Introduction
Strongyloidiosis by Strongyloides stercoralis (Rhabditida: Strongyloididae) is a parasitic disease of humans and dogs, which may bear the brunt of this parasitosis (Nutman, Reference Nutman2017; Thamsborg et al., Reference Thamsborg, Ketzis, Horii and Matthews2017). The infection is typical of, but not exclusive for, tropical and subtropical areas where the environmental conditions favour the free-living phase of this nematode (Ferreira-Júnior et al., Reference Ferreira-Júnior, Goncalves-Pires, Silva, Goncalves and Costa-Cruz2006; Martins et al., Reference Martins, Barros Cda, Bier, Marinho, Figueiredo, Hoffmann, Molento and Biondo2012; Bisoffi et al., Reference Bisoffi, Buonfrate, Montresor, Requena-Mendez, Munoz, Krolewiecki, Gotuzzo, Mena, Chiodini, Anselmi, Moreira and Albonico2013; Schär et al., Reference Schär, Trostdorf, Giardina, Khieu, Muth, Marti, Vounatsou and Odermatt2013; Thamsborg et al., Reference Thamsborg, Ketzis, Horii and Matthews2017). The adult nematodes have a complex, rather unique, life cycle consisting of parasitic female worms residing in the host small intestine reproducing via parthenogenesis, and free-living male and female in the environment, reproducing sexually (Viney, Reference Viney2017). In the host the new-born larvae are shed through feces and may cause a systemic migration (autoinfective cycle), allowing the parasite to persist in healthy individuals or to disseminate in chronically infected and immunocompromised hosts (Schär et al., Reference Schär, Trostdorf, Giardina, Khieu, Muth, Marti, Vounatsou and Odermatt2013; Buonfrate et al., Reference Buonfrate, Formenti, Perandin and Bisoffi2015). Evidence for zoonotic transmission suggests that dogs may play a role as reservoirs of the parasite to humans mainly in poor socioeconomic context with low hygiene standards (Jaleta et al., Reference Jaleta, Zhou, Bemm, Schär, Khieu, Muth, Odermatt, Lok and Streit2017; Thamsborg et al., Reference Thamsborg, Ketzis, Horii and Matthews2017).
Despite the increased attention for human strongyloidiosis in developed and less developed countries (Schär et al., Reference Schär, Inpankaew, Traub, Khieu, Dalsgaard, Chimnoi, Chhoun, Sok, Marti, Muth and Odermatt2014; Anselmi et al., Reference Anselmi, Buonfrate, Guevara Espinoza, Prandi, Marquez, Gobbo, Montresor, Albonico, Racines Orbe, Martin Moreira and Bisoffi2015; Cabezas-Fernández et al., Reference Cabezas-Fernández, Salas-Coronas, Lozano-Serrano, Vazquez-Villegas, Cabeza-Barrera and Cobo2015; Buonfrate et al., Reference Buonfrate, Baldissera, Abrescia, Bassetti, Caramaschi, Giobbia, Mascarello, Rodari, Scattolo, Napoletano and Bisoffi2016), data on the epidemiology of canine infection is restricted to tropical and subtropical geographical areas, such as Brazil and Cambodia (Gonçalves et al., Reference Gonçalves, Machado, Gonçalves-Pires, Ferreira-Júnior, Silva and Costa-Cruz2007; Schär et al., Reference Schär, Inpankaew, Traub, Khieu, Dalsgaard, Chimnoi, Chhoun, Sok, Marti, Muth and Odermatt2014; Jaleta et al., Reference Jaleta, Zhou, Bemm, Schär, Khieu, Muth, Odermatt, Lok and Streit2017). In Europe, canine strongyloidiosis has been reported in Germany (Epe et al., Reference Epe, Ising-Volmer and Stoye1993), Finland (Dillard et al., Reference Dillard, Saari and Antitila2007), Greece (Papazahariadou et al., Reference Papazahariadou, Founta, Papadopoulos, Chliounakis, Antoniadou-Sotiriadou and Theodorides2007), France (Cervone et al., Reference Cervone, Giannelli, Otranto and Perrucci2016) and Italy (Paradies et al., Reference Paradies, Iarussi, Sasanelli, Capogna, Lia, Zucca, Greco, Cantacessi and Otranto2017). The medical and veterinarian relevance of this parasite has probably been largely underestimated due to the difficulty of diagnosis. Indeed, one of the major limitations in the assessment of this infection in dogs is represented by the diagnostic procedures for the detection of S. stercoralis (Buonfrate et al., Reference Buonfrate, Paradies, Iarussi, Formenti, Perandin, Otranto and Bisoffi2017a).
