INTRODUCTION
Medulloblastoma is a highly malignant tumour in the cerebellum, a part of the posterior fossa that coordinates all motor functions. It is the second most common central nervous system cancer in children, making up about 20% of all paediatric brain tumours. In adults (>15 years old), medulloblastoma is less common and accounts for <1% of adult brain tumours.Reference Brodin, Munck Af Rosenschold and Aznar 1 , 2 The current clinical practice involves surgery followed by postoperative radiotherapy to the cranial–spinal axis with adjuvant chemotherapy due to propensity to spread within the neuraxis.Reference Lopez Guerra, Marrone and Jaen 3 Recent treatment strategies for medulloblastoma patients have been relatively successful with 5-year survival rates of 70–80%.Reference Packer, Gajjar and Vezina 4 , Reference Ostrom, Gittleman and Liao 5 However, despite improved survival rates, patients experience long-term side-effects from the radiotherapy.Reference Fossati, Ricardi and Orecchia 6 These toxicities include impaired neurocognitive development, growth retardation, endocrine dysfunction, cataract formation, hearing problems, cardiomyopathy, impaired fertility and secondary malignancies.Reference Brodin, Munck Af Rosenschold and Aznar 1
Modern craniospinal irradiation (CSI) techniques have been developed with the aim of minimising long-term side-effects in children. Conventionally, two lateral cranial fields and one or two posterior spinal fields are arranged to treat the entire craniospinal axis.Reference Myers, Mavroidis, Papanikolaou and Stathakis 7 Due to field-size constrictions, this type of linear accelerator-based 3D-conformal radiotherapy (Linac-3DCRT) requires the matching of junctions. The need for separate isocentres unfortunately reduces homogeneity at junction points and increases planning complexity.Reference Barrett 8 Tomotherapy plans allow for the continuous treatment of long targets without the need for field matching. TomoHelical (Accuray Inc., Madison, WI, USA) radiotherapy, the most widely used form of tomotherapy, delivers dose from any of 360° and uses intensity-modulated radiotherapy (IMRT). The delivery of TomoHelical plans for CSI is well documented.Reference Penagaricano, Moros, Corry, Saylors and Ratanatharathorn 9 – Reference Sugie, Shibamoto and Ayakawa 12 Compared with Linac-3DCRT, TomoHelical treatments for CSI in the supine position at the host institute as well as others have demonstrated increased patient comfort and dose conformity.Reference Penagaricano, Moros, Corry, Saylors and Ratanatharathorn 9 , Reference Huang, Parker and Freeman 10 However, due to the nature of 360° TomoHelical beam arrangements, there are concerns about increased integral doses (ID) which potentially leads to increased secondary malignancies.Reference Brodin, Munck Af Rosenschold and Aznar 1 , Reference Lopez Guerra, Marrone and Jaen 3 , Reference Fossati, Ricardi and Orecchia 6 The 30–60-minute-long treatment duration is also a major concern for patient comfort and those who require sedation.
In contrast to TomoHelical radiotherapy, TomoDirect (Accuray Inc.) is a treatment option that enables the user to apply specific beam angles for treatment planning. There are two types of TomoDirect plans: TomoDirect-3DCRT and TomoDirect-IMRT. Forward planning in 3DCRT delivers dose to the target without regions-at-risk constraints while inverse planning in IMRT uses multi-leaf collimators (MLCs) to create highly conformal dose to targets. 13
TomoDirect treatments for CSI have the potential to shorten treatment times and decrease ID. This new technology available from tomotherapy offers the potential to achieve lower dose to all non-target tissues without compromising the target dose. Despite the publication of two recent articles, there is a lack of evidence to support the use of TomoDirect for CSI treatments.Reference Kim, Jeong and Chung 14 , Reference Langner, Molloy, Gleason and Feddock 15 This paper aims to address this gap in knowledge by comparing TomoDirect radiotherapy with TomoHelical radiotherapy and Linac-3DCRT in a dosimetric study of CSI treatment.
