Introduction
Green gram [Vigna radiata (L.) Wilczek] (Fabaceae), commonly known as mung bean, is a low altitude crop grown from sea level to ca. 2000 m (Akpapunam, Reference Akpapunam, Nwokolo and Smartt1996). The plant is cultivated for production of pulses in India, Bangladesh, Srilanka, Pakistan, China, Thailand, Philippines, Indonesia, Burma and Bangladesh as well as hot and dry regions of South Europe and Southern USA (Dikshit et al., Reference Dikshit, Jyoti, Aski, Mishra and Akanksha2017). India is the largest pulse producer in the world and occupies a unique position in the agricultural economy of India (Singh & Ahlawat, Reference Singh and Ahlawat2005). The pulse is an important source of easily digestible high-quality protein for vegetarians and sick persons. The plant has been shown to possess antioxidant, antimicrobial, anti-inflammatory, antidiabetic, antihypertensive and antitumor activities (Bhatty et al., Reference Bhatty, Gilani and Nagra2000).
Insect pests are one of the major constraints limiting yield potential of mung bean throughout the world (Devesthali & Joshi, Reference Devesthali and Joshi1994; Atwal & Dhaliwal, Reference Atwal and Dhaliwal2005). Spilosoma obliqua Walker (Syn. Diacrisia obliqua) (Lepidoptera: Arctiidae), commonly known as Bihar hairy caterpillar, is one of the major phytophagous pests of this crop in India, Bangladesh, Bhutan, Srilanka, Pakistan and south-eastern Afghanistan (Varatharajan et al., Reference Varatharajan, Singh, Keisa, Singh and Selvasundaram1998). The insect is also a serious pest of sunflower (Varatharajan et al., Reference Varatharajan, Singh, Keisa, Singh and Selvasundaram1998), sesame (Biswas, Reference Biswas2006), soybean (Chaudhary, Reference Chaudhary2009), jute (Selvaraj et al., Reference Selvaraj, Gotyal, Satpathy and Meena2015), black gram and cowpea (Haribhai, Reference Haribhai2015). The first to third instar larvae feed voraciously on the lower epidermis of leaves, while fourth to sixth instar larvae consume whole leaf tissue, leaving only the ribs, and the plants may be completely defoliated (Gupta & Bhattacharya, Reference Gupta and Bhattacharya2008). Further, in case of severe attack, the top shoots of the plant are eaten up (Gupta & Bhattacharya, Reference Gupta and Bhattacharya2008). Till date information on the biology of S. obliqua on green gram is meager.
Applications of synthetic pesticides are the chief control strategy of S. obliqua. A number of researchers' demonstrated that plant extracts might be employed for the development of bio-pesticides to control this insect pest (Haribhai, Reference Haribhai2015; Jashvantbhai, Reference Jashvantbhai2015; MahaLakshmi et al., Reference MahaLakshmi, Sreekanth and Adinarayana2018). However, bio-pesticides are not yet commercialized. Host plant resistance is an alternate safe strategy instead of synthetic insecticides for limiting herbivores, which is economically as well as environmentally secure (Zehnder et al., Reference Zehnder, Gurr, Kuhne, Wade, Wratten and Wyss2007; Golizadeh et al., Reference Golizadeh, Ghavidel, Razmjou, Fathi and Hassanpour2017a, Reference Golizadeh, Jafari-Behi, Razmjou, Naseri and Hassanpour2017b). Among the three mechanisms (antixenosis, antibiosis and tolerance) involved in plant resistance to pests, antibiosis is the most important, because it has a direct effect on the survival and development rates, adult longevity and fecundity (Golizadeh et al., Reference Golizadeh, Jafari-Behi, Razmjou, Naseri and Hassanpour2017b). Host plant cultivars can vary in the context of various physiological and morphological characteristics as well as leaf structure, trichome number, wax content, nutritional values and secondary metabolites (Sarfraz et al., Reference Sarfraz, Dosdall and Keddie2007; Golizadeh et al., Reference Golizadeh, Ghavidel, Razmjou, Fathi and Hassanpour2017a, Reference Golizadeh, Jafari-Behi, Razmjou, Naseri and Hassanpour2017b), which can influence survival, development time, adult longevity and fecundity of an insect pest.
The basic information on the biology of an insect pest is necessary before deciding the strategy to combat the pest (Chi, Reference Chi1990; Chi & Su, Reference Chi and Su2006; Huang & Chi, Reference Huang and Chi2011; Akca et al., Reference Akca, Ayvaz, Yazici, Smith and Chi2015; Zheng et al., Reference Zheng, Tao, Chi, Wan and Chu2017). Life table is a powerful tool for analyzing and understanding the effect of different hosts or cultivars of a host on the growth, survival, reproduction and intrinsic rate of insect populations (Chi & Getz, Reference Chi and Getz1988; Chi, Reference Chi1990; Marouf et al., Reference Marouf, Amir-Maafi and Shayesteh2013; Zheng et al., Reference Zheng, Tao, Chi, Wan and Chu2017; Karimi-Pormehr et al., Reference Karimi-Pormehr, Borzoui, Naseri, Dastjerdi and Mansouri2018). But the traditional life table, which is based on only the female population, ignores the male population, different development stages and individual differences. The age-stage, two-sex life table can eliminate many of the inherent error characteristics of female-based life tables (Huang & Chi, Reference Huang and Chi2011; Akca et al., Reference Akca, Ayvaz, Yazici, Smith and Chi2015; Zheng et al., Reference Zheng, Tao, Chi, Wan and Chu2017).
