Introduction
Like all organisms, insect pests evolve through heritable genetic variation and selection imposed by environmental factors, some of which can be challenging to identify. However, the evolution of adaptive traits such as insecticide resistance is not only of great practical and economic significance, but takes place in response to strong unambiguous selection pressures resulting from intense agricultural activity. Intuitively, in these situations, insecticide-resistant individuals should suffer fitness costs in the absence of insecticide pressure compared with wild-type susceptible ones because, if they didn't, resistance alleles would be more common prior to insecticide exposure (Crow, Reference Crow1957). Thus, adaptive changes associated with exposure to insecticides should be constrained by deleterious pleiotropic costs of genes conferring resistance (Roush & McKenzie, Reference Roush and McKenzie1987). To support this, there is growing evidence of handicaps caused by disruptive side-effects on fitness traits, including behaviour, probably because insecticide resistance mutations occur in highly-conserved proteins in the nervous system or because they interfere with other biological functions. Such ‘fitness trade-offs’ associated with resistance are probably exacerbated by the phenomenon evolving during a period of rapid environmental change, whereas characters that show high overall adaptation or co-adaptation are thought normally to take a long time to evolve (McKenzie, Reference McKenzie1996).
Previous work on peach-potato aphids, Myzus persicae (Sulzer), has revealed negative pleiotropic effects of two separate insecticide resistance mechanisms on survival that appear to be mediated through altered behaviour (Foster et al., Reference Foster, Harrington, Devonshire, Denholm, Devine, Kenward and Bale1996, Reference Foster, Harrington, Devonshire, Denholm, Clark and Mugglestone1997). These mechanisms are carboxylesterase resistance, primarily to organophosphates and conferred by overproduction of an insecticide-detoxifying enzyme (Field et al., Reference Field, Devonshire and Forde1988), and knock-down resistance (kdr) to pyrethroids conferred by a single point mutation in the voltage-gated sodium channel in the nervous system (Martinez-Torres et al., Reference Martinez-Torres, Foster, Field, Devonshire and Williamson1999). Both mechanisms are widely distributed in M. persicae in the UK and mainland Europe and are probably present in this species globally. One dramatic form of altered aphid behaviour associated with insecticide resistance is a much lower propensity to respond to aphid alarm pheromone, (E)-β-farnesene (Foster et al., Reference Foster, Woodcock, Williamson, Devonshire, Denholm and Thompson1999, Reference Foster, Young, Williamson, Duce, Denholm and Devine2003, Reference Foster, Denholm, Thompson, Poppy and Powell2005), a semio-chemical released from the cornicle secretions exuded by aphids when disturbed by natural enemies (Pickett et al., Reference Pickett, Wadhams, Woodcock and Hardie1992; Hardie et al., Reference Hardie, Pickett, Poe, Smiley, Hardie and Minks1999). (E)-β-farnesene causes neighbouring aphids to withdraw their mouthparts from the plant and disperse away from the pheromone source and has been shown to act as a strong kairomone that attracts female parasitoids (Foster et al., Reference Foster, Denholm, Thompson, Poppy and Powell2005). Parthenogenetic insecticide-susceptible M. persicae clones (high alarm responders) and resistant clones (low alarm responders) were used to test the intriguing hypothesis that the evolution of metabolic and target-site insecticide resistance can result in greater vulnerability to parasitoids (Foster et al., Reference Foster, Tomiczek, Thompson, Denholm, Poppy, Kraaijeveld and Powell2007). This demonstrated, for the first time, the existence of a fitness trade-off expressed through interactions with another trophic level. Small cohorts of aphids were exposed to adult females of Diaeretiella rapae (McIntosh) (a frequent parasitoid of M. persicae) on leaf discs in small-scale, Petri dish assays. Observations, made through a binocular microscope, showed that second/third instar aphids from insecticide-susceptible clones were encountered and attacked by parasitoids significantly less than resistant clones. Insecticide-susceptible aphids also dispersed more readily after parasitoid attacks, with these differences culminating in them suffering significantly lower levels of mummification (parasitism).