METHODS
Five consecutive medulloblastoma patients previously treated with TomoHelical radiotherapy at the host hospital were replanned with 3DCRT, TomoHelical and TomoDirect. This included all three paediatric and two adult patients treated from 2008 to 2014. All five patients underwent computed tomography (GE LightspeedTM, Chicago, IL, USA) simulation in the supine position (Table 1). They were fitted with customised full body vacuum immobilisations to stabilise body positioning, as well as standardised head-rests and thermoplastic masks to immobilise the head and neck area.
Table 1 Patient demographics and CT parameters

Abbreviation: CT, computer tomography; PTV, planning target volume.
Organs-at-risk (OARs) and planning target volumes (PTVs) were contoured using the Eclipse 10 (Varian, Palo Alto, CA, USA) planning station. The target volumes were contoured by the oncologist to include the cranium and spinal axis. During planning, the PTV was split into PTVbrain (cranial contents) and PTVspine (inferiorly from C1) to further improve dosimetry. A list of OARs were contoured by trained contouring radiation therapists and adjusted by the primary researcher for consistency. These organs include brainstem, pituitary, optic nerves, optic chiasm, eyes, lenses, hearing apparatus (cochlea and middle ear), parotids, mandible, larynx, oesophagus, lungs, heart, breasts, liver, kidneys, bowels, testes, ovaries and uterus. The body was defined as the entire external contour down to the top third of the femur.
To permit comparisons, a prescription for high-risk medulloblastoma patients (36 Gy in 20 fractions) was applied for all plans.Reference Langner, Molloy, Gleason and Feddock 15
PLANNING
All TomoHelical plans were replanned for this study. A standardised planning protocol was applied to all patients. This protocol closely followed the guidelines set out by the host department. For medulloblastoma patients, nearly 15–20% of recurrences occur at the cribriform plate due to excessive shielding to protect ocular structures.Reference Patil, Oinam, Chakraborty, Ghoshal and Sharma 16 , Reference Patel, Drodge, Jacques, Warkentin, Powell and Chafe 17 Therefore, in achieving adequate target coverage in the cribriform plate between the eyes, ocular structures unavoidably received unwanted dose from lateral opposing cranial fields. In this study, MLCs were used to shield the lenses and facial structures away from PTVbrain.
Linac-3DCRT
For conventional 3DCRT, a fixed-beam geometry and 6 MV X-rays were used. Two lateral opposing cranial fields were employed, half-beam blocked inferiorly to match the junction of a posterior spinal field. MLC positions were adjusted to block high-risk OARs. Prescription dose was normalised to the reference point located at the geometric centre of PTVbrain. Spinal field dose normalisation point was placed in the central axis, at depth of the spinal canal.Reference Nakamura, Shikama and Wada 18 , Reference Gupta and Sarin 19 For patients with PTV lengths within 65 cm, one spinal field was used using a source-to-skin distance (SSD) of 100–110 cm. For patients with longer spinal lengths, two direct posterior spinal fields (SSD 100 cm) were dosimetrically matched at spine depth. All junctions were then shifted 1 cm each on a 3-day cycle to feather the dose.
Tomotherapy
Posterior and lateral blocks were added for TomoDirect to limit gantry angles of 90 and 270° to the brain and 180° to the spine. An overlap of 1·25 cm (half field width) between the posterior and lateral fields was introduced to allow for continuous dose distribution at the junction (Figure 1). For TomoDirect planning, a complete block was applied to restrict beam entry and exit through both lenses.
