Introduction
Although it is the most isolated continent on Earth, Antarctica is not free of pollution. Fishing, tourism and research activities bring thousands of people, boats and various environmental impacts to specific sites and coastal areas of the continent every year. Antarctica also receives continuous continent-wide deposition of windborne pollutant-containing particles (Sheppard et al. Reference Sheppard, Claridge and Campbell2000). Among substances harmful to the environment, heavy metals merit special attention because of their high toxicity to organisms.
Some researchers have already documented contamination sources related to hydrocarbons (Ferguson et al. Reference Ferguson, Franzmann, Revill, Snape and Rayner2003) and heavy metals (Sheppard et al. Reference Sheppard, Claridge and Campbell2000, Santos et al. Reference Santos, Silva-Filho, Schaefer, Albuquerque-Filho and Campos2005, Townsend et al. Reference Townsend, Snape, Palmer and Seen2009), introduced by human activity. Sheppard et al. (Reference Sheppard, Claridge and Campbell2000) analysed some areas near Scott Base, Antarctica, and at least one sample in each area had Pb levels above the background levels of the site. This was attributed to battery leaks and paint waste containing the metal. Santos et al. (Reference Santos, Silva-Filho, Schaefer, Albuquerque-Filho and Campos2005) studied soils and coastal sediments of Admiralty Bay, King George Island, and found that house paint and oil were the main sources of heavy metals in sediments. Soils collected near the Commandante Ferraz station on King George Island had low Pb levels (11.5 mg kg-1). In the sub-Antarctic, soils from Atlas Cove, Heard Island, showed contamination by heavy metals around the old station but not at levels likely to produce a significant potential toxic impact on local ecosystems (Stark et al. Reference Stark, Gardner, Snape and McIvor2003). At Casey Station Cunningham et al. (Reference Cunningham, Raymond, Snape and Riddle2005) found a strong relationship between metal concentrations in soil and water and the composition of diatom communities. The Polar Regions are different to other parts of the world, for example, toxicology data using local species, are not available for the Antarctic and setting of triggers and targets are particularly significant in remote regions, where the costs of site clean-up may be orders of magnitude greater than in more populated parts of the world (Snape et al. Reference Snape, Riddle, Filler and Willians2003).
No studies to date have examined the relationship between the composition of Antarctic soils and their adsorption of heavy metals. Studies focusing on adsorption of pollutants in different soil constituents can provide information about their interactions with soil colloids and their partition among various soil constituents (Appel et al. Reference Appel, Ma, Rhue and Reve2008). Such data can help to predict the degree of vulnerability of Antarctic soil in response to the growing human occupation.
The vulnerability of the environment is directly proportional to the capacity of a soil for retaining pollutants. Environmental risks become greater when the amount of heavy metals exceeds the adsorptive capacity of the soil, which leads to leaching and groundwater contamination. Among the tools used to evaluate the adsorption of heavy metals in soils are the Langmuir and Freundlich isotherms (Sinegani & Araki Reference Sinegani and Araki2010). Adhikari & Singh (Reference Adhikari and Singh2003) studied the Pb and Cd adsorption capacity of different Indian soils and found maximum adsorption ranging from 62.5–123.4 mg kg-1 from the Langmuir equation. The authors suggested that the most important factors related to sorptive capacity of soils were cation exchange capacity, pH, organic matter, clay and CaCO3 content, presenting positive statistical correlations between these parameters and adsorption data.
Sinegani & Araki (Reference Sinegani and Araki2010) evaluated Pb adsorption in soils from temperate and semi-arid regions of Iran and observed a good fit of the experimental data to the Langmuir and Freundlich isotherms. The concentrations added to the soils ranged from 2–150 mg l-1, and the values of maximum adsorption estimated by the Langmuir equation ranged from 23.8–39.4 mmol kg-1. Higher levels of adsorption were observed in temperate than in semi-arid soils, as result of high clay content, cation exchange capacity, organic matter and Fe and Al crystalline and Al amorphous minerals in this environment (Sinegani & Araki Reference Sinegani and Araki2010).