Proper diagnostic tools are pivotal to identify infected individuals and to evaluate the prevalence of the infection amongst host populations. Though some serological (i.e. immunofluorescence antibody test (IFAT), enzyme-linked immunosorbent assay (ELISA)) and molecular tests (i.e. real time-polymerase chain reaction (rt-PCR)) have been employed for the diagnosis in dogs (Ferreira-Júnior et al., Reference Ferreira-Júnior, Goncalves-Pires, Silva, Goncalves and Costa-Cruz2006; Buonfrate et al., Reference Buonfrate, Paradies, Iarussi, Formenti, Perandin, Otranto and Bisoffi2017a, Reference Buonfrate, Requena-Mendez, Angheben, Cinquini, Cruciani, Fittipaldo, Giorli, Gobbi, Piubelli and Bisoffi2018), the detection of the parasites by Baermann method remains the most common technique. Nonetheless, the latter tool is characterized by a low sensitivity and requires multiple testing to unequivocally rule out the presence of larvae in faecal samples (Bisoffi et al., Reference Bisoffi, Buonfrate, Sequi, Mejia, Cimino, Krolewiecki, Albonico, Gobbo, Bonafini, Angheben, Requena-Mendez, Muñoz and Nutman2014; Buonfrate et al., Reference Buonfrate, Formenti, Perandin and Bisoffi2015). Again, the comparison of the accuracy of serological, molecular and coprological tests for S. stercoralis diagnosis in dogs has never been conducted. This study assessed the occurrence of S. stercoralis infection in shelter dogs by comparing the sensitivity and specificity of serological, coprological and molecular methods and the larval shedding by weekly examination of feces in a cohort of infected dogs.
Materials and methods
Study design
From April to September 2017, feces and blood samples were collected from 100 dogs living in a shelter in the province of Bari (41°04′47″N, 16°55′17″E, Apulia region, Italy) where cases of S. stercoralis infection were described (Paradies et al., Reference Paradies, Iarussi, Sasanelli, Capogna, Lia, Zucca, Greco, Cantacessi and Otranto2017). The municipal shelter is divided into two main areas (i.e. A1 and A2), formed by 6 and 10 units, respectively. Each unit is composed of wire mesh cages (approximately 3 × 3 m2), housing about 800 dogs according to their gender and existing hierarchies within each group. Stool and blood from healthy animals or with clinical signs (i.e. diarrhoea, weight loss, reduced appetite) were sampled directly from the rectal ampulla and from the cephalic vein, respectively. Additionally, from dogs scored positive to S. stercoralis at least at one coprological technique, faecal samples were weekly collected for 1 month and analysed by parasitological and molecular tests. The enrolled dogs were not treated with any anthelmintic drugs at least over the month prior to sample collection. Animals were grouped according to their age in younger and elder than 7-years-old. Dogs with severe health condition were treated with ivermectin 200 µg kg−1/sid/os for 2 consecutive days as reported for human strongyloidiosis (Bisoffi et al., Reference Bisoffi, Buonfrate, Angheben, Boscolo, Anselmi, Marocco, Monteiro, Gobbo, Bisoffi and Gobbi2011).
Faecal examination
The coprological diagnosis of S. stercoralis infection was performed through direct microscopy, Baermann and culturing technique. For the Baermann examination, 5 g of feces were analysed and any recovered larvae were identified according to their morphology (Little, Reference Little1966) and molecularly processed through partial cytochrome c oxidase subunit 1 (cox1) gene amplification and sequencing (see Hasegawa et al., Reference Hasegawa, Sato, Fujita, Nguema, Nobusue, Miyagi, Kooriyama, Takenoshita, Noda, Sato, Morimoto, Ikeda and Nishida2010). The Koga agar plate test (Koga et al., Reference Koga, Kasuya, Khamboonruang, Sukhavat, Ieda, Takatsuka, Kita and Ohtomo1991) was performed by plating 2 g of fresh stool on the agar plate and observed the live larvae and adult worms at the microscope after incubation at 27 ± 2 °C for 2 days. In addition, faecal flotation was performed on 2 g of feces to diagnose other helminthic infections. Presence of Tritrichomonas foetus was evaluated microscopically, followed by DNA isolation and amplification of marker genes to rule out other trichomonads and confirm the genotype identity as previously described by Šlapeta et al. (Reference Šlapeta, Craig, McDonell and Emery2010, Reference Šlapeta, Müller, Stack, Walker, Lew-Tabor, Tachezy and Frey2012).