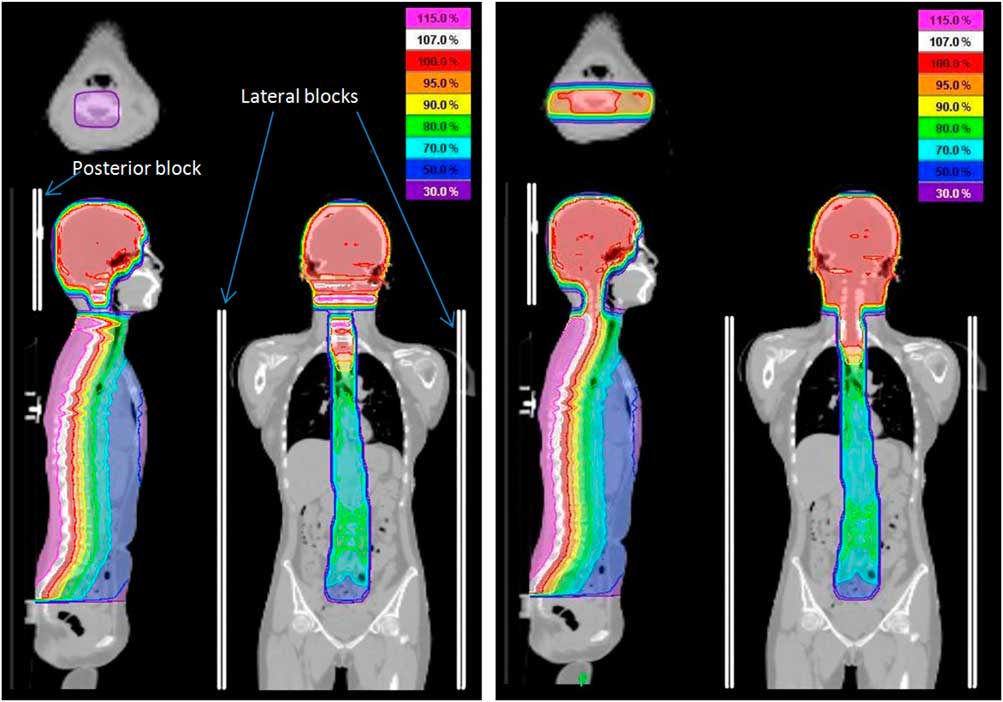
Figure 1 TomoDirect dose distribution with and without overlap. Notes: Dose distribution for TomoDirect plan with no overlap of posterior and lateral fields (left image) exhibits gap in dose. The gap was eliminated by creating an overlap of 1·25 cm between posterior and lateral fields (right image).
For all tomotherapy plans, field width, the axial thickness of the fan beam, was set to 2·51 cm and pitch, the couch travel distance for one complete gantry rotation relative to axial beam width was set to 0·287. A fine dose calculation grid was used, meaning that dose–volume calculation is based on a planning image that is not downsampled.
One generalised set of dose objectives was prepared as a starting point for TomoDirect-IMRT and TomoHelical plans. This template was created based on previously published data for OAR dose constraints.Reference Brodin, Munck Af Rosenschold and Aznar 1 , Reference Marks, Yorke and Jackson 20 – Reference Verellen and Vanhavere 23 After every 50 iterations, planning objectives were manually adjusted to tailor to the patient anatomy. Modulation factor is the maximum divided by average leaf-opening time. It estimates plan complexity. A modulation factor was first set to 2, increasing to 2·7 as needed to improve dose conformity at the expense of increased beam-on time. 13 All beam-on times were kept within 25 minutes as per departmental protocol.
Data analysis
Data collection was performed on the imaging software MIM 6.4.9 (MIM Software Inc., Cleveland, OH, USA). Plans were compared based on homogeneity index (HI), ID, overall treatment time and tissue sparing endpoints for all OARs including maximum dose (D max), mean dose (D mean), volumes of OARs receiving 5, 10, 20 and 30 Gy (V 5 Gy, V 10 Gy, V 20 Gy, V 30 Gy). Slice-by-slice isodose distributions and dose–volume histograms (DVHs) were compared for all plans.
The HI is calculated using
where D 2% and D 98% are the dose to 2 and 98% of the PTV, and D Rx the prescription dose. A lower HI indicates better homogeneity.Reference Yoon, Shin and Kim 24
ID measures the amount of physical damage induced by irradiation. It incorporates all biological effects produced in an irradiated volume of tissue and is represented as total absorbed energy.Reference Mayneord 25 , Reference Nguyen, Rubino and Guerin 26 In this study, this was computed for the non-target body as follows:
where D mean and V are the mean dose and volume of the non-target body, and ρ mean is estimated to be the density of water.