Because of the adverse formation conditions, which include low temperatures and low availability of liquid water, Antarctic soils are rich in poorly crystalline minerals (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006) such as allophane and imogolite. These minerals have high specific surface area and high pH-dependent surface charge density (due to aluminol (Al-OH) and silanol (Si-OH) groups), which may determine the high adsorption capacity of these colloids.
Thus, the objective of this study was to estimate the maximum adsorption capacity of Pb in the clay fraction of two soil profiles associated with ornithogenic activities from King George Island, using the Langmuir isotherm. Correlations between adsorption parameters estimated by the isotherm and mineralogical soil attributes were also calculated.
Material and methods
Study area and soil sampling and characterization of two soil profiles
The study was carried out on the Fildes Peninsula, the largest ice-free area of King George Island, South Shetland Islands, Maritime Antarctica (Fig. 1). At each site pits were dug to bedrock or to the permafrost table (Table I) and soil in all genetic horizons sampled. Collected soils were air-dried and sieved at 2-mm to obtain fine earth for further analysis.

Fig. 1 Location of the study site on the Fildes Peninsula of King George Island (large map) and its location in Antarctica (inset).
Table I Overview and clay and total organic carbon (TOC) contents of soil profiles from Fildes Peninsula, Maritime Antarctica.

1Structure: type (L = lumps, SG = simple grains, AB = angular blocks, SB = subangular blocks, Ma = massive); development degree (S = strong, M = moderate); size (Sm = small, VS = very small, Me = medium).
2Dry consistency (So = soft, SH = slightly hard, H = hard); wet consistency (VF = very friable, Fr = friable, St = steady); plasticity (Pl = plastic, NPl = non plastic, SPl = slightly plastic); stickiness (S = sticky, NS = not sticky, SS = slightly sticky).
These soil were developed from andesitic basalts or moraines formed by similar volcanic rock fragments related to the advances and retreats of the Collins Glacier. Soils were moderately drained and erosion was not apparent, with gentle relief and slopes of less than 5%. Soils were classified using the World Reference Base of Soil Resources (ISSS 1998) (Table I). Clay content (pipette method - Gee & Bauder Reference Gee and Bauder1986) and total organic carbon content (wet combustion method - Yeomans & Bremner Reference Yeomans and Bremner1988) were determined in soil samples (Table I).
Clay fraction characterization
Forty grams of < 2 mm air-dried soil samples were shaken in 100 ml of pH 10.0 de-ionized water (1 g of Na2CO3 in 10 l of de-ionized water) using an orbital shaker at 120 oscillations min-1. We avoided using basic substances, such as 0.2 M NaOH, because of the predominance of amorphous mineral material in the Antarctic soils (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006). The sand fraction was retained in a 0.053-mm mesh sieve, and silt and clay fractions collected in 1000 ml cylinders and separated by sedimentation (Gee & Bauder Reference Gee and Bauder1986). Due to the small amount of clay (Table I), this procedure was repeated an average of ten times to obtain enough clay for all analytical procedures. Untreated clay samples were subjected to the following sequential extraction (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006): 0.1 M sodium pyrophosphate (PYR) (Dahlgren Reference Dahlgren1994), 0.2 M ammonium oxalate (AO) (Schwertmann Reference Schwertmann1973), 0.5 M NaOH (Jackson et al. Reference Jackson, Lim and Zelazny1986, modified by Melo et al. Reference Melo, Schaefer, Novais, Singh and Fontes2002).
The Al, Fe and Si concentrations were determined by inductively coupled plasma - atomic emission spectrometry (ICP-AES) in a Perkin Elmer, Optima 3300 DV model, with axial vision, radio frequency power of 1300 W and 40 MHz, plasma gas flow of 15 l min-1 and auxiliary gas flow of 0.7 l min-1. The wavelengths used were (nm): Al - 308.2, Fe - 238.2 and Si - 251.6. The results were converted to percentages of Al, Fe and Si oxides (Table II).
Table II Contents of oxides obtained by sequential extractions with sodium pyrophosphate (PYR), ammonium oxalate (AO) and NaOH in the clay fraction of soils from the Fildes Peninsula, Maritime Antarctica.

