Introduction
Plant resistance to pests and diseases often occurs as the expression of a variety of innate or constitutive defence systems, triggered by the presence of certain plant resistance (R) genes. For instance, the cloned gene Mi-1 confers resistance to many tomato Lycopersicon esculentum cultivars against the three most common and damaging species of root-knot nematodes, Meloidogyne incognita, M. javanica and M. arenaria (Roberts & Thomason, Reference Roberts and Thomason1986); the potato aphid Macrosiphum euphorbiae (Rossi et al., Reference Rossi, Goggin, Milligan, Kaloshian, Ullman and Williamson1998); and the B and Q biotypes of the main whitefly vector of plant viruses, Bemisia tabaci (Nombela et al., Reference Nombela, Williamson and Muñiz2003).
In addition to this constitutive resistance, plants can activate protective mechanisms against a pest or pathogen upon contact with a previous invader. This is termed induced or acquired resistance, and it can be systemically expressed (systemic acquired resistance, or SAR, and induced systemic resistance, or ISR) or confined only to previously infested plant parts (local acquired resistance, or LAR). Although there is wide variation in the terminology, it is currently agreed by a good part of the research community that induced resistance is the general term by which all types of elicited responses that lead to enhanced protection against disease can be designated (Hammerschmidt et al., Reference Hammerschmidt, Métraux and van Loon2001). In addition, this term is used to denote reduced plant damage by herbivorous insects after a previous attack, when plant-insect interactions are considered (Hammerschmidt et al., Reference Hammerschmidt, Métraux and van Loon2001). Some biological agents, such as certain bacteria, fungi or viruses, can induce plant resistance to other pathogens (Agrawal et al., Reference Agrawal, Tuzun and Bent1999; Hammerschmidt et al., Reference Hammerschmidt, Métraux and van Loon2001; Siddiqui & Shaukat, Reference Siddiqui and Shaukat2004). Similarly, positive and negative associations as a result of cross-talk between insect- and pathogen-induced defence pathways have been widely reported (reviewed by Hunter, Reference Hunter2000), including the induction of plant resistance to insects due to a previous attack by the same or another organism. More specifically, over 100 plant species have been found to respond to past or current herbivory by increasing their resistance to herbivores (Karban & Baldwin, Reference Karban and Baldwin1997). In these interactions, the initial attack acts as a cue to predict a risk of future herbivory for the plant, which changes its defensive phenotype to produce reduced fitness and/or preference of insect feeding (Karban et al., Reference Karban, Agrawal, Thaler and Adler1999). On the contrary, there may also be opposing effects induced by a previous infestation, making the plant more attractive and/or susceptible to other herbivores (Prado & Tjallingii, Reference Prado and Tjallingii1997; Thaler et al., Reference Thaler, Stout, Karban and Duffey2001).
There are results to support that induced resistance could be attributable to changes in the emission of volatile compounds by plants previously infested by insects. Infestation of cotton by leaf chewing beet armyworm, Spodoptera exigua, strongly induced plant volatile emission, whereas infestation with whitefly B. tabaci (B-biotype) did not induce volatile emissions (Rodríguez-Saona et al., Reference Rodríguez-Saona, Crafts-Brandner and Cañas2003). Whereas many of these compounds attract natural enemies of the herbivores responsible for the damage, other plant volatiles are known to elicit priming mechanisms which deter insects directly (reviewed by Hunter, Reference Hunter2002; Engelberth et al., Reference Engelberth, Alborn, Schmelz and Tumlinson2004; Heil & Kost, Reference Heil and Kost2006).
