, Del Fiore, Degueldre and Salmon2005), enhanced connectivity within the dmPFC subsystem may lead to a state of constant comparison with others and introspection about one's own mental state, especially related to evaluating one's present state. Our observations are consistent with several previous studies. By using independent component analysis, Zhu et al. demonstrated that increased FC in the ACC and dmPFC was associated with rumination and decreased FC in the posterior medial regions of the DN, including the PCC, precuneus and angular gyrus (Zhu et al., Reference Zhu, Wang, Xiao, Liao, Zhong, Wang and Yao2012). In an antidepressant treatment study, the anterior subnetwork of the DN showed persistent increased FC both before and after antidepressant treatment in MDD subjects (Li et al., Reference Li, Liu, Friston, Shen, Wang, Zeng and Hu2013).
Additionally, several previous studies have reported hypoactivity in the dorsal/rostral ACC and dlPFC (Davidson, Pizzagalli, Nitschke, & Putnam, Reference Davidson, Pizzagalli, Nitschke and Putnam2002; Pizzagalli, Peccoralo, Davidson, & Cohen, Reference Pizzagalli, Peccoralo, Davidson and Cohen2006; Roiser, Elliott, & Sahakian, Reference Roiser, Elliott and Sahakian2012). Since the dorsal/rostral ACC is thought to monitor processing conflicts that could disrupt performance and recruit the dlPFC to reallocate attentional resources (Fales et al., Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen and Sheline2008), abnormally enhanced FC with the DN and decreased FC with the dlPFC may induce cognitive control dysfunction in the dlPFC and influence the regulation of emotion processing in the affective network (including the amygdala and subgenual/perigenual ACC). The amygdala and subgenual/perigenual ACC are involved in mediating negative emotions such as fear and sadness. This may explain the abnormal connectivity of the subgenual/perigenual ACC and negative emotion bias in patients with MDD.
Our results also revealed reduced connectivity between the caudal ACC and SPL. Their FC was negatively associated with HAMA scores in the MDD patients. Since the caudal ACC is mainly involved in the cingulo-opercular network (Sadaghiani & D'Esposito, Reference Sadaghiani and D'Esposito2014), the interaction between the caudal ACC and SPL (which belongs to the dATN) may relate to the hierarchical flow of information within the sensory-motor pathway (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). Our results are in line with a previous network-based statistical study that found large-scale hypoconnectivity in MDD, including in the attention network, the somatosensory network and the DN. Specifically, reduced intra-network connectivity of the dATN was significantly correlated with a longer duration of depression (Sacchet et al., Reference Sacchet, Ho, Connolly, Tymofiyeva, Lewinn, Han and Frank2016). This abnormal connectivity in parieto-occipital regions is thought to be related to biases in attention and inhibitory control of irrelevant sensory or internally generated information (Gotlib & Joormann, Reference Gotlib and Joormann2010).
There is a limitation that deserves mention. The meta-analysis in this study was performed using Neurosynth, which automatically generates activity maps based on the given search terms and pre-extracted coordinates from articles. Although it is convenient and useful for large-scale analyses, the specificity of this method is somewhat less than manual meta-analytic approaches.
In summary, the present study examined the rsFC of ACC sub-regions in first-episode medication-naïve patients with MDD. Compared with the healthy controls, the MDD patients demonstrated decreased subgenual/perigenual ACC-PCC/precuneus, subgenual ACC-pIPL, and caudal ACC-SPL connectivity and increased rostral ACC-dmPFC connectivity, suggesting dysfunctional interactions between midline cores (amPFC and PCC) and subsystems (MTL and dmPFC) of the DN. Additionally, the current results revealed that these abnormal levels of FC were associated with depressive symptom severity, further indicating that depression might arise from abnormal functional interactions between brain regions in the DN. These findings may provide new insights into the roles of specific sub-regions of the ACC and the DN in the pathophysiology of MDD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720000434
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 81730049; 61473221; 31671144] and Hunan Province Natural Science Foundation of China [grant numbers 2019JJ40362].
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.