1SUM = sum of the contents of Al, Fe and Si oxides of the corresponding extraction.
2TCMR = total clay mass removal by sequential treatments with PYR, AO and NaOH, following the method presented by Simas et al. (Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006).
The choice of this sequential extraction method was to characterize quantitatively and chemically the amorphous minerals present in high concentration in the clay fraction of Antarctic soils (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006), since the X-ray diffraction (XRD) is not, by itself, able to provide numerical data which can be analyzed quantitatively. Such extraction allowed us to divide the different phases of amorphous minerals: linked to the organic matter (extracted by PYR); Fe, Al and Si oxides (extracted by AO) and aluminosilicates (extracted by NaOH). Classical methods of sequential analysis, such as Tessier (Tessier et al. Reference Tessier, Campbell and Bisson1979), are not used for mineralogical characterization but for partial extraction of the crystalline minerals, in studies of speciation of the total content of heavy metals from soil and clay samples. In these classical methods, each sequential step only determines different forms of heavy metals (soluble, exchangeable, carbonates, organic matter, Fe and Mn oxides, and residual).
Approximately 0.5 g of untreated clay and part of the residue of sequential extractions (PYR, AO and NaOH) was used for mineralogical characterization of samples by XRD (powder method). X-ray diffraction patterns were obtained in a diffractometer with a vertical goniometer, using an angular velocity of 0.5° 2θ min-1, range 2–50° 2θ, equipped with a Cu tube and a Ni filter. The X-ray tube was operated at 20 kV and 40 mA. Differential XRD (DXRD) was obtained by subtracting the patterns of the clay treated with PYR and AO and also by subtracting the patterns of AO and NaOH (Dahlgren Reference Dahlgren1994).
To achieve differentiation of 2:1 phyllosilicate minerals, such as smectite, vermiculite and chlorite, samples of untreated clay (Whittig & Allardice Reference Whittig and Allardice1986) were subjected to the following treatments: Mg saturation, Mg saturation and ethylene glycol solvation, K saturation and air drying, K saturation and drying at 550°C. After these treatments, samples were mounted on glass slides (oriented samples) and analysed by XRD in a range from 3–15° 2θ. Mineralogical clay composition is presented in Table III.
Table III Mineralogical composition of the clay fraction determined by X-ray diffraction (XRD) and differential X-ray diffraction (DXRD) of soils from the Fildes Peninsula, Maritime Antarctica.
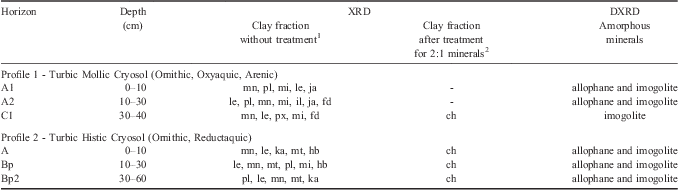
1ch = chlorite, ka = kaolinite, fd = feldspar, hb = hydrobiotite, il = ilite, ja = jarosite, mt = metavariscite, mn = minyulite, le = leucophosphite, pl = plagioclase, px = pyroxene, mi = mica. The ordering of the minerals in the clay sample without treatments followed the decreasing intensity of their reflections by XRD.
2Treatment for identifying 2:1 clay minerals: Mg saturation and ethyleneglycol solvation, K saturation and heating (Whittig & Allardice Reference Whittig and Allardice1986).
Pb adsorption in the clay fraction
The clay samples used in the Pb adsorption experiment were standardized by the following procedures: pH adjusted to 6.0, freeze-dried by lyophilisation, and sieved through a 0.053-mm sieve. Samples of 0.3 g of clay was used, in duplicate, in polyethylene (PET) tubes. The samples were suspended in 15 ml of 0.001 M Na(NO)3 (used as a support electrolyte) and ten Pb concentrations in the form of Pb(NO)3: 0, 200, 1000, 2000, 5000, 10 000, 20 000, 30 000, 40 000, and 60 000 mg kg-1 (Dias et al. Reference Dias, Alleoni, Casagrande and Camargo2001, with modifications). Preliminary tests indicated that the high doses used in this experiment were necessary.