Here, we describe laboratory experiments investigating whether the vulnerability of insecticide-susceptible and -resistant clones of M. persicae to endoparasitism also occurs over larger spatial scales ranging from individual leaves to groups of whole plants in large chambers, more closely simulating a field crop.
Materials and methods
Rearing of Myzus persicae clones
All experiments and insect rearing regimes used a 21°C per 16 h photoperiod and Chinese cabbage (Brassica napus L var chinensis cv Wong Bok) as host plants. Eight green-bodied parthenogenetic M. persicae clones, from the UK and mainland Europe, chosen because they represented the extremes of carboxylesterase and kdr insecticide resistance, were used (table 1). These had been used previously in Petri dish assays by Foster et al. (Reference Foster, Tomiczek, Thompson, Denholm, Poppy, Kraaijeveld and Powell2007). The clones were categorised into four resistance genotypes each represented by two clones:
(i) fully insecticide-susceptible (high alarm responders);
(ii) extreme carboxylesterase (R3) lacking kdr (low alarm responders);
(iii) carboxylesterase-susceptible (S), homozygous kdr resistant (RR) (low alarm responders); and
(iv) extreme carboxylesterase (R3), kdr RR (low alarm responders)
The third category were full carboxylesterase-revertants, i.e. phenotypically carboxylesterase-susceptible through loss of expression of highly-amplified, carboxylesterase genes (Field et al., Reference Field, Devonshire, ffrench-Constant and Forde1989). Revertants were used because ‘true’ carboxylesterase-susceptible clones, carrying single copies of the carboxylesterase genes together with the kdr mechanism, have not been found in M. persicae so far. Each clone had been originally established from an individual female and then reared parthenogenetically on excised leaves in small plastic box-cages (8 cm high×5 cm wide×2 cm deep). New generations were produced by transferring young adult apterae, using a wetted fine paintbrush, to each box. After several days, the parents were removed, leaving age-synchronised cohorts of nymphs.
Table 1. Myzus persicae clones used in experiments at different spatial scales.
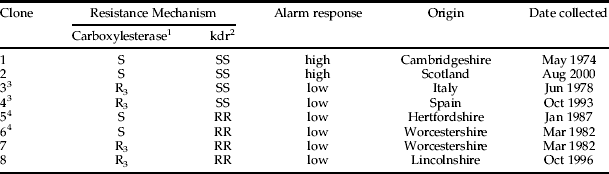
1 Based on a total esterase assay (Grant et al., Reference Grant, Bender and Hammock1989).
2 Based on a Taqman method (Anstead et al., Reference Anstead, Williamson and Denholm2004). SS, homozygous-susceptible; RR, homozygous-resistant.
3 Mainland Europe clones, chosen because this genotype has not been found in the UK to date.
4 Phenotypically carboxylesterase-S but genotypically carboxylesterase-R3 (Field et al., Reference Field, Devonshire, ffrench-Constant and Forde1989).
Clonal integrity was checked regularly using a kinetic total esterase assay (Grant et al., Reference Grant, Bender and Hammock1989) for the carboxylesterase mechanism and a real-time PCR DNA assay (Anstead et al., Reference Anstead, Williamson and Denholm2004) for kdr genotype.
Rearing of Diaeretiella rapae
A laboratory culture of the parasitoid D. rapae, established from a UK field sample collected in 1995, was maintained on a fully insecticide-susceptible clone of M. persicae on young Chinese cabbage plants housed in wood-framed cages (60 cm high×28 cm wide×53 cm deep), enclosed with glass on the top and door and fine-mesh nylon gauze on the remaining sides. Subsequent parasitoid generations were set up by inoculating plants with excised leaves supporting abundant M. persicae adults and nymphs. Several days later, at least 30 adult male and female parasitoids were added from an older cage. Under these conditions, parasitoid generation time was approximately two weeks with aphid mummies beginning to appear about nine days after the addition of parasitoids.