Available information on induced resistance to arthropods mostly refers to chewing herbivores, which usually cause extensive leaf damage to infested plants. A less studied phenomenon is the induction of plant resistance to/by phloem-feeding insects, such as whiteflies or aphids, which maintain a longer interaction with their host plant but cause only limited direct damage to the plant tissues with their stylets (Walling, Reference Walling2000). Most reports on phloem-feeding insects have focused on induced responses (positive or negative) to aphids, acquired by the plant upon a previous infestation by the same or another aphid species (Wool & Hales, Reference Wool and Hales1996; Quiroz et al., Reference Quiroz, Petterson, Pickett, Wadhams and Niemeyer1997; Messina et al., Reference Messina, Taylor and Karren2002; Sauge et al., Reference Sauge, Lacroze, Poëssel, Pascal and Kervella2002), among other biotic or abiotic inducers. However, little is known to date about plant responses to whiteflies induced after previous attacks by arthropods or by other inducer factors (Inbar et al., Reference Inbar, Doostdar, Leibee and Mayer1999; Agrawal et al., Reference Agrawal, Karban and Colfer2000; Mayer et al., Reference Mayer, Inbar, Mckenzie, Shatters, Borowicz, Albrecht, Powell and Doostdar2002; Murugan et al., Reference Murugan and Dhandapani2006). In a recent study from our laboratory, it was reported that treatment with Benzothiadiazole (BTH), which mimics the biological activation of SAR by necrogenic pathogens (Kunz et al., Reference Kunz, Schurter and Maetzke1997) and induces resistance in different cultivated plants against a broad spectrum of fungal, bacterial and viral diseases (Oostendorp et al., Reference Oostendorp, Kunz, Dietrich and Staub2001; Smith-Becker et al., Reference Smith-Becker, Keen and Becker2003), also induced local resistance in tomato to both B and Q biotypes of B. tabaci (Nombela et al., Reference Nombela, Pascual, Aviles, Guillard and Muñiz2005). Previously, it was demonstrated that BTH induced resistance in tomato to M. euphorbiae (Cooper et al., Reference Cooper, Jia and Goggin2004), which suggests the possible existence of overlap between plant defences against these two insect pests.
Several bioassays were carried out under controlled conditions in the present work to test if resistance to whitefly B. tabaci could be induced in susceptible tomato plants (lacking the Mi-1 gene) by a previous infestation with the potato aphid M. euphorbiae. Moreover, the question whether the induced response to B. tabaci would be local or systemically expressed in other parts of the plant was also addressed. Conversely, other assays were performed to evaluate the effect of a preinfestation with B. tabaci on the tomato responses to two different aphid clones of M. euphorbiae.
Materials and methods
Plant material
Tomato plants (L. esculentum cv. Marmande) were used in this study. Marmande plants lack the Mi-1 gene which is responsible for innate resistance to both B. tabaci and M. euphorbiae, so this cultivar is highly susceptible to these insect pests. Tomato seeds were germinated in a climatic chamber maintained at a day:night temperature regime of 26:20°C and a photoperiod of 16:8 h L:D. Plants were grown in vermiculite in one-litre plastic pots irrigated every ten days with a 3 gr l−1 solution of the nutritive complex 20-20-20 (Nutrichem 60; Miller Chemical, Hanover, PA, USA) and with tap water when needed in the meantime. One-month-old plants were used in all assays and, at this stage, plants had six or seven fully expanded true leaves.
Insect populations
Adult female whiteflies (B-biotype of B. tabaci) were used in this study. A population had been initially obtained from cucumber plants and, after several years, it was transferred to tomato cv. Marmande where it has been reared for more than 50 generations.
A potato aphid clone (M. euphorbiae) was established from a single virginoparous aptera female collected in 1999 on lettuce in Villa del Prado, Madrid (Sp clone). The colony was reared on tomato plants cv. Marmande kept in cages at a day:night temperature of 22:16°C and a photoperiod of 14:10 h L:D. A second aphid clone (Nt clone) was obtained from potato plants in The Netherlands and reared on tomato cv. Marmande for six months. Only young adult aphids, one- to three-days old, were used for infesting tomato plants.
Tomato response to whiteflies: preinfestations with aphids
Three no-choice assays were performed in a growth chamber at a constant temperature of 24°C, a photoperiod of 16:8 h L:D and a relative humidity of 68–75%. For the first and second assay, each of ten tomato plants was infested with 20 apterous adult aphids confined in a transparent plastic cage (6.5×2 cm) attached to a leaf in a similar way as described by Fereres et al. (Reference Fereres, Lister, Araya and Foster1989). The leaf used was the upper-most fully expanded leaf of every plant. Another ten plants without aphids or cages were used as control. In addition, cages with no aphids were placed on four other plants of the first assay to test for the possible influence of the plastic cages on plant response. Aphids where kept on the plants for three days, and then adult aphids and laid nymphs were gently removed using a soft little brush. No dead aphids were observed on the plants. Plants of the first and second assays were maintained in the growth chamber for one hour or four days, respectively, until whitefly infestation.