In summary, the factorial arrangement was as follows: four samples were analysed (two horizons of profile 1 and two horizons of profile 2) using, for this case, two PET tubes (i.e. two replicates) and ten concentrations, following a strict analytical control to prevent contamination between samples and to ensure the reliability of results. In total, analysis was made of 80 clay samples.
The tubes were shaken in an orbital shaker at 120 oscillations min-1 for 24 hours at 22°C. The contents of the tubes were centrifuged at 24 456 xg for 10 min. Pb concentrations were determined by atomic absorption spectrophotometry, in a Varian AA240FS model, with wavelength range of 185–900 nm, focal length of 250 mm and wavelength slew rate of 2000 nm min-1. The adsorbed concentration was calculated based on the amount of Pb (in mg) added to each sample, on the amount of Pb in solution (equilibrium concentration) and on the mass of the sample used in the experiment. To obtain the adsorption parameters we used the Langmuir isotherm: x = (K C b) / (1 + K C), in which x is the amount of Pb adsorbed per unit of mass of clay, C is the concentration of Pb in equilibrium solution, b is the maximum adsorption, and K is affinity constant. The contents of Al2O3, SiO2 and Fe2O3 obtained from sequential extractions, and the total mass of the clay fraction removed by extraction with PYR, AO and NaOH were correlated with the Langmuir isotherm parameters (K and b) using the program Statistica for Windows® (StatSoft 2007).
Results
Considering that PYR, AO and NaOH preferentially extract amorphous minerals, the levels of these minerals in the clay fraction should be between 376 and 705 g kg-1 (Table II). These contents were estimated by mass reduction of the clay samples after sequential treatments with PYR, AO and NaOH.
The maximum dose used in the adsorption experiment (60 000 mg kg-1) was not sufficient to attain the plateau in the isotherm for two samples, collected in the most superficial soil layers of two profiles (A1 and A). Since the horizons A1 and A of profiles 1 and 2, respectively, did not attain the plateau in the Pb adsorption, the results presented in this work do not show the results of these two horizons. For the other four soil samples, the coefficients of determination of Langmuir isotherms were higher than 0.9 (Figs 2 & 3).

Fig. 2 Pb adsorption on clay fractions in the a. A2, and b. C1 horizons of profile 1 from Fildes Peninsula, Maritime Antarctica.

Fig. 3 Pb adsorption on clay fractions in the a. Bp, and b. Bp2 horizons of profile 2 from Fildes Peninsula, Maritime Antarctica.
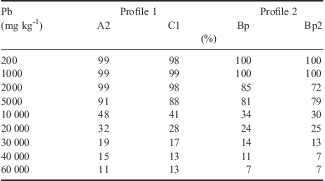
The highest percentages of Pb adsorption were found when low metal concentrations were added to the soil sample (Table IV). In profile 1, almost 100% of added Pb was adsorbed, up to the concentration of 2000 mg kg-1. On the other hand, only 7% of total added Pb remained adsorbed in Bp and Bp2 horizons (profile 2) at the highest concentration (60 000 mg kg-1).
Table IV Percentage of adsorbed Pb in relation to added concentrations in the clay fraction of soils from the Fildes Peninsula, Maritime Antarctica.
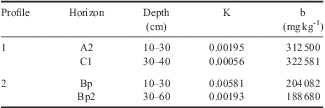
The values of maximum Pb adsorption (b parameter in the Langmuir equation) were extremely high, with a maximum value of 322 581 mg kg-1 to the C1 horizon of profile 1 (Table V). For the affinity constant (K) the values were higher for soil samples from the upper horizons of both profiles (0.00581 for Bp and 0.00195 for A2) (Table V).
Table V Langmuir constants (affinity constant (K) and maximum adsorption (b)) for Pb adsorption in the clay fraction of soils from the Fildes Peninsula, Maritime Antarctica.