Complete blood count (CBC)
The CBC was performed by using the ADVIA® 2120 SIEMENS analyser (Siemens Healthineers, Italy) and supported by blood smear evaluation. Dogs with 1.2–1.8 × 103 eosinophils μL−1 at CBC were considered affected by mild eosinophilia, while severe eosinophilia was documented by >1.8 × 103 eosinophils μL−1.
Molecular assay
For DNA extraction, 200 mg of feces were suspended in 200 µL of phosphate-buffered saline containing 2% polyvinylpolypyrolidone (Sigma-Aldrich) and freezed overnight at −20 °C until the extraction. After thawing, 2 µL of a 1:1000 dilution of Phocin Herpes Virus type-1 (PhHV-1) with Cq = 18, was added to each sample to serve as an internal control (the virus stock was kindly provided by Dr Pas S., ErasmusMC, Department of Virology, Rotterdam). Subsequently, the samples were boiled and extracted using MagnaPureLC.2 instrument (Roche Diagnostics), following the protocol DNA I Blood Cells High performance II, using the DNA isolation kit I (Roche Diagnostics). DNA samples were stored at −20 °C until further rt-PCR analysis. The real-time assay was performed as described by Verweij et al. (Reference Verweij, Canales, Polman, Ziem, Brienen, Polderman and van Lieshout2009). The small-subunit rRNA gene sequence was chosen as an amplification target and primers and probes synthesized by MWG Biotech S.r.l. (Ebersberg, Germany). Amplification reaction was performed in a volume of 25 µL; the PCR cycle protocol consists of 3 min at 95 °C followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C and 30 s at 72 °C. Appropriate positive and negative controls were included in all the experiments. As a control for PCR inhibitors and amplification quality, the PhHV-1 control DNA was amplified with the appropriate primers/probe mix (see Verweij et al., Reference Verweij, Canales, Polman, Ziem, Brienen, Polderman and van Lieshout2009) in the same reaction in a multiplex PCR. The reactions, detection and data analysis were performed with the CFX96 detection system (Bio-Rad Laboratories, Milano, Italy).
Serological assays
The ELISA is a commercial kit (Bordier, Affinity Products, Switzerland) based on somatic antigens from Strongyloides ratti larvae. The results were expressed in optical density (OD) considering as positive an OD of the tested sample higher than that of the positive control. The OD of the positive control could change among runs, hence a result expressed as a normalized ratio was preferable for study purpose, so that the results of the tests conducted in different runs were comparable. Positive tests were defined by OD values ⩾1 (normalized ratio). The IFAT is an in-house technique based on somatic antigens from S. stercoralis larvae (obtained by stools of infected donors placed in agar plate culture) as previously described by Boscolo et al. (Reference Boscolo, Gobbo, Mantovani, Degani, Anselmi, Monteiro, Marocco, Angheben, Mistretta, Santacatterina, Tais and Bisoffi2007). Samples were scored as positive when an antibody titre ⩾1:20 was detected.
Statistical analysis
The accuracy of the diagnostic methods was assessed against a panel of true positives defined as the samples positive at any coprological techniques and/or rt-PCR. However, this method has a low sensitivity of coprological techniques, entailing the risk of misclassification of samples, in particular, an exceeding proportion of samples might be erroneously classified as negative. Hence, a composite reference standard (CRS) was also used, as a statistical method suggested in absence of a diagnostic gold standard (Rutjes et al., Reference Rutjes, Reitsma, Coomarasamy, Khan and Bossuyt2007). The CRS permits to classify the samples as negative/positive according to the combination of the results of different tests. Here, for this purpose, for the CRS, true positives were defined as samples positive either to coprological methods (i.e. Baermann, direct smear and culture) and/or rt-PCR (first CRS) and to any faecal method and/or positive to both serological tests (second CRS).