A third assay was carried out under similar conditions but, in this case, 15 plants were infested for three days with 20 adults of M. euphorbiae in one leaf as previously described; and 15 other non-infested plants with empty cages were used as control. For this assay, 18 h passed between aphid removal and whitefly infestation.
Tomato response to whiteflies: whitefly infestations
Every preinfested or control plant from the first and second assays was covered with a transparent plastic cylinder (27 cm high, 12 cm diameter) with a thin polypropylene insect mesh attached by paraffin wax to the top and two other holes on the cylinder surface to allow ventilation. Five adult female whiteflies were released on the tomato plant inside the cylinder through another hole sealed with a small cork. For both assays, whiteflies were kept on the plants for 27 days, and then the numbers of third (L3) and fourth-stage (L4) nymphs and adult whiteflies on every plant were recorded. The number of adults was deduced from the observed number of empty pupal cases.
In the third assay (18 h after aphid removal), five adult female whiteflies were confined to a plastic truncated cone clip-cage (3.6 cm×2.6 cm diameter; 4 cm high) (Muñiz & Nombela, Reference Muñiz and Nombela2001). The clip-cage was attached to the same previously caged leaf of every aphid-infested or control plant, such that whiteflies had access only to the abaxial surface of the leaf. In addition, to test if plant resistance was systemically acquired (SAR), another similar clip-cage with five whiteflies was attached to the contiguous upper leaf of every plant. Five days later all female whiteflies and clip-cages were removed from the plants, and the corresponding leaflets were marked with small paper rings attached to their petioles. Eggs were allowed to develop for 22 more days, and then the numbers of L3, L4 and adult whiteflies were counted as detailed for the other assays.
Data from aphid-infested and control plants or from infested and uninfested leaves were log10 (x+1) transformed and analyzed by a one-way ANOVA and means compared by the Tukey HSD test (Statgraphics, 1997).
Tomato response to aphids: preinfestations with whiteflies
Two no-choice assays were performed in a growth chamber under conditions similar to those previously used for the study of the tomato responses to whiteflies. Every tomato plant was covered by a transparent plastic cylinder (27×12 cm) as previously described. Ten and 27 covered plants were infested in the first and second assays, respectively, with ten whitefly adults (B-biotype). In all experiments, a similar number of covered but uninfested (without whiteflies) plants were used as controls. Whiteflies and cylinders were removed after 48 h.
Tomato response to aphids: aphid infestations
One hour after whiteflies were removed in the first assay, two adult winged aphids (Sp clone) were placed on the upper-most fully expanded leaf of every preinfested or control plant. The plants were covered by plastic cylinders. The adults were removed after a 96 h period and only two nymphs were maintained on the leaves. Ten and 20 days later, the total numbers of adults and nymphs per plant were counted.
Similar methodology was followed in the second assay. We used a different aphid clone (Nt clone). The adults were removed after 24 h and all nymphs born during this period were kept on the same leaf. After 11 days, the numbers of adults and nymphs were counted and only two adult aphids were kept on the plant. Counting was repeated 11 days later (the same time interval as the pre-reproductive period).
The numbers of adult aphids and nymphs per treatment were log10 (x+1) or ![]() transformed, then analyzed by a one-way ANOVA and means compared by the Tukey HSD or by the Mann-Whitney U test for data not adjusted to a normal distribution (Statgraphics, 1997).
transformed, then analyzed by a one-way ANOVA and means compared by the Tukey HSD or by the Mann-Whitney U test for data not adjusted to a normal distribution (Statgraphics, 1997).
Results
Response to B. tabaci induced by aphids
The averaged total numbers of individuals of B. tabaci per plant 27 days after whitefly infestation were significantly (F 1.18=6.72, P=0.02) lower on plants previously infested by M. euphorbiae than those observed on uninfested control plants when whiteflies infested the plants one hour after aphids were removed (fig. 1a). Moreover, differences between aphid-infested and control plants were significant for all three B. tabaci developmental stages: L3 (F 1.18=5.51, P=0.03), L4 (F 1.18=7.86, P=0.01) and newly emerged adults (F 1.18=5.64, P=0.03) (fig. 1a).

Fig. 1. Mean numbers of L3, L4 or adults (empty pupal cases) of B. tabaci (B-biotype) observed on the aphid-infested and control plants at 27 days after infestation with five female whiteflies. Aphids had been in contact with the plants for three days and were removed (a) one hour, (b) 18 hours or (c) four days prior to whitefly infestation. Lower case letters compare values for every life stage separately; capital letters compare the total numbers of whiteflies. Different letters on bars from the same graphic indicate significant (P<0*"/>, L4; ■, adults).