Planning and treatment times for each method was obtained and compared. Beam-on time was calculated for Linac-3DCRT using a dose rate of 400 MU/minute.
RESULTS
Dose distributions for patients 1 and 5 are shown in Figures 2 and 3 to represent paediatric and adult patients, respectively, for Linac-3DCRT, TomoHelical, TomoDirect-3DCRT and TomoDirect-IMRT plans.

Figure 2 Sample dose distributions for one paediatric patient. Notes: Isodose lines of 115, 110, 107, 100, 95, 90, 80, 70, 50 and 30% of the prescribed dose are displayed for linear accelerator-based 3D-conformal radiotherapy (Linac-3DCRT), TomoHelical, TomoDirect-3DCRT and TomoDirect-intensity-modulated radiotherapy (IMRT) plans.

Figure 3 Sample dose distributions for one adult patient. Notes: Isodose lines of 115, 110, 107, 100, 95, 90, 80, 70, 50 and 30% of the prescribed dose are displayed for linear accelerator-based 3D-conformal radiotherapy (Linac-3DCRT), TomoHelical, TomoDirect-3DCRT and TomoDirect-intensity-modulated radiotherapy (IMRT) plans.
For Linac plans, dose at junction sites appear inhomogeneous despite feathering, with underdose and overdose posterior and anterior to the spine, respectively. Slice-by-slice comparisons of the isodose maps between TomoDirect-3DCRT and TomoDirect-IMRT plans displayed little visible differences in the cranium while the posterior beam is narrower in the lung regions.
Figure 4 shows the DVH for the PTV and lungs of patient 1. Sparing of the lungs was best achieved with TomoDirect planning, as is shown in the sample DVH for lungs.

Figure 4 Planning target volume (PTV) dose–volume histogram (DVH) and total lung DVH for patient 1. Abbreviations: Linac-3DCRT, linear accelerator-based 3D-conformal radiotherapy; IMRT, intensity-modulated radiotherapy.
Table 2 shows the summary of HI and non-target integral dose (NTID) for Linac-3DCRT, TomoHelical and TomoDirect plans. TomoHelical plans achieved the lowest mean HI and better comformity compared with Linac-3DCRT, TomoDirect-3DCRT and TomoDirect-IMRT. In contrast, TomoDirect-IMRT achieved the lowest NTID compared with TomoDirect-3DCRT, TomoHelical and Linac-3DCRT. The dose–volume information for OARs are shown in Table 3.
Table 2 Homogeneity index and non-target integral dose for all patients

Notes: Data are shown for linear accelerator-based 3D-conformal radiotherapy (3D), TomoHelical (TH), TomoDirect-3D-conformal radiotherapy (TD3) and TomoDirect-intensity-modulated radiotherapy (TDI) plans.
Abbreviation: CSI, craniospinal irradiation.
Table 3 Average volumes of organs-at-risk (OARs) receiving over 5, 10, 20 and 30 Gy

Notes: volumes are shown for linear accelerator-based 3D-conformal radiotherapy (3D), TomoHelical (TH), TomoDirect-3D (TD3) and TomoDirect-intensity-modulated radiotherapy (TDI) plans. Average volumes for two female patients is shown for breasts, ovaries and uterus. Average volumes for three male patients is shown for testes. For other organs, average from five patients is shown.
TomoHelical plans achieved the lowest D max in all organs except the breasts. TomoHelical plans achieved the lowest D mean for all OARs, except in the mandible, lungs, breasts, liver, kidneys, bowels and non-target body, where both TomoDirect methods triumphed (Figure 5). In Linac plans, the highest OAR D mean were observed in all OARs except for the breasts.

Figure 5 Average D mean (Gy) for organs-at-risk. Notes: Average D mean for two female patients is shown for breasts, ovaries and uterus. Average D mean for three male patients is shown for testes. For other organs, average from five patients is shown. Abbreviations: Linac-3DCRT, linear accelerator-based 3D-conformal radiotherapy; IMRT, intensity-modulated radiotherapy.