Inexperienced, virgin adult D. rapae females (1–2 days old) were used throughout the experiments to eliminate any potential heterogeneity in behaviour caused by prior contact with aphids or other parasitoids. Individual mummies were cut from plants and isolated, on small sections of leaf, in sealed, small, glass vials that were checked for adult parasitoid emergence twice daily. Any adults that eclosed were sexed, and the females allowed to feed from small pieces of compacted tissue paper, soaked with 30% honey solution, added to each vial.
Movement and mummification of Myzus persicae clones at different spatial scales
Experiments on parasitism and aphid dispersal were done at three spatial scales. These used single excised leaves, single whole plants or groups of plants. In the latter, parasitism was assessed in a larger, more complex spatial environment containing a choice for parasitoids of either all eight M. persicae clones or just two clones. Assessments of mummification were done at least 11 days after exposure to parasitoids as this period was shown to be sufficient to allow all symptoms of parasitism to develop (Foster et al., Reference Foster, Tomiczek, Thompson, Denholm, Poppy, Kraaijeveld and Powell2007). Any second-generation aphids that were subsequently produced were not included in the assessments since they had not been born during the period of exposure to parasitoids.
In all the experiments, aphid movement was measured in the presence and absence (controls) of parasitoids at the end of the period of exposure. An additional assessment of parasitoid emergence was done in the experiment using single plants.
Experiments with single excised leaves
Thirteen separate experiments were done, each containing up to ten replicates of each M. persicae clone. Some experiments did not contain all eight clones. For each experiment, primary leaves (about 5 cm in length) were cut at the petiole from two-week-old plants and placed, abaxial-side up, into small plastic box cages. Each leaf was then inoculated on the abaxial side with three young adult M. persicae apterae, which were removed two days later after they had produced first generation (G1) nymphs. These were allowed to develop to the second/third instar stage when a surplus was removed, leaving a cohort of approximately 15 nymphs per replicate (on the abaxial side of leaves). Each cohort was then exposed to D. rapae attack by aspirating parasitoid females (one per replicate) in random order into each box cage, which were then stored vertically for two hours in the light. Afterwards, all parasitoids were removed and an assessment of any aphid movement off the excised leaf was made. The boxes were then left in trays of water until the number of mummies forming in each replicate was recorded.
Experiments with single plants
Eleven separate experiments were done, each containing one replicate of each M. persicae clone. Some experiments did not contain all eight clones. For each experiment, eight two-week-old plants, grown singly in plastic pots (11.5 cm in diameter), were taken from a glasshouse, and each clone was allocated randomly to one plant. Twelve second or third instar nymphs (depending on experiment) were added to the first and second leaves (six nymphs per leaf) of each plant. The plant was then covered with an inverted clear plastic pot (19 cm high×13 cm diameter) containing gauze-covered aeration holes, and the aphids were left to settle for 24 h. One parasitoid female was then added, using an aspirator, to each plant and the cover replaced. The parasitoids were left for 6 h (a period shown in preliminary experiments to lead to adequate mummification) in the light, and then removed using an aspirator. At this point, the number of aphids in each replicate that had moved to other parts of the plant or completely off the plant was recorded. All aphids were then transferred to an excised leaf in a small plastic box (one replicate per box) until mummification was recorded. In these experiments, all mummies and surrounding small sections of leaf were isolated in glass vials to measure successful parasitoid adult emergence. Four additional experiments (controls) were also done to assess aphid movement in the absence of parasitoids.