The agreement between the different diagnostic tests alone or in combination was measured through Cohen's κ (Cohen, Reference Cohen1960). Test sensitivity was calculated as the proportion of positive results over all positive samples, for both reference methods. Uncertainty was quantified with the 95% confidence interval. The corresponding receiver operating characteristic (ROC) curves were plotted for IFAT serology. Prevalence of the infection in the study population was calculated as the proportion of dogs diagnosed with S. stercoralis infection over all dogs screened. All analyses were done in R, version 3.4.0 (R Core Team, 2017) and Stata s.e. 14.0.
The association between S. stercoralis infection and dog data (i.e. location, gender, age, clinical signs and eosinophilia) was investigated using a logistic regression model. The statistical significance of the model was assessed by χ 2 statistic and P value. The odds ratio of S. stercoralis infection, as defined by CRS, was calculated for each level of the categorical variables. The association between infection by S. stercoralis and other intestinal parasites was evaluated by χ 2 test. The percentage of co-infection with other intestinal parasites compared with dogs positive and negative to S. stercoralis (as defined by CRS) was evaluated by Fisher exact test with permutational adjustment of P values. The same analysis was performed to compare the occurrence of symptomatic dogs. Logistic regression was performed with MedCalc Statistical Software version 18, adjustment of P values was performed with the PROC MULTTEST of the software SAS V9.4 for a personal computer.
Results
Of the 100 dogs sampled (i.e. 51 females and 49 males) 69% were younger and 31% older than 7-years-old. Thirty-six dogs (36%) scored positive to S. stercoralis at coprological methods and/or rt-PCR, with 22.3% (19 of 85) animals positive to Baermann, 6.9% (3 of 43) to Koga agar culture, 4% to the smear (n = 100) and 30% by rt-PCR (n = 100). Feces of 15 animals were not examined by Baermann because of the small amount (<5 g). The agreement between the two tests was assessed by Cohen's κ and resulted fair: 0.40 (95% CI 0.19–0.62). The morphological identification of S. stercoralis collected by Baermann was confirmed by the molecular analysis by the sequencing of the partial cox1 gene, which showed 100% nucleotide identity with a free-living adult of S. stercoralis (GenBank accession n. AJ558163). The infection intensity was evaluated by the Baermann method and a mean of three S. strongyloides larvae per gram of feces was recorded.
Sensitivity and specificity of each diagnostic method is reported in Table 1. According to the CRS2, the most sensitive test was IFAT (93.8%; 82.8–98.7), while the most specific the Baermann (100; CI 91–100) and rt-PCR (100%; CI 92.7–100). When the second CRS was considered (hence the results of serology was added into the analysis), 11 positive cases were further identified and the overall prevalence of infected dogs raised to 56%. All but one case resulting positive to ELISA (n = 30) were also positive to IFAT, which identified further 36 positive samples. The two CRSs showed substantial agreement: 0.72 (95% CI: 0.59–0.85). The ROC curves were plotted for IFAT and they showed an upward trend with specificity for increasing titres, reaching values of around 98% for titres ⩾1:320, when the calculation was assessed against either the first CRS or the second CRS (Fig. 1a and b).

Fig. 1. ROC curves for IFAT assessed against the faecal (a) or faecal and serological CRS (b) composite reference standard.
Table 1. Estimates of diagnostic accuracy of several techniques for the diagnosis of Strongyloides stercoralis infection in 100 dogs
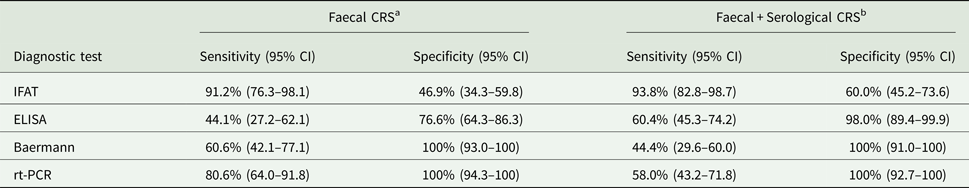
CRS, composite reference standard; rt-PCR (real time polymerase chain reaction).
a Sample is considered as true positive if positive to any of the following method: Baermann, direct smear, culture, rt-PCR.
b Sample is considered as true positive if positive to any faecal method or positive to both ELISA and IFAT.