OAR constraints were met in most cases. Exceptions include the eyes (D max exceeded 20 Gy for all Linac and TomoDirect plans, and two TomoHelical plans), parotids (D mean exceeded 25 Gy for four Linac, three TomoDirect-3DCRT and one TomoDirect-IMRT plan), lung (D mean exceeded 10 Gy for four Linac plans) and heart (D mean exceeded 3·5 Gy for all Linac and TomoDirect plans and one TomoHelical plan).
Table 4 summarises the planning and total treatment times for each treatment type.
Table 4 Estimated planning and treatment times for each techniques

Notes: planning durations were established by adding up stopwatch-timed procedures at each step of planning. Average time taken from the host department for linear accelerator-based 3D-conformal radiotherapy (Linac-3DCRT) and tomotherapy procedures are shown.
Abbreviation: IMRT, intensity-modulated radiotherapy.
DISCUSSION
In this study, four different methods for CSI treatments were evaluated: TomoDirect-3DCRT, TomoDirect-IMRT, TomoHelical radiotherapy and Linac-3DCRT. This study aims to fill the gap in CSI treatment options to help guide future treatment decisions.
Dose homogeneity and ID
TomoHelical radiotherapy achieved higher dose homogeneity compared with Linac-3DCRT and TomoDirect methods (Table 2). These study results are consistent with existing literature. Langner et al.Reference Langner, Molloy, Gleason and Feddock 15 reported the same pattern of PTV homogeneity: HI of 4, 17 and 18% for TomoHelical, TomoDirect-3DCRT and Linac-3DCRT, respectively. Similarly, Kim et al.Reference Kim, Jeong and Chung 14 noted that dose homogeneity for PTV was higher for TomoHelical and TomoDirect-3DCRT plans (dose homogeneity index (DHI) given by the ratio of D 95% to D 5%, of around 0·95) compared with Linac-3DCRT (DHI of 0·79).Reference Kim, Jeong and Chung 14 The differences between endpoint values in this study and in others may be attributed to varying planning parameters and procedures, dose constraints and prescription dose used. For instance, Kim et al.Reference Kim, Jeong and Chung 14 used two posterior oblique beams for TomoDirect plans. As this would incur exit dose to the lungs and double the treatment time in the spine region, multiple beam angles to the spine were not used in this study.
One of the primary motives for investigating the use of TomoDirect radiotherapy was to reduce NTID compared with Linac-3DCRT and TomoHelical. This was observed for all five patients (Table 2). The low ID is a result of a smaller irradiated volume especially in the spine, where a direct posterior beam orientation avoids all non-target tissues lateral to PTVspine. The clinical implications of lowered NTID is the potential to reduce chances of radiation-induced secondary malignancies.Reference Brodin, Munck Af Rosenschold and Aznar 1 , Reference Lopez Guerra, Marrone and Jaen 3 , Reference Fossati, Ricardi and Orecchia 6
Although ID for the TomoDirect CSI technique has never been explored in other studies, many have compared ID between Linac-3DCRT and TomoHelical radiotherapy. As attention first shifted from conventional radiotherapy to IMRT, studies evaluated the potential increase in secondary malignancies due to increased irradiated volumes. Correlation was drawn between ID and increased risk of secondary cancers.Reference Brodin, Munck Af Rosenschold and Aznar 1 , Reference Penagaricano, Moros, Corry, Saylors and Ratanatharathorn 9 , Reference Nguyen, Rubino and Guerin 26 Several authors found that IMRT and TomoHelical IMRT increases ID and therefore, risk of radiation-induced cancers, compared with conventional treatments.Reference Verellen and Vanhavere 23 , Reference Hall and Wuu 27 This conclusion was challenged by various subsequent studies which showed that ID in conventional 3DCRT was comparable with IMRT techniques, including TomoHelical radiotherapy.Reference Penagaricano, Moros, Corry, Saylors and Ratanatharathorn 9 , Reference Patel, Drodge, Jacques, Warkentin, Powell and Chafe 17 , Reference Barra, Gusinu and Cavagnetto 28
In this study, TomoHelical plans resulted in lower NTID than Linac-3DCRT. This result varied between other studies. In a CSI study by Penagaricano et al.,Reference Penagaricano, Moros, Corry, Saylors and Ratanatharathorn 9 healthy tissue ID was found to be lower in TomoHelical than Linac-3DCRT in one patient and the opposite for two patients. In a study by Patel et al.,Reference Patel, Drodge, Jacques, Warkentin, Powell and Chafe 17 NTID was lower for Linac-3DCRT than for TomoHelical in all of the five patients’ plans. The varying results comparing Linac and TomoHelical are due to different Linac planning protocols. In the study by Patel et al.,Reference Patel, Drodge, Jacques, Warkentin, Powell and Chafe 17 MLCs were used to shrink the lateral borders of the posterior spine field. This reduced the irradiated volume and subsequently, NTID. Unfortunately, as these studies contained five or less samples, results were not statistically significant.