Experiments using groups of plants (one aphid clone per plant)
Eight separate experiments (replicates) were done each containing one replicate for each of the eight M. persicae clones. For each experiment, eight plants, grown as for the single plant experiments, were placed in large chambers (1 m high×1 m wide×2 m long) (Foster & Devonshire, Reference Foster and Devonshire1999) and arranged into a circular ‘clock face’ design (approximately 60 cm in diameter) on the chamber floor, which was covered with blue tissue paper. Each clone was then allocated to one plant in each of the experiments using a Latin square design. Each plant was inoculated with 12 second or third instar nymphs on the first and second leaves (six nymphs per leaf), and the aphids were left to settle for 24 h. Five parasitoid females were then released from a Petri dish placed in the centre of the circle of plants. After 6 h, the numbers of aphids in each plant replicate that were (i) remaining on the inoculation leaves, (ii) retrieved on other parts of the plant or (iii) found off the plant (either on the surface of the compost, the pot or the floor of the chamber) were recorded. The three groups of aphids from each plant replicate were then reared separately on excised leaves until mummification was recorded.
Experiments using groups of plants (two aphid clones per plant)
Eight separate experiments (replicates) were done using similar methods to the previous study but allowing parasitoids a choice of aphid clones on each plant. Only two M. persicae clones in total were assessed; one fully insecticide-susceptible (Clone 1) and the other with both resistance mechanisms (Clone 8) (table 1). Two large chambers were used in each experiment. Six plants were placed into one chamber (subsequently using parasitoids) and four into the other. Each plant was inoculated with ten second or third instar nymphs from both clones (five per leaf), and the aphids were left to settle for 24 h. Aphids in the first chamber were then exposed to attack by five parasitoid females. These were again released from a Petri dish in the centre of the plants. The aphids in the other chamber were used as controls and not exposed to parasitoids. Treatments were alternated between chambers for subsequent experiments. After six hours, the position of retrieved aphids was recorded, and they were transferred to excised leaves (three per plant as before) until mummification was recorded. However, because two aphid clones were inoculated onto each plant, aphids also needed to be distinguished. This was done using a biochemical test, validated to ensure that resistance testing could be extended to parasitized aphids up to a certain age (see below).
Total esterase test distinguishing carboxylesterase resistance level of mummified aphids
Although intact adult aphids from carboxylesterase-S and R3 M. persicae are easily identified using a total esterase assay (Grant et al., Reference Grant, Bender and Hammock1989), it was not known how robust this is for mummified aphids. To test this, 18 box-cages per clone, each containing two young adult apterae on an excised leaf, were set up for Clone 1 (fully susceptible) and Clone 8 (est-R3/kdr-RR) giving 36 boxes in total. All adults and any surplus nymphs were removed two days later, leaving a synchronised cohort of about 15 first instar offspring in each box. After a further three days, when the aphids were at the second/third instar stage, single virgin parasitoid females were aspirated into nine of the boxes of each clone (designated ‘P’ boxes: aphids exposed to parasitoids). The remaining boxes were designated ‘C’ boxes (controls not exposed to parasitoids). Parasitoids were removed seven hours later, and all boxes were left for seven days. Aphids in the P boxes, thereafter, were assessed daily for signs of parasitism, and a random selection of the darker individuals (symptomatic of parasitism) were placed individually into wells of a microplate containing 50 ul of Tween buffer. A random selection of the control aphids was also plated. This process was repeated up until day 15 after exposure to parasitoids. All plated aphids were assessed for their total esterase content.
Statistical analysis
All the parameters were analysed as proportions by analysis of deviance using a generalised linear model (McCullagh & Nelder, Reference McCullagh and Nelder1994), assuming a probit link function and a binomial error distribution. Associations of the parameters with high or low alarm response (table 1) were investigated only if the M. persicae clones in resistance categories 2, 3 and 4 (low alarm responders) were statistically homogeneous and, therefore, could be pooled for comparison with clones in category 1 (high alarm responders). This proved possible in all comparisons of aphid movement and mummification.