A significant and positive correlation was observed between maximum Pb adsorption capacity and Fe2O3 and total oxides (Al2O3 + Fe2O3 + SiO2) extracted by PYR. Other clay mineralogical parameters did not correlate significantly with maximum adsorption and the affinity constant (Table VI). A high and positive correlation was also observed between Fe2O3 extracted by AO and b Langmuir parameter (r = 0.90), however, these data were not statistically significant due to the low number of observations in this work (n = 4).
Table VI Correlation coefficients of regression analysis between mineralogical attributes and the adsorption parameters K (affinity constant) and b (maximum adsorption) estimated by the Langmuir isotherms of soils from the Fildes Peninsula, Maritime Antarctica. Values and meanings of mineralogical parameters are presented in Tables II & III.

*Significant at 5%.
PYR = sodium pyrophosphate, AO = ammonium oxalate, ns = not significant.
Samples with higher concentrations of Fe-AO had the lowest levels of Si-AO (Table II), resulting in negative correlations between these soil attributes (Table VI) and between Si-AO levels and maximum Pb adsorption values (r = -0.93; Table VI).
Discussion
The two profiles can be considered a good indication of soil behaviour in the case of a Pb contamination in the selected area. Michel (Reference Michel2011) studied the morphology of 50 soil profiles scattered over the Fildes Peninsula and only 10% showed any difference in the parent material observed here (andesitic basalts related to the advances and retreats of the Collins Glacier), with the influence of sulfide-bearing in the andesites. The formation of almost 50% of these soils was affected by ornithogenic activities, a condition also observed in the two profiles in the present study. In Maritime Antarctica ornithogenic activity (mainly penguins) accelerates soil genesis, formation of clay and poorly crystalline minerals and induces the process of phosphatization and precipitation of crystalline phosphates (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006).
Some authors have observed a similarly good fit of the Langmuir isotherm to adsorbed Pb in different soils (Veeresh et al. Reference Veeresh, Thipathy, Shaudhuri, Hart and Powell2003, Zuhairi Reference Zuhairi2003, Serrano et al. Reference Serrano, Garrido, Campbell and García-González2005). The progressive decrease in the percentages of Pb adsorption (Table IV) as a response to increasing rates of added metal is related to continuous saturation of the binding sites of colloids. Other authors have reported the same behaviour for adsorption of heavy metals in Indian soils (Dutta & Singh Reference Dutta and Singh2011) and in amorphous iron oxyhydroxide (Benjamin & Leckie Reference Benjamin and Leckie1981). Dutta & Singh (Reference Dutta and Singh2011) observed that when more Pb is available in solution, the amount of Pb adsorption increased too. At the same time, the percentage of Pb adsorbed decreased because the quantity of binding sites decreases.
The results for maximum Pb adsorption (Table V) highlight the high cation adsorptive capability of the clay fraction of analysed soils from Antarctica. Adhikari & Singh (Reference Adhikari and Singh2003) used Pb concentrations ranging from 0–90 mg l-1 in 24-hour equilibrium experiments and two temperatures (24 and 45°C) in soils from five different and representative agro-ecological zones in India and found Pb maximum adsorption (b Langmuir values) ranging only from 62.5–123.4 mg kg-1. The larger b value was observed in a Vertic Ustochrept Soil (USDA Soil Taxonomy Classification), which the authors associated with soil physical properties, such as pH, cation exchange capacity and organic matter content.
The Pb adsorption capacity of the clay fraction of these ornithogenic soils (Table V) is very favourable from an environmental standpoint because it represents a high capacity for filtering heavy metals, which may compensate for the low levels of this colloidal fraction in the soils (≤ 200 g kg-1; Table I). It may also reduce the potential of human activities in Antarctica for contaminating groundwater in the studied soils. Simas et al. (Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006) worked with soil profiles scattered in King George Island, and found lower concentration of amorphous minerals extracted with the same methods (PYR, AO and NaOH) in basaltic non-ornithogenic soils (mean of 198 g kg-1) in relation to ornithogenic ones (mean of 566 mg kg-1, close to the present study value of 533 mg kg-1; Table II). These data indicate that the non-ornithogenic soils in Antarctica must have a lower potential for heavy metal adsorption and other studies must be done to confirm this tendency. The main problem in Antarctica in relation to Pb leaching must be the soils formed almost exclusively by physical weathering. Most soils studied by Navas et al. (Reference Navas, López-Martínez, Casas, Machín, Durán, Serrano, Cuchi and Mink2008) have their development mainly associated with physical weathering and cryogenic processes causing the rock to disintegrate. In addition to various mineralogical constituents, other characteristics important for the retention of pollutants in soil are, for example, the presence (or absence) of vegetation and the level of organic matter.