Of the 20 dogs positive to S. stercoralis at least at one faecal examination test (19 by Baermann and 1 by direct microscopy) at the baseline (T0), a subset of 17 dogs were studied over a 4-week period (T1–T4) by coprological (i.e. Baermann, direct microscopy, culture) and rt-PCR to evaluate intermittency of larval shedding (Table 2). Eleven of the 17 dogs were positive at rt-PCR, whereas of the 6 negative, S. stercoralis larvae were isolated by Baermann and molecularly identified by conventional PCR and sequencing. Ten (58.8%) out of the 17 dogs scored negative at the Baermann at either T3 and/or T4, though rt-PCR confirmed the parasitological results in only five animals (3 of which were already negative at T0) (Table 2). Only two dogs of the above 10 animals, were treated with ivermectin per os soon after the diagnosis because of their severe health conditions. Out of the 17 Baermann positive dogs at T0, 12 were retested four times (T1–T4) showing repeated positive result by Baermann and/or rt-PCR in 50–75% (6–9 dogs), whereas 6 dogs had at least one Baermann test negative before returning positive again (Table 2). Ten dogs rt-PCR positive at T0 were retested four times (T1–T4) with repeated positive result in 60–80% (6–8 dogs) and 5 dogs had at least one test negative before returning positive result again (Table 2).
Table 2. Results of faecal analyses (i.e. Baermann and rt-PCR) in a cohort of 17 infected dogs by weekly (T1–T4) examination for 1 month after S. stercoralis diagnosis by parasitological and serological tests (T0)

a Dogs n 4 and 17 were treated with ivermectin soon after the diagnosis at T0 because of their severe health condition.
The risk of S. stercoralis infection in dogs was not significantly associated to any of the two areas of the shelter (A1 vs A2: OR = 0.61, 95% CI 0.26–1.4, P = 0.2491). The units of each areas of the shelter had no any role in determining risk of S. stercoralis infection neither according to A1 (χ 2 = 3.701, df = 5, P = 0.5931) nor to A2 (χ 2 = 6.116, df = 9, P = 0.7283). Dogs elder than 7 years were not related to higher risk of S. stercoralis infection than the younger (OR = 1.27, 95% CI 0.52–3.13, P = 0.6017). Same result was observed for gender, in particular, the risk of female respect to male dogs was OR = 0.44 (95% CI 0.19–1.02) and was not statistically significant (P = 0.052). Eosinophilia, analysed as continuous variable, was not related to higher risk of S. stercoralis infection (OR = 0.99, 95% CI 0.99–1, P = 0.6529).
Of the 36 dogs positive to S. stercoralis at coprological methods and/or rt-PCR, 44.4% (16/36) were symptomatic at least at one clinical sign showing no statistical significant difference with the asymptomatic dogs (χ 2 = 0.982, df = 1, P = 0.0986). In dogs infected by S. stercoralis, cachexia and/or weight loss are the most common clinical signs (47.2%, 17/36), followed by diarrhoea (13.9%, 5/36). Diarrhoea and weight loss were present in 8.3% dogs (3/36), while diarrhoea and colitis in only one dog (2.7%). In S. stercoralis negative dogs, diarrhoea was observed in 10.9% dogs (7/64), cachexia and weight loss in 14.1% (9/64). The differences between the two groups are not statistically significant for any symptom.
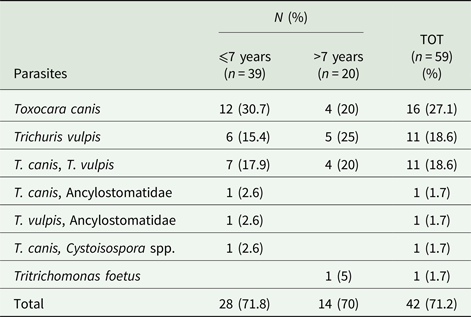
Of the 59 samples available for flotation examination, 42 (71.2%) dogs scored positive for at least one species of intestinal parasite. Among dogs infected by S. stercoralis, 65.4% (17/26) were co-infected with other intestinal parasites, showing no statistical significance compared with S. stercoralis negative dogs (72.7%, 24/33) (χ 2 = 0.364, df = 1, P = 0.5465).