Results
Aphid retrieval
There was consistently very high aphid retrieval at the assessment points for movement (at the end of exposure to parasitoids) and mummification (after aphid incubation) in both the control and the parasitoid exposure replicates, i.e. very few aphids were missing or died due to causes other than mummification during the course of the experiments.
Aphid movement
In all the control replicates (without parasitoids) at the three spatial scales, there was very little aphid movement from their inoculation leaves. In these, no aphids were found off their inoculation leaves in the single excised leaf experiments. Furthermore, very few aphids moved from their inoculation leaves to other parts of the plant in the experiments using single plants and groups of plants.
When aphids were exposed to parasitoids, no significant differences were seen between the insecticide-susceptible and -resistant clones in movement off their inoculation leaves in the excised leaf experiments (F1,106 0.30, P=0.58; table 2). In the single plant experiments, there were no significant differences between susceptible and resistant aphids in either movement from their inoculation leaves to another part of the plant (F1,20 0.23, P=0.64; table 2) or in the number of aphids being found completely off their plants, i.e. on the compost or pot (F1,27 0.16, P=0.70; table 2).
Table 2. Percent movement of insecticide-susceptible (S) and -resistant (est-R3, kdr-RR and est-R3/kdr-RR) Myzus persicae from inoculation leaves or plants after exposure to parasitoids in experiments at different spatial scales. Figures in bold show significant differences (P<0.05) for comparisons between S aphids versus the other categories (pooled). Standard errors are shown in parenthesis.
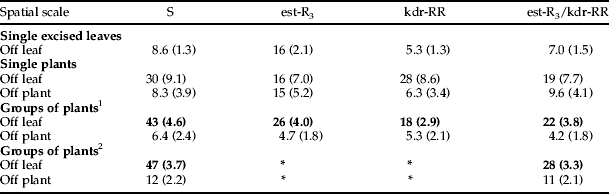
1 One clone per plant.
2 Two clones per plant (only clone 1, insecticide-S, and clone 8, est-R3/kdr-RR, were tested).
When aphids were exposed to foraging parasitoids on groups of plants, no aphids were found on the chamber floor in any of the experiments, suggesting no movement of aphids between plants. In contrast to the experiments at smaller spatial scales, a significantly greater proportion of aphids from insecticide-susceptible clones moved from their inoculation leaves to other leaves on the same plant or off the plant compared to the insecticide-resistant aphids in both sets of experiments (one clone per plant: F1,42 19.3, P<0.0001; two clones per plant: F1,47 14.7, P<0.001; table 2). However, very few aphids had moved completely off the plants, i.e. were retrieved on the compost or the pot itself, and there was no difference between the insecticide-susceptible and -resistant clones for this parameter (one clone per plant: F1,42 0.44, P=0.51; two clones per plant: F1,47 0.05, P=0.83; table 2). This suggests that M. persicae, irrespective of insecticide resistance, displays low complete dislodgment from plants after attack by D. rapae, at least under the conditions used.
Parasitoid emergence
The success of parasitoids emerging from aphid mummies was measured in the experiment using single plants. This proved to be consistently very high (>95%) for all the M. persicae clones. In all but one case, these parasitoids were male, as would be expected with virgin mothers. The one female offspring produced from a virgin mother (from Clone 8) represents a very rare phenomenon that has been reported previously in Lysiphlebus testaceipes (Wesmae) (Whiting, Reference Whiting1918).
Mummification
A small number of replicates in the single excised leaf study (3.1%) and the single plant study (7.9%) were found not to have produced any mummies. These were excluded from the analyses as they probably reflected parasitoids that were sedentary during the period of exposure. All of the replicates in experiments using groups of plants produced at least one mummy (probably because more than one parasitoid was released).