Sequential extraction allowed the quantification of amorphous minerals in different phases, such as those linked to organic matter (extracted by PYR); Fe, Al and Si oxides (extracted by AO) and aluminosilicates (extracted by NaOH) (Table II). Differential X-ray diffraction was also effictive at detecting allophane and imogolite in the clay fraction (Table III). The high contents of these minerals phases in Antarctic soils highlights the differences in pedogenetic conditions, for example, in comparison to humid tropics. Melo et al. (Reference Melo, Schaefer, Novais, Singh and Fontes2002) studied clay samples of Bw horizons of Oxisols from different regions of Brazil, using a sequential extraction procedure similar to the present study, and found that the content of amorphous minerals estimated by AO and 0.5 mol l-1 NaOH amounted to only 36 g kg-1. The allophane and imogolite detected by DXRD in the clay fraction (Table III) have high specific surface area and a high density of hydroxyl groups (Al-OH and Si-OH) whose surface charge is pH dependent (Perrot Reference Perrot1977).
Some work carried out on Fildes Peninsula and near that area, suggests that the clay content is relatively low (Jie et al. Reference Jie, Zitong and Blume2000) and the origin of the clay minerals is generally more from physical weathering than from chemical weathering, although the climate is warmer and more humid than other regions of Antarctica (Jeong & Yoon Reference Jeong and Yoon2001). The major classes of soil minerals in Fildes Peninsula, generally, are smectite, chlorite and interstratified illite-smectite and chlorite-smectite (Jeong et al. Reference Jeong, Yoon and Lee2004). However, these studies did not use specific chemical or physical methods to study amorphous minerals in the clay fraction.
The correlations between maximum adsorption capacity and total oxide contents extracted by PYR (Table VI) highlight the importance of amorphous metal-complex organic matter extracted by Na pyrophosphate (Dahlgren Reference Dahlgren1994) in heavy metals adsorption. The high positive correlation found between the levels of Fe oxide extracted by AO and maximum Pb adsorption was due to the high specific surface area of the oxides extracted by AO (levels ranging between 29.6 and 51.9 g kg-1; Table II). The zero point of charge (ZPC) of such oxides is high, ranging from 7–9 (Schwertmann & Taylor Reference Schwertmann and Taylor1989), and the predominance of protoned ferrol groups (-FeOH-0,5 and -FeOH2+0,5) favours the isotopic exchange of H and H2 by Pb(OH)+ and Pb2+ (chemisorption or specific adsorption) (Backers et al. Reference Backers, McLaren, Rate and Swift1995). The pH of the clay samples was adjusted to 6.0 before the Pb adsorption experiment (pH less than ZPC of the oxide). The negative correlation between the maximum adsorption and SiO2-AO (Table VI) was attributed to low contents of this oxide (Table II), which means lower formation of silanol groups (Si-OH) and the formation of negative charge for non-specific adsorption of Pb.
According to the Langmuir isotherm, the adsorption of ions to soil colloids occurs as a monolayer on the surface and maximum adsorption occurs when the surface is completely covered (Fontes & Alleoni Reference Fontes and Alleoni2006). However, adsorption may have exceeded the double diffuse layer due to the large number of adsorption sites (high density of surface negative charge and high specific surface area of amorphous minerals), so that the stoichiometry between the negative charge of colloids and the positive charges of Pb2+ or Pb(OH)+ was not achieved.