Twenty-eight animals (47.4%) were infected by only one species (27.1% Toxocara canis and 18.6% Trichuris vulpis), while 14 (23.7%) by two species (Table 3). In dogs infected by S. stercoralis, 26.9% (7/26) dogs were co-infected with T. canis, followed by T. vulpis in 15.4% (4/26). The coinfection of T. canis and T. vulpis was observed in 19.2% (5/26) of dogs positive to S. stercoralis. The differences between S. stercoralis infected and non-infected animals are not statistically significant at the Fisher exact test, adjusted for multiplicity. Intestinal parasites were diagnosed in 71.8% and 70% dogs younger and elder than 7-years-old, respectively (χ 2 = 0.0204, P = 0.8864) (Table 3). Among gastrointestinal parasites, Toxocara canis (49%) and T. vulpis (39%) were most frequently identified. Toxocara canis was the most common nematode in animals up to 7 years of age (53.8%; P = 0.946) while T. vulpis in dogs elder than 7 years (35.9%; P = 0.814). At the direct microscopy, one dog resulted positive to Tritrichomonas foetus, typed as ‘feline genotype’ because of ITS rDNA region and eight protein-coding genes matching 100% the ‘feline genotype’ T. foetus (GenBank accession numbers MH647728–MH647736).
Table 3. Number (n) and percentage (%) of dogs positive for other intestinal parasites at the flotation examinations according to age classes
Discussion
The infection by S. stercoralis has been evaluated in a population of shelter dogs by comparing coprological, serological and molecular tests in order to assess their efficiency in diagnosis and monitoring. The occurrence and assessment of prevalence for canine strongyloidiosis still represent a conundrum, because of (i) the lack of a gold standard diagnostic technique, (ii) the low parasite burden and (iii) the intermittent larval shedding (Buonfrate et al., Reference Buonfrate, Paradies, Iarussi, Formenti, Perandin, Otranto and Bisoffi2017a). According to the approach employed, the prevalence of strongyloidiosis detected in dog population ranged from 22.3% by coprology (including Baermann, direct microscopy and culture) to 30% by rt-PCR. This data indicate that the infection is endemic in the shelter as it is further confirmed by the higher prevalence recorded by serology (56%) which indicate that more than half of animal population was exposed to the parasite. Prevalence data are higher than those registered by Baermann in owned (i.e. 0.2–0.9%) (Ferreira-Júnior et al., Reference Ferreira-Júnior, Goncalves-Pires, Silva, Goncalves and Costa-Cruz2006; Gates and Nolan, Reference Gates and Nolan2009; Riggio et al., Reference Riggio, Mannella, Ariti and Perrucci2013; Paradies et al., Reference Paradies, Iarussi, Sasanelli, Capogna, Lia, Zucca, Greco, Cantacessi and Otranto2017) and shelter dogs from Italy, Brazil and the USA (0.6–8.1%) (Gonçalves et al., Reference Gonçalves, Machado, Gonçalves-Pires, Ferreira-Júnior, Silva and Costa-Cruz2007; Paradies et al., Reference Paradies, Iarussi, Sasanelli, Capogna, Lia, Zucca, Greco, Cantacessi and Otranto2017). Such a higher prevalence could be due to the peculiarity of the studied population since such a large number of dogs (i.e. 800) living in a confined environment and with wicked health conditions, are at high risk of infection. Indeed, in studies carried out in different epidemiological contexts the prevalence of strongyloidiosis is similar to that recorded in dogs living in villages from Cambodia and Brazil (e.g. 14.9–26.3%) (Martins et al., Reference Martins, Barros Cda, Bier, Marinho, Figueiredo, Hoffmann, Molento and Biondo2012; Schär et al., Reference Schär, Inpankaew, Traub, Khieu, Dalsgaard, Chimnoi, Chhoun, Sok, Marti, Muth and Odermatt2014). Current data demonstrate that coupling of rt-PCR with Baermann is an optimal approach to detect patent infection and therefore reservoir dogs, although missing diagnoses could occur. Our data show that rt-PCR should be performed at least twice a week apart, while Baermann should be repeated three times to improve the identification of intermittent larval shedding.