Significantly fewer insecticide-susceptible aphids compared to resistant aphids became mummified in the single excised leaf study (F1,106=32.9, P<0.0001; fig. 1) and the single plant study (F1,44=29.0, P<0.0001; fig. 2). This trend was also apparent in experiments using groups of plants, both with one clone per plant (F1,42=15.5, P<0.001; fig. 3) and two clones per plant (F1,47=7.74, P<0.01; fig. 4). Interestingly, in the latter study, an assessment of parasitisation, with respect to aphid retrieval position after exposure to parasitoids, showed that higher proportions of mummies developed in aphids that had moved to other leaves or off the plant, compared to those aphids retrieved on their initial inoculation leaves, in both clones (fig. 5). Thus, although overall mummification was significantly lower in the insecticide-susceptible aphids, they still suffered a comparatively similar increase in mummification as the resistant aphids when they moved from their inoculation leaves, possibly in response to a parasitoid attack.

Fig. 1. Proportion of aphids mummified in study using excised leaves. Limits of bars indicate ±1 standard error. □, high alarm responders; ▪, low alarm responders.

Fig. 2. Proportion of aphids mummified in study using single plants. Limits of bars indicate ±1 standard error. □, high alarm responders; ▪, low alarm responders.

Fig. 3. Proportion of aphids mummified in study using groups of plants (one clone per plant). Limits of bars indicate ±1 standard error. □, high alarm responders; ▪, low alarm responders.

Fig. 4. Proportion of aphids mummified in study using groups of plants (two clones per plant). Limits of bars indicate ±1 standard error. □, high alarm responders; ▪, low alarm responders.
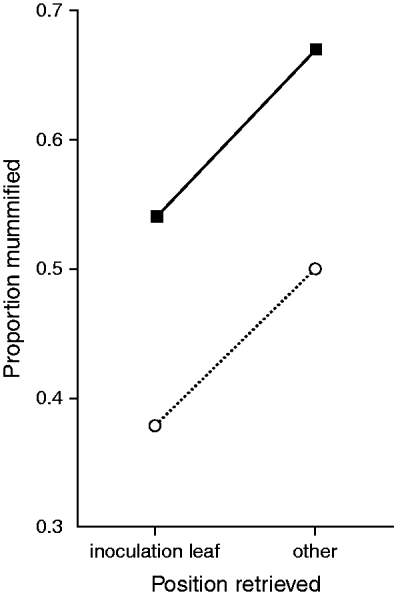
Fig. 5. Proportion of aphids mummified in those remaining on the inoculation leaves and those that moved to another part of the plant or off the plant in study using groups of plants (two clones per plant) (- - -○- - -, susceptible; ▪, est-R3/kdr-RR).
Total esterase test distinguishing carboxylesterase resistance level of mummified aphids
The first signs of darker, parasitised M. persicae were seen nine days after exposure to D. rapae, and by day ten mummies could be clearly seen (fig. 6). This suggests that timescales earlier than ten days should not be used to assess parasitism under the experimental conditions used in this study. No new mummies were recorded after day 11. Mean total esterase values for parasitised and control aphids plotted versus day after exposure to parasitoids demonstrated that mummified carboxylesterase-S and -R3 M. persicae could be distinguished clearly up until 12 days after exposure to parasitoids (fig. 7). Beyond this period, there was a sharp fall in total esterase titres of parasitized aphids to levels comparable with susceptible aphids.

Fig. 6. Development of mummies (% of aphids) in Myzus persicae versus time after exposure to Diaeretiella rapae. □, high alarm responder; ▪, low alarm responder.

Fig. 7. Total esterase activity (mOD/min) in (a) carboxylesterase-S and (b) carboxylesterase-R3 Myzus persicae between eight and 15 days after exposure to Diaeretiella rapae. ○, carboxylesterase-S un-mummified; ●, carboxylesterase-S mummified; □, carboxylesterase-R3 un-mummified; ▪, carboxylesterase-R3 mummified.