When infestation by B. tabaci occurred 18 h after aphids were eliminated from the plants, the total number of individuals (F 1.20=13.24, P=0.00) and the averaged number of adults (F 1.20=13.01, P=0.00) were significantly lower on preinfested than on control plants, but no significant differences in the numbers of L3 or L4 were detectable (fig. 1b). In contrast, no significant differences were detected between aphid-infested and control plants when whitefly infestation occurred four days after aphid removal (fig. 1c).
Moreover, control plants which had empty plastic cages hosted similar numbers of whiteflies to those plants without any aphid or cage on their leaves (data not shown). This indicates that using plastic cages to confine aphids in these assays did not affect subsequent whitefly infestation results.
Systemic response to B. tabaci induced by aphids
In the 18-h assay with the whiteflies confined into clip-cages, fewer adults (F 1.18=6.56, P=0.02) and total whiteflies (F 1.18=6.47, P=0.02) were counted on the upper leaf contiguous to the aphid-infested leaf of the preinfested plants than those found on the corresponding upper leaf, contiguous to the non-infested leaf, of the control plants (fig. 2a).

Fig. 2. Mean numbers of L3, L4 or adults of B. tabaci (B-biotype) observed at day 27 (a) on the leaf contiguous to the aphid-infested leaf of the aphid-infested plants or contiguous to the equivalent uninfested leaf of the control plants, and (b) on the aphid-infested and the uninfested contiguous leaves of plants previously infested by aphids. Aphids had been in contact with the plants for three days and were removed 18 h prior to whitefly infestation. Lower case letters compare values for every life stage separately; capital letters compare the total numbers of whiteflies. Different letters on bars indicate significant (P<0*"/>, L4; ■, adults).
In plants infested with B. tabaci 18 h after aphid removal, statistically significant differences in the averaged numbers of L3, L4, adult or total whiteflies were not detectable between the aphid-infested leaves and the uninfested contiguous leaves of the same plants (fig. 2b).
Responses to M. euphorbiae induced by whiteflies
In the first assay (aphid clone Sp), the mean number of nymphs per plant (Nph 1 per Pl) and per adult (Nph 1 per Ad 0) ten days after adult aphid removal were significantly higher than on uninfested control plants (fig. 3a). Twenty days after adult removal the differences between whitefly preinfested and uninfested plants disappeared (fig. 3a). On the contrary, in the second assay with aphid clon Nt, no statistically significant differences were detected at any time point on the parameters analyzed (fig. 3b).