In studies where more than one diagnostic method has been used for the diagnosis of S. stercoralis the prevalence of the infection recorded by serology was higher in both owned and shelter dogs (20.9–24.3%) than that by Baermann (0.6–0.9%) (Ferreira-Júnior et al., Reference Ferreira-Júnior, Goncalves-Pires, Silva, Goncalves and Costa-Cruz2006; Gonçalves et al., Reference Gonçalves, Machado, Gonçalves-Pires, Ferreira-Júnior, Silva and Costa-Cruz2007).
Amongst the parasitological techniques, the Baermann detected the highest number of S. stercoralis infected animals (22.3%), followed by culture (6.9%) and direct microscopy (4%), suggesting that the former is the most suitable tool for the diagnosis of strongyloidiosis. Nonetheless, rt-PCR successfully diagnosed further positive samples leading to an overall prevalence of 30%.
The prevalence of positive animals herein detected by coprological methods is lower than that recorded in human surveillance studies of populations with high risk of infection such as children community or immigrants with clinical signs or those living in less developed countries (Glinz et al., Reference Glinz, Silué, Knopp, Lohourignon, Yao, Steinmann, Rinaldi, Cringoli, N'Goran and Utzinger2010; Schär et al., Reference Schär, Inpankaew, Traub, Khieu, Dalsgaard, Chimnoi, Chhoun, Sok, Marti, Muth and Odermatt2014; Cabezas-Ferdández et al., Reference Cabezas-Fernández, Salas-Coronas, Lozano-Serrano, Vazquez-Villegas, Cabeza-Barrera and Cobo2015).
Higher prevalence of human strongyloidiosis was recorded when similar methodologies were employed such as the Kato-Katz technique by multiple examinations of the stool sample (Glinz et al., Reference Glinz, Silué, Knopp, Lohourignon, Yao, Steinmann, Rinaldi, Cringoli, N'Goran and Utzinger2010) or when each stool sample from the same individual was plated onto ten agar plates (Buonfrate et al., Reference Buonfrate, Perandin, Formenti and Bisoffi2017b).
The good performance of rt-PCR and the good specificity of IFAT with high IgG titres in the diagnosis of human infection were already recorded in a retrospective study on 223 human patients (Buonfrate et al., Reference Buonfrate, Perandin, Formenti and Bisoffi2017b). Therefore, although faecal examination presents inherent limitations due to the collection of fresh feces, the combination of parasitological and molecular tests improves the diagnosis of dogs infected by S. stercoralis. The accuracy of serology, molecular and coprological tests for S. stercoralis is detected against a primary CRS (using faecal-based positivity) and a secondary CRS (which includes serological positivity). Through Baermann examination, the risk of any false positive results was excluded considering both the morphological identification of larvae and their confirmatory sequencing. Nonetheless, the lower performance of Baermann than rt-PCR suggests that a single stool examination by Baermann may fail to detect S. stercoralis larvae in infected hosts, also due to the low viable worm burden. Conversely, rt-PCR may detect also unviable larvae, when present.
Even considering the IFAT limitations for the diagnosis of strongyloidiosis in dogs (i.e. difficulty in setting up the test, in result interpretation and lack of validation in dogs), the test was the most sensitive according to the CRS2 analysis (Table 1), as demonstrated for the diagnosis in humans (Requena-Méndez et al., Reference Requena-Méndez, Chiodini, Bisoffi, Buonfrate, Gotuzzo and Muñoz2013; Bisoffi et al., Reference Bisoffi, Buonfrate, Sequi, Mejia, Cimino, Krolewiecki, Albonico, Gobbo, Bonafini, Angheben, Requena-Mendez, Muñoz and Nutman2014; Buonfrate et al., Reference Buonfrate, Perandin, Formenti and Bisoffi2017b). Although the usefulness of IFAT for both diagnosis and prevalence studies has been scantly investigated in dogs (Ferreira-Júnior et al., Reference Ferreira-Júnior, Goncalves-Pires, Silva, Goncalves and Costa-Cruz2006; Gonçalves et al., Reference Gonçalves, Machado, Gonçalves-Pires, Ferreira-Júnior, Silva and Costa-Cruz2007), a higher prevalence of infected animals was recorded by serology than by coprology. In spite of the low specificity of the IFAT (i.e. up to 60% in CRS2), this technique could be useful for screening animal populations also considering the chronic status of the disease and its lethality in immunocompromised individuals. The diagnostic performance of IFAT provided by ROC analysis indicated that the specificity increased for higher titers (⩾1:320), which could be used for a certain diagnosis of strongyloidiosis without the support of any faecal-based tests. Data confirming that the specificity of IFAT increases for high titres has been reported also in humans (Bisoffi et al., Reference Bisoffi, Buonfrate, Sequi, Mejia, Cimino, Krolewiecki, Albonico, Gobbo, Bonafini, Angheben, Requena-Mendez, Muñoz and Nutman2014; Buonfrate et al., Reference Buonfrate, Perandin, Formenti and Bisoffi2017b).