Regarding the affinity constant (K value of the Langmuir isotherm), some researchers have observed a strong affinity between Pb and Cd (Serrano et al. Reference Serrano, Garrido, Campbell and García-González2005) and Cs (Campbell & Davies Reference Campbell and Davies1995) and soil samples from superficial horizons, as observed in the present work for Pb in both soil profiles (Table V). In the clays of profile 1, due to the higher adsorption capacity in relation to profile 2, adsorption may have occurred in more layers of Pb around the particles, which increased the maximum adsorption (b value), but reduced the energy or adsorption affinity (K value) (Table V). One piece of evidence for this behaviour was the opposite signs of the coefficients of correlation between K and b values and the mineralogical parameters. For example, once the occurrence of complexes of Fe-organic matter (Fe2O3-PYR) was greater, maximum adsorption increased (r = 0.98) and binding affinity decreased (r = -0.74) (Table VI). Appel & Ma (Reference Appel and Ma2002) also observed that the amount of Pb sorbed was much larger than the amount of negative surface charge for samples of an Oxisol, suggesting both inner- and outer-sphere reactions. According to the authors, Pb had a higher affinity for soil sorption sites, as confirmed by its ability to take part in inner-sphere surface reactions, and the adsorption of Pb was divided into two steps. In the first step, Pb was retained by specific forces, such as chemisorption. In the second step, the remaining Pb was retained by simple electrostatic attraction in the diffuse double layer. In the present study, the following groups present in the amorphous minerals must have improved specific adsorption: aluminol (-AlOH) in the allophane and imogolite, ferrol (-FeOH) in the Fe oxides (AO extraction), aluminol in the aluminosilicates (NaOH extraction) and carboxylic and phenolic in the organic matter (PYR extraction).
Veeresh et al. (Reference Veeresh, Thipathy, Shaudhuri, Hart and Powell2003) and Serrano et al. (Reference Serrano, Garrido, Campbell and García-González2005) observed an inflection point in their adsorption data of Pb, Cd and Ni, with two clearly defined stages. In the first phase, a rapid adsorption at low metal concentrations was found in sites with strong affinity for the element (chemisorption) and high K Langmuir value. This was followed by a phase at high metal concentrations, in which the adsorption occurred on low-energy sites (reversible adsorption). In samples from all soil horizons of the present study, a significant decrease (about 50%) was observed in the percentage of Pb adsorption as a response to increasing concentration of the metal from 5000 to 10 000 mg kg-1 (Table IV). Specific Pb adsorption in the soil colloids reflects lower environmental risk, since this stronger binding makes Pb desorption and the contamination of groundwater less likely. In Antarctica, ornithogenic activity (penguins and skuas) accelerates soil genesis and the formation of clay and poorly crystalline minerals (Simas et al. Reference Simas, Schaefer, Melo, Guerra, Saunders and Gilkes2006) and should result in environments that are less vulnerable to leaching of contaminants such as Pb.
Conclusions
The Langmuir isotherm was a suitable tool for analysis and the values of maximum Pb adsorption of the clay fractions of the ornithogenic soils were very high compared to the other regions of the planet, making Antarctica a unique environment. This behaviour reflected the richness of the clay fraction in amorphous minerals.
It is clear that Pb adsorption occurs in more than one stage: the first when a higher content is adsorbed on the clay fraction and the second when the percentage decreases, until stabilization. The adsorption in the first stage is more environmentally positive, since this stronger binding makes Pb desorption less likely.
From an environmental point of view, soils rich in amorphous minerals, usually associated with ornithogenic activity in Antarctica, should have greater efficiency in filtering Pb, thus reducing Pb leaching and groundwater contamination. Further studies must be done in order to check this behaviour in non-ornithogenic Antarctic soils, which normally are less developed and have lower amorphous minerals in the clay fraction. Only after study of a great number of soil profiles in a specific Maritime Antarctic region, such as Fildes Peninsula, will it be possible to predict the degree of vulnerability of those soils in response to human occupation.
Acknowledgements
We are grateful to the Brazilian National Research and Technology Council (CNPq) and to the Coordination for the Improvement of Higher Level Personnel (CAPES) for financing this research. We are also grateful to the Brazilian Navy and the Brazilian Antarctic Program for overseeing logistics during the Antarctica expedition. The constructive comments of the reviewers are also gratefully acknowledged.