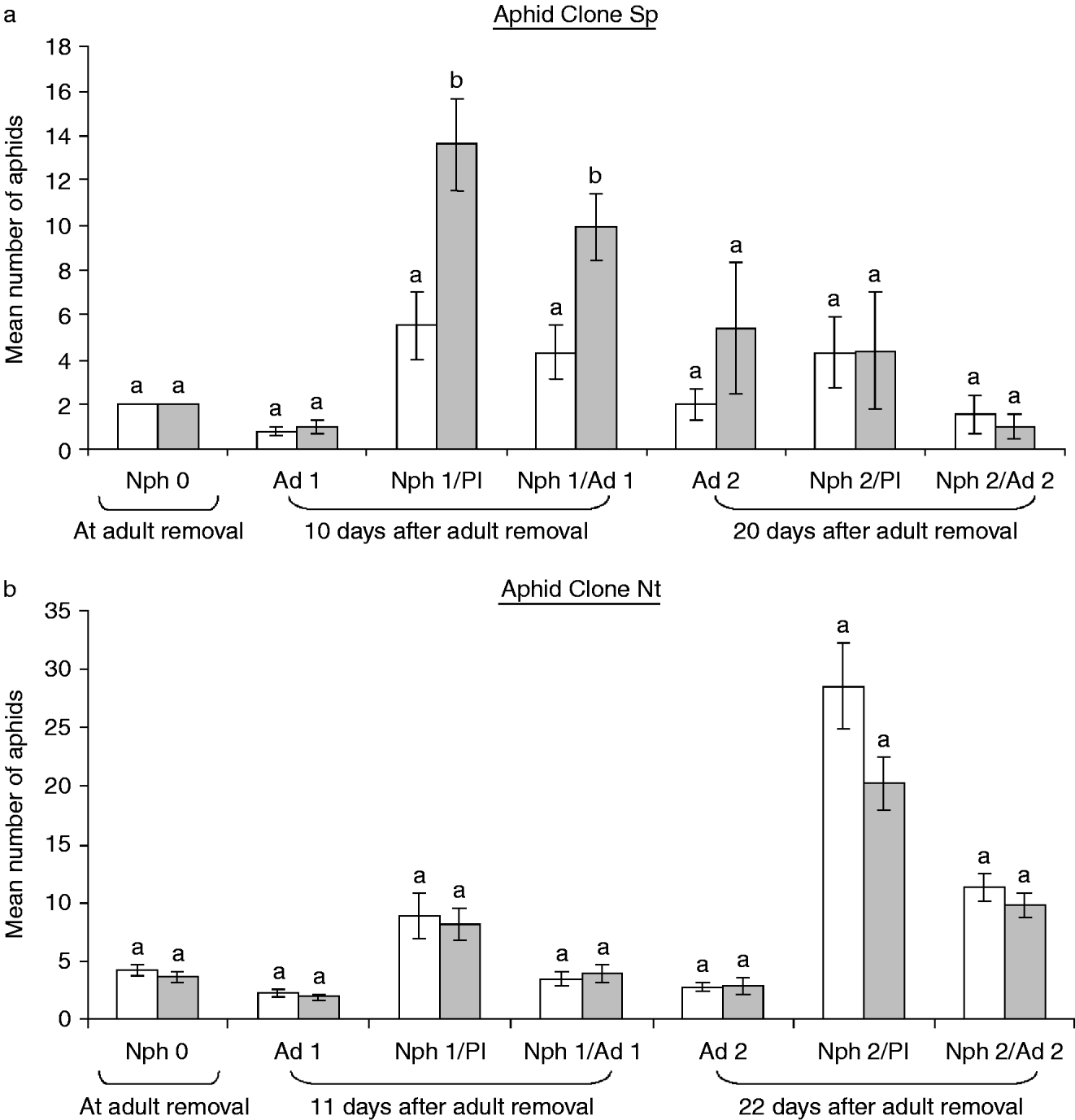
Fig. 3. Mean (±SE) numbers of aphid nymphs and adults per plant and per adult with and without previous infestation with B. tabaci during 48 h. Different letters on bars indicate significant (P<0*"/>, whitefly preinfested plants).
Discussion
Induction of plant resistance to B. tabaci by aphid feeding
Results from the present work demonstrate that resistance to B. tabaci is induced in susceptible tomato plants after a previous infestation by the potato aphid M. euphorbiae. Three days of previous contact with 20 apterous-adult aphids were enough for the plants to acquire a certain level of resistance to the B biotype of B. tabaci. Similarly, Agrawal et al. (Reference Agrawal, Karban and Colfer2000) observed that whitefly populations in cotton were directly and negatively affected by a previous infestation with approximately 30 Tetranychus turkestani spider mites. However, the present study is the first report (to our knowledge) where resistance to whiteflies induced by other insects has been demonstrated. Other studies have reported that feeding by aphids Myzus persicae, Aphis gossypii or Rhopalosiphum padi induced resistance to the same aphid species (Wool & Hales, Reference Wool and Hales1996; Quiroz et al., Reference Quiroz, Petterson, Pickett, Wadhams and Niemeyer1997; Messina et al., Reference Messina, Taylor and Karren2002; Sauge et al., Reference Sauge, Lacroze, Poëssel, Pascal and Kervella2002). In contrast to these effects, feeding by R. padi had limited effect on Diuraphis noxia, while a prior D. noxia infestation did not affect subsequent infestations by any of both aphid species (Messina et al., Reference Messina, Taylor and Karren2002). Furthermore, previous infestation of the susceptible peach cultivar GF305 slightly enhanced larviposition of M. persicae females (Sauge et al., Reference Sauge, Lacroze, Poëssel, Pascal and Kervella2002). Also, Stout et al. (Reference Stout, Workman, Bostock and Duffey1998) observed that the relative growth rates of chewing S. exigua larvae were 10–50% higher when fed on leaves preinfested with M. euphorbiae than when fed on control leaves. These variable results make plant responses to aphids a clear example of specificity of effect as defined by Karban & Baldwin (Reference Karban and Baldwin1997).