Evaluating different tomotherapy treatment techniques
TomoDirect-3DCRT and TomoDirect-IMRT are similar in principle and mode of delivery and are expected to deliver similar endpoint results. For TomoDirect-3DCRT, the planning process took into account only the prescription to PTV, without allowing the user to apply constraints to regions-at-risk. Therefore, dose to the target is achieved at the expense of irradiating surrounding OARs. For TomoDirect-IMRT, dose constraints could be applied to regions-at-risk, enabling some dose modulation to lower the dose to OARs surrounding the PTV. 13
For this study, little difference was observed between DVHs for the two sets of TomoDirect plans, with slightly lower OAR mean doses observed for TomoDirect-IMRT plans. In particular, the parotids received a D mean of over 25 Gy for three out of five TomoDirect-3DCRT plans which exceeds the dose constraint for parotids (75% reduced salivary flow).Reference Marks, Yorke and Jackson 20 , Reference Roesink, Moerland, Battermann, Hordijk and Terhaard 21
In the dosimetric analysis, it was demonstrated that TomoDirect-IMRT resulted in better NTID and important normal tissue sparing compared with TomoDirect-3DCRT. This is attributed to the use of inverse planning and the ability to increase dose modulation.
For better PTV homogeneity, TomoHelical radiotherapy is still the method of choice. One notable advantage of TomoHelical planning in the cranial region is the ability to achieve prescription dose to the region between the eyes, especially the cribriform plate, without compromising dose to ocular structures such as the eyes and optic nerves. Organs that are directly anterior to the spine, such as the oesophagus, larynx, heart, ovaries and uterus benefit from TomoHelical planning.
On the other hand, organs where the majority of the volume lies lateral to the single posterior field are thus better spared in TomoDirect compared with TomoHelical radiotherapy. Due to the larger margins and open posterior field arrangement of the traditional Linac-3DCRT technique, the benefit of organ sparing seen in TomoDirect was not observed except in the case of the breasts. This agrees with other studies where breast dose was lower in Linac-3DCRT compared with TomoHelical.Reference Myers, Mavroidis, Papanikolaou and Stathakis 7 , Reference Yoon, Shin and Kim 24 , Reference Sharma, Gupta, Jalali, Master, Phurailatpam and Sarin 29
Despite differences in OAR doses between plans, almost all tomotherapy plans achieved OAR dose constraints. However, only TomoHelical plans achieved D max of eyes below 20 Gy and heart D mean below 3·5 Gy.
Recommendations
This study demonstrated that both TomoHelical and TomoDirect plans offered better OAR sparing endpoints, homogeneity and ID than the traditional Linac-3DCRT technique. It is more difficult to single out a clear winner in the tomotherapy family.
For centres with only tomotherapy, the author recommends the use of TomoDirect technique as an alternative for CSI treatments due to its potentially lower NTID. Also, although TomoHelical treatments offer all around better OAR sparing and homogeneity it should be used with caution as the longer treatment time may lead to increased chances of patient movement due to discomfort. Hence the precise dose targeted to the PTV may not be the actual dose delivered.