Discussion
The experiments done at three spatial scales showed that insecticide-resistant M. persicae clones (low alarm responders) consistently suffered significantly greater levels of mummification by D. rapae than insecticide-susceptible clones (high alarm responders). Smaller differences in mummification were recorded in the single excised leaf experiments, probably because the limited areas and relatively long exposure time reduced the opportunity for aphid escape and the impact of any advantages/disadvantages associated with differential behaviour amongst the aphid genotypes. In other words, it may have been harder for aphids to escape from parasitoids.
At all the spatial scales, few aphids moved completely off their food source (leaves or plants), and there were no statistical differences amongst the resistance genotypes. This suggests that insecticide-susceptible and -resistant M. persicae show similarly low amounts of complete dislodgment from plants after attack by D. rapae and, therefore, are probably not under subsequent differential selection due to predation by ground-dwelling predators, such as carabid beetles, in the wild (Chau & Mackauer, Reference Chau and Mackauer1997; Sunderland et al., Reference Sunderland, Axelsen, Dromph, Freier, Hemptinne, Holst, Mols, Petersen, Powell, Ruggle, Triltsch and Winder1997). In contrast, movement to another part of the plant, which was possible to measure in the single plant and multi plant experiments, was greater for insecticide-susceptible M. persicae, but there were only significant differences in this behaviour in the latter possibly as a result of the larger spatial scale. Such differences in behaviour associated with insecticide resistance may be magnified further for aphids in the wild, as there will be even greater scope for dispersal and avoidance of parasitism.
The applicability of our findings for M. persicae with a broader geographical origin remains to be established. However, assays for aphid alarm response done on M. persicae clones with a wide range of origins, including Europe and New Zealand, and with different micro-satellite genotypes have implied that the R3 carboxylesterase and kdr mechanisms are consistently associated with low responses to the aphid alarm pheromone (E)-ß-farnesene (Foster et al., Reference Foster, Woodcock, Williamson, Devonshire, Denholm and Thompson1999, Reference Foster, Young, Williamson, Duce, Denholm and Devine2003, Reference Foster, Denholm, Thompson, Poppy and Powell2005; van Toor et al., Reference van Toor, Foster, Anstead, Mitchinson, Fenton and Kasprovicz2008), which previous (Foster et al., Reference Foster, Tomiczek, Thompson, Denholm, Poppy, Kraaijeveld and Powell2007) and current experiments show to be associated with vulnerability to parasitoids. Because of the consistency of the phenomenon, we hypothesise that reduced alarm response is directly attributable to insecticide resistance, rather than the alternative explanation that resistance genes are closely linked to ones affecting behaviour. However, this will continue to be tested using aphids carrying new micro-satellite genotypes as they become available. These are most likely to come from abroad, as the UK M. persicae population appears to have limited genetic variation (Fenton et al., Reference Fenton, Malloch, Woodford, Foster, Anstead, Denholm, King and Pickup2005), primarily due to the lack of sexual reproduction in this country.
Our findings strengthen evidence that insecticide resistance genes can have negative pleiotropic effects on overall biological fitness, including important behavioural traits, by producing maladaptive behaviours. The results also support a view that aphid alarm behaviour has evolved in the context of selection pressure exerted by natural enemies. The phenomenon provides the first demonstration that interactions with a higher trophic level (parasitoids) can potentially have inhibitory effects on the evolution of insecticide resistance in herbivorous pests. Parasitoids, therefore, may play an important role in maintaining genetic diversity, in this case by influencing the dynamics of resistance and countering a progressive increase in its frequency in response to insecticide pressure. In addition to parasitoids, a number of specialised predators, such as ladybirds and hoverfly larvae, also forage for aphids on plants. These too could preferentially attack and kill insecticide-resistant aphids showing a reduced alarm response. This merits further investigation.
Acknowledgements
The authors thank Diana Cox for testing the aphid clones for carboxylesterase resistance and kdr. The work was supported by the Biotechnology and Biological Sciences Research Council of the United Kingdom.