It has been described that phloem-feeding insects induce a number of plant responses as a result of transcriptional reprogramming in their host plants (reviewed by Thompson & Goggin, Reference Thompson and Goggin2006), but the proximate mechanisms of induction of resistance to insects by aphid feeding remain unclear. Reduced reproductive performance of A. gossypii on cotton seedlings, which had survived to a previous infestation by the same aphid species, was identified as a result of cumulative plant damage and reduced nutritional quality after aphid feeding, rather than to the production of some chemicals in the plant (Wool & Hales, Reference Wool and Hales1996). However, the aphid-induced resistance to whiteflies observed in the present study cannot be easily attributable to direct plant damage because young seedlings were not tested, but one-month old tomato plants. Moreover, aphid populations for the initial infestation were allowed to freely increase in size during more than 20 days on cotton seedlings; meanwhile, aphids in our assays stayed on the tomato plants for three days only. In accordance with our observations in the present study, it is currently agreed that aphid feeding causes limited direct plant damage (Zhu-Salzman et al., Reference Zhu-Salzman, Bi and Liu2005) although Rabbinge et al. (Reference Rabbinge, Drees, van Der Graaf, Verberne and Wesselo1981) suggested that indirect damage due to the secretion of honeydew that eventually induces a reduction in stomatal conductance and photosynthesis rate can be serious.
In contrast to the above-mentioned effects on B. tabaci by aphids and spider mites, infestation of tomato by whitefly B. argentifolii (corresponding to the B-biotype of B. tabaci) was not affected by previous infestations with chewing insects, such as the vegetable leafminer Liriomyza trifolii or the corn earworm Helicoverpa zea (Inbar et al., Reference Inbar, Doostdar, Leibee and Mayer1999). Conversely, different behaviour (oviposition, feeding preference) and reduction of the survival rates and developmental times of these chewing insects were observed by the same authors in tomato plants previously infested by B. argentifolii. These results can be explained by differences in the feeding behaviour (sucking or chewing) of the first-infesting arthropods in these two sets of experiments. Phloem-feeding insects use their stylets to penetrate between the epidermal and parenchymal cells intercellularly to reach phloem sieve tubes. This results in limited plant damage that is distinct from that of chewing insects (Zhu-Salzman et al., Reference Zhu-Salzman, Bi and Liu2005). It has been postulated that whiteflies and other phloem-feeders are not as sensitive as chewing insects to many plant defences because secondary metabolites or proteins are usually not expressed or available in the phloem (Mayer et al., Reference Mayer, Inbar, Mckenzie, Shatters, Borowicz, Albrecht, Powell and Doostdar2002). Using the electrical penetration graph (EPG) technique (Tjallingii, Reference Tjallingii, Minks and Harrewijn1988), our group had previously demonstrated that innate resistance to B. tabaci in tomato is due to factors in the epidermis and/or mesophyll that inhibit or delay the whiteflies from reaching phloem sieve elements (Jiang et al., Reference Jiang, Nombela and Muñiz2001).
Similarly to the results shown in the present work, exogenous treatment of leaves with BTH induced local resistance to B. tabaci in tomato and cucumber (Correa et al., Reference Correa, Moraes, Auad and Carvalho2005; Nombela et al., Reference Nombela, Pascual, Aviles, Guillard and Muñiz2005) and to M. euphorbiae in tomato (Cooper et al., Reference Cooper, Jia and Goggin2004). Previous studies demonstrated that M. euphorbiae and M. persicae aphids are potent inducers of PR proteins, similarly to host responses observed with pathogens or salicylic acid (SA)/BTH treatment (Bostock et al., Reference Bostock, Karban, Thaler, Weyman and Gilchrist2001). Both SA- and jasmonate (JA)-ethylene-dependent pathways have been demonstrated to be activated in tomato in response to feeding by M. euphorbiae (Walling, Reference Walling2000; Martínez de Ilarduya et al., Reference Martínez de Ilarduya, Xie and Kaloshian2003).