The shorter TomoDirect-3DCRT treatment times may be chosen for patients requiring anaesthesia or for those patients who are claustrophobic. Moreover, TomoDirect radiotherapy should be considered for cases where sparing certain organs completely is desirable, especially laterally situated organs such as the breasts. The breasts should receive the lowest dose possible to reduce chances of a secondary malignancy. A peer-reviewed study showed that minimising the dose to the breast is inversely related to age and is therefore particularly essential in younger patients.Reference Stovall, Smith and Langholz 30 For better homogeneity, NTID and OAR sparing, TomoDirect-IMRT is recommended over TomoDirect-3DCRT.
Conversely, TomoDirect or Linac-3DCRT should not be used if the PTV is covered by the eyes in beam’s eye view of the lateral cranial fields. This is because complete coverage of the cribriform plate becomes impossible without significant damage to ocular structures. The TomoHelical technique should be chosen instead.
Although Linac-3DCRT seemed the best in terms of resource distribution due to its short planning and treatment time, these benefits do not outweigh problems in dose homogeneity, NTID and organ sparing. Therefore, for clinics with only Linacs, it may be worth exploring other methods for CSI treatments, such as volumetric-modulated arc therapy (VMAT). VMAT offers a new promising treatment method with decreased ID while offering comparable normal tissue sparing to TomoHelical radiotherapy.Reference Myers, Mavroidis, Papanikolaou and Stathakis 7 , Reference Patel, Drodge, Jacques, Warkentin, Powell and Chafe 17 , Reference Srivastava, Saini and Sharma 31 However, this technique does not address the field-size limitation of a Linac, still necessitating junction matching between the cranial and spinal fields, and between two spinal fields for longer PTVs.
LIMITATIONS AND FUTURE DIRECTIONS
There are several limitations in this study. First, biases and confounding variables such as age and gender differences may be present. Furthermore, dose to normal tissue is based on dosimetric information without considering potential clinical variances such as radiation leakage from the machine.
Moreover, due to the limited sample size (n=5), statistical significance could not be achieved. Patterns observed may be due to chance. This is a common problem with CSI studies, as medulloblastoma is a rare disease. In the future, the author hopes to collect more samples for further statistical analyses.
Limitations in estimating planning and treatment times must be noted. All planning times are subject to variances in computer speeds. Similarly, treatment setup and imaging times vary for each patient and each fraction. They should only be used as a reference.
Finally, limitations in the use of theoretical parameters such as HI, conformation number and ID to compare plans apply. There is limited published data regarding the possible correlations between clinical outcome and these indices proposed in the literature. Further research of population-based studies and prospective studies are needed to correlate dosimetric results and ID to late effects and incidences of secondary malignancy.
CONCLUSIONS
This study showed that although TomoDirect plans lacked the PTV dose homogeneity present in TomoHelical plans, they perform better in those aspects than traditional Linac-3DCRT plans. The TomoDirect technique may also reduce PTV inhomogeneity compared with Linac-3DCRT. TomoDirect treatment time is shorter than that of TomoHelical treatments and may be considered for patients requiring anaesthetics. In all, TomoDirect may be an option for cases where the patient needs to be treated supine, require shorter treatment times or better sparing of the laterally situated OAR.
More importantly, TomoDirect plans resulted in the lowest NTID, a factor linked with radiation-induced secondary malignancies. Interestingly, NTID for TomoHelical radiotherapy is also lower than Linac-3DCRT. Conclusions regarding ID should be drawn with caution as the results from comparisons between Linac-3DCRT and TomoHelical radiotherapy differ between studies, suggesting that findings are department specific. Thus it is recommended that each radiotherapy centre conduct its own planning study to obtain results based on their in-house protocols, software, hardware and expertise. In the future, extensive research is needed to evaluate the clinical implications of these findings in the reduction of treatment toxicities and secondary malignancies.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or non-for-profit sectors.
Conflicts of Interest
None.