The study on the 4-week follow-up on dogs positive to S. stercoralis at the parasitological examination (Table 2) indicated the inconsistent shedding of L1, suggesting the importance of multiple collections of feces for a reliable diagnosis, mostly if animals screened positive at the serology. The parasitological positivity of dogs at T0 overlaps the results of rt-PCR, except for six animals, which, scored positive to IFAT only with titres ⩾1:80 (Table 2).
The only two dogs scored positive to S. stercoralis at T0 and negative at 3 and/or 4 weeks follow-up at both Baermann and rt-PCR may depend only on the sensitivity of the parasitological tests. Of the two dogs treated with ivermectin soon after the strongyloidiosis diagnosis, one dog died despite the treatment, whereas the other dog discontinued to shed L1 in the feces during the follow-up period as detected by Baermann and rt-PCR, suggesting the usefulness of both tests in monitoring the treatment efficacy (Mansfield and Schad, Reference Mansfield and Schad1992; Yang et al., Reference Yang, Gebeyehu, Jung, Kwon and Kwak2013).
The gastrointestinal worms remain an important and common finding in shelter dogs, including those of zoonotic concerns (Martínez-Carrasco et al., Reference Martínez-Carrasco, Berriatua, Garijo, Martínez, Alonso and de Ybáñez2007; Martins et al., Reference Martins, Barros Cda, Bier, Marinho, Figueiredo, Hoffmann, Molento and Biondo2012; Neves et al., Reference Neves, Lobo, Simões and Cardoso2014; Schär et al., Reference Schär, Inpankaew, Traub, Khieu, Dalsgaard, Chimnoi, Chhoun, Sok, Marti, Muth and Odermatt2014). The overall prevalence of intestinal helminths (71.2%) reflects the living conditions in a kennel where overcrowding and environmental contamination favour parasite transmission and maintenance of infestations (Otranto et al., Reference Otranto, Dantas-Torres, Mihalca, Traub, Lappin and Baneth2017). In addition, the high prevalence of S. stercoralis along with T. canis (49.1%) and Ancylostomatidae (3.4%) is of concern as it represents a potential hazard for shelter workers. Therefore, more effective treatment strategies and improvement in measures for the prevention and control of these infections are required. The detection of Tritrichomonas foetus ‘feline genotype’ in dogs is unexpected (Šlapeta et al., Reference Šlapeta, Müller, Stack, Walker, Lew-Tabor, Tachezy and Frey2012; Gookin et al., Reference Gookin, Hanrahan and Levy2017). The single case of T. foetus in a shelter dog microscopically and molecularly identified is of relevance because the epidemiology of feline trichomonosis and the spectrum of reservoir hosts is not known.
In conclusion, the use of rt-PCR and Baermann tests is an optimal approach to detect S. stercoralis patent infection, and therefore the reservoir dogs, although intermittent larval shedding may result in a false negative result. Therefore, IFAT assay, highly sensitive for screening and specific for diagnosis with IgG titres ⩾1:320, should be used in combination with parasitological exams for diagnosis of strongyloidiosis. Under the above circumstances and considering the tight human-dog bounds, an improvement in the control and prevention of parasite infections is mandatory. The finding of an effective therapy in dogs, the monitoring of larval shedding and antibodies titres in infected animals after treatment, would deserve further investigations.
Acknowledgements
We are grateful to Dr Vito Colella (University of Bari) for his suggestions and comments to the manuscript and to the shelter's workers for their invaluable support.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The protocol of this study was approved by the Ethical Committee of the Department of Veterinary Medicine of the University of Bari, Italy (Prot. Uniba 6/17).