Current results also indicate that resistance of tomato plants against B. tabaci was both local (LAR) and systemically (SAR) expressed. On the contrary, the expression of the resistance induced by treatment of tomato with BTH was limited to treated leaves (Nombela et al., Reference Nombela, Pascual, Aviles, Guillard and Muñiz2005). Moreover, whitefly numbers in the present work were significantly reduced when infestation occurred between one and 18 h after aphid removal. However, reduction was not detectable when four days passed between aphid removal and whitefly infestation, which was equivalent to seven days after aphid infestation as these insects fed on the plants for three days. So, whitefly resistance was transiently induced by M. euphorbiae. This effect was even shorter lasting than the previously observed resistant response to B. tabaci locally induced in tomato plants by BTH, which did not last longer than ten or 11 days after treatment (Nombela et al., Reference Nombela, Pascual, Aviles, Guillard and Muñiz2005). Plant responses to aphid feeding are rapid (Smith & Boyko, Reference Smith and Boyko2006). M. persicae feeding induces resistance responses in foliage of apple (Malus) within as little as two hours, which persist as long as 48 h (Kfoury & Masonie, Reference Kfoury and Masonie1995; Sauge et al., Reference Sauge, Lacroze, Poëssel, Pascal and Kervella2002). It has been demonstrated that levels of PR-1 and GluB transcripts in susceptible tomato plants significantly decrease one week after potato aphid infestation, but systemic accumulation of transcripts from any of these PR-genes in adjacent non-infested leaflets of the same plant was not detected (Martínez de Ilarduya et al., Reference Martínez de Ilarduya, Xie and Kaloshian2003). For this reason, it is unlikely that these defence-response genes should be the best candidates to entirely explain the systemic resistance against B. tabaci induced by aphids in susceptible tomato in the current work.
Variable plant responses to M. euphorbiae induced by B. tabaci
The responses to M. euphorbiae of the plants previously infested by whiteflies were shown to be dependent on the aphid clone tested. Sp-clone adults, obtained ten days after adult removal, on tomato plants preinfested with whiteflies, produced a significantly higher number of nymphs than those on uninfested control plants. However, we did not observe significant differences when the aphid clone Nt was used. These results indicate that changes in plant properties provoked by a previous infestation by whitefly are probably beneficial to clone Sp of M. euphorbiae. This confirms that feeding by similar insects can produce changes in plant quality (Walling, Reference Walling2000; Messina et al., Reference Messina, Taylor and Karren2002), improving the development of other species. A positive effect on aphids was previously observed by other authors. Aphis fabae has beneficial effects when living in colonies (Prado & Tjallingii, Reference Prado and Tjallingii1997). These authors observed that some changes during stylet route towards the phloem can be considered as increased host plant acceptance. Sauge et al. (Reference Sauge, Lacroze, Poëssel, Pascal and Kervella2002) observed that a previous infestation with M. persicae in susceptible peach increases larviposition by adult aphid females. Dugravot et al. (Reference Dugravot, Brunissen, Létocart, Tjallingii, Vicent, Giordanengo and Cherqui2007) evaluated the influence of previous infestation by conspecific M. persicae and heterospecific M. euphorbiae on M. persicae feeding activities on potato plants. They observed that the effects of previous infestation occurring at the local level were opposite to those observed at the systemic level. M. persicae food acceptance was slightly enhanced on previously infested leaves, whereas it was inhibited on no-infested leaves of infested potato plants.
The variability of tomato response induced by whitefly feeding depending on the aphid clone tested presents a certain parallelism with the isolate-specific innate resistance to aphids mediated by the Mi-1 gene; this tomato gene conferred resistance to a red isolate of M. euphorbiae but had no effect on a green isolate of the same species (Rossi et al., Reference Rossi, Goggin, Milligan, Kaloshian, Ullman and Williamson1998). Similarly, plants carrying the Mi-1 gene were resistant to M. euphorbiae isolates from France and The Netherlands, were susceptible to two isolates from California, one from New Jersey and one from North Carolina, with mixed results against two other isolates from California and no effect on two isolates of M. persicae (Goggin et al., Reference Goggin, Williamson and Ullman2001). These authors postulated that this great variability (which is independent of geographical origin, aphid colour or original host) could be due to the presence of certain virulence or avirulence factors in the different aphid isolates. Whether B. tabaci feeding induces, in the Mi-lacking plants, a compound that mimics the recognition of the virulence or avirulence factor from the aphid clone is a stimulating hypothesis, which is not possible to be confirmed until the above-mentioned virulence or avirulence factors can be identified. Moreover, clear interclonal variation in the aphid performance was observed in the present work. The adult aphids of Sp clone were left 96 h on the tomato plant to obtain enough number of nymphs, but only 24 h was enough for the Nt-clone in order to reach the same purpose. Also, the number of aphids in the control plants was consistently different between the both aphid clones. The interclonal variation in aphid performance has been observed by other authors. De Barro et al. (Reference De Barro, Sherratt, David and Maclean1995) provided evidence of genetic variation in performance on host and evidence for clonal adaptation to particular host species. Goundoudaki et al. (Reference Goundoudaki, Tsitsipis, Margaritopoulos, Zarpas and Divanidis2003) proposed that the interclonal variation in performance is possibly related to the colour of M. persicae. The genetic variability of aphid clones suggests that the evaluation of induced or constitutive resistance should be based on data obtained from more that one aphid clone.
It was also observed in our study that the duration of tomato response to a previous infestation by B. tabaci is apparently limited; within ten days, the reproduction (mean number of nymphs) of M. euphorbiae increased significantly when tomato plants had been previously infested by B. tabaci. However, after 20 days, these differences were not observed. Similarly to the resistance to B. tabaci, induced by aphid preinfestation, the increased susceptibility to the Sp-clone aphids was transiently induced by the whiteflies. Other transient plant responses to aphids have been induced by external biotic or abiotic agents (Sauge et al., Reference Sauge, Lacroze, Poëssel, Pascal and Kervella2002; Cooper et al., Reference Cooper, Jia and Goggin2004).
Asymmetry in the induced responses to/by piercing-sucking insects
The interaction between the two piercing-sucking insect species of this work was asymmetric because aphid feeding induced plant resistance to B. tabaci; meanwhile, preinfestation by whiteflies induced variable tomato responses against M. euphorbiae, depending on the aphid clone tested. Asymmetrical interactions between insects are not uncommon; whitefly feeding induced behavioural differences (oviposition, feeding preferences) and reduced survival rates and development times of cabbage looper (Trichoplusia ni) and leaf miner (Liromyza trifolii). Meanwhile, previous exposition to leaf miners had little or no effect on silverleaf whitefly oviposition (Mayer et al., Reference Mayer, Inbar, Mckenzie, Shatters, Borowicz, Albrecht, Powell and Doostdar2002). The asymmetrical interactions between whiteflies and aphids observed in the present study are likely due to different salivary components that may elicit different responses from their host. The general and species-specific elicitors may correspond to one of the known salivary constituents or may be an uncharacterized component of the saliva (Walling, Reference Walling2000). Moreover, the general and species-specific elicitors may be directly synthesized by the insect or may be a product of endosymbiotic bacteria (Costa et al., Reference Costa, Westcot, Ullman, Rosell, Brown and Johnson1995; Douglas, Reference Douglas1998).
In addition, whitefly and aphid populations sometimes display different behaviours in response to the same external factor affecting the host plant. So, feeding by spider mite T. turkestani in cotton plants, which induced resistance to B. tabaci, had a positive effect on populations of M. persicae (Agrawal et al., Reference Agrawal, Karban and Colfer2000). The key to understand such asymmetric interactions probably lies on the assumption that plant resistance to phloem-feeding insects is mediated by two types of plant genes; it is known that plant genes participating in the recognition of aphid herbivory act in concert with other plant genes involved in defence against herbivores. It has been proved that similarities exist in the types of plant genes expressed in response to feeding by different species of aphids. However, numerous differences in plant signalling and defence responses unique to specific aphid-plant interactions have been identified (Smith & Boyko, Reference Smith and Boyko2006).
The complexity of the understanding of induced plant responses to herbivory needs to be addressed using both holistic and mechanistic approaches (Agrawal, Reference Agrawal2005). The present study tried to take into account both types of approaches, as multi-species interactions in highly controlled environments were considered.
Acknowledgements
This research was partially funded by a CICYT Project (AGL2000-1591-C02-01). G. Nombela was financially supported by a contract I3-P2001-1 from the ‘Programa de Incorporación de Investigadores’ CSIC-FSE, Spain, and by another contract from the ‘Programa Ramón y Cajal 2003’ of the MCyT, Spain. E. Garzo was financially supported by a fellowship I3P-BPG2001 from the program ‘Postgrado para formación y especialización en líneas de investigación de interés para el sector industrial’ CSIC-FSE, Spain. Authors wish to thank Prof. Tjallingii for providing the Nt aphid clone.