Thus, objectives of the present study were to (i) investigate the development, longevity and reproductive potential of S. obliqua using age-stage, two sex life table in relation to the three green gram cultivars (PDM 54, PUSA BAISAKHI and SAMRAT which are currently grown by farmers in West Bengal, India due to high yielding potential because genetic make-up of these three cultivars are suitable in the present conditions), (ii) study the food utilization efficiency measures of S. obliqua by feeding on the three green gram cultivars, and (iii) understand the possible impact of different nutritional factors (total carbohydrates, proteins, lipids, amino acids and nitrogen content) and antinutritional factors (total phenols, flavonols and tannins) present in the three green gram cultivars on the growth, survival and reproduction of S. obliqua. Therefore, findings of the current study can help in the selection of partially resistant green gram cultivar, provide useful data for further green gram breeding program and contribute to effective management of S. obliqua.
Materials and methods
Host plants
Six plots [each plot 3.05 m × 3.05 m; soil organic matter 5.3 ± 0.2% (± standard error), pH 7.7] were prepared for cultivation of three green gram cultivars in the Crop Research Farm (CRF), University of Burdwan (23°16′N, 87°54′E), West Bengal, India during April–July 2018 under a photoperiod of 13 L:11 D at 30–35°C. Three cultivars of green gram seeds, PDM 54, PUSA BAISAKHI and SAMRAT were separately germinated on moistened filter papers. Each green gram cultivar was grown in two side by side plots and there was a gap of 0.91 m between two plots. There was a space of 6.10 m for the cultivation of another green gram cultivar and in this space bottle gourds were grown. Each cultivar of green gram plants was naturally infected by S. obliqua during early May in the field.
For preparation of uninfested plants of each green gram cultivar, each seed with cotyledon was planted in a pot containing ~1500 cm3 of soil collected from the field of CRF, and grown in natural condition in the CRF during May–August 2018 under a photoperiod of 13 L:11 D at 30–35°C. The whole plant with the pot was covered with a fine mesh nylon net [120 cm (height) × 80 cm (diameter)] to prevent insect attacks and unintentional infection. Forty plants were prepared for each green gram cultivar. Plants were provided with water every other day. Mature leaves from 5 to 6-week old plants were collected and provided as food to the larvae of S. obliqua.
Insect mass culture
Sixth instar larvae (50) of S. obliqua were collected from each cultivar of green gram plants growing in the CRF of the University of Burdwan during mid June 2018 and were taken into the laboratory and placed on the leaves of the same cultivar in glass jars (20 cm d × 30 cm h) from which they were collected. A pair of newly emerged male and female was placed in a glass jar (20 cm d × 30 cm h), containing the same leaves of green gram cultivar from which sixth instar larvae were collected, for egg laying (N = 20 pairs of male and female for each cultivar of green gram leaves). Eggs (100) laid by newly emerged females on the second day were collected from each cultivar of green gram leaves (PDM 54, PUSA BAISAKHI and SAMRAT). Hundred eggs [ten eggs in a glass jar (20 cm d × 30 cm h)] were separately used for culture in each green gram cultivar for three generations for conditioning of the insects in each cultivar of green gram leaves at 27 ± 1°C, 65 ± 5% relative humidity (RH) and a photoperiod of 12:12 (L/D) h.
Development time, oviposition period, fecundity and longevity
This experiment was conducted by taking the fourth generation newly emerged adults (males: identified by the presence of bipectinate antenna and females: the presence of club-shaped antenna) that had been reared for three generations on the same cultivars. For each cultivar, a pair of newly emerged male and female (N = 20) was paired (newly emerged males and females mate on the same day of emergence) in a fine mesh nylon net cage (25 × 25 × 25 cm) containing a small cotton ball soaked with 10% honey solution for adults' feeding, and eggs laid by females on the second day were collected in order to obtain the same aged eggs. Hundred eggs collected from females, on which cultivar of green gram leaves they were reared, were used separately to construct age-stage, two-sex life table of S. obliqua on each cultivar of green gram leaves; each egg was considered as a replicate on each cultivar of green gram leaves. Each cultivar of green gram leaves was replaced daily with fresh one. The petiole of each fresh mature leaf was inserted into a moist piece of cotton, which was wrapped with aluminum foil to prevent moisture loss, and provided daily for feeding. Each larva was placed in a glass jar (5 cm diameter × 8 cm length) containing a particular cultivar of green gram leaves until pupation. Each pupa was placed in a separate glass jar (8 cm diameter × 10 cm length) and covered with fine mesh nylon net. Mortality and larval moulting including pupation time and adult emergence of each individual were recorded daily at 24 h interval. The individual longevity of adults, i.e., from adult emergence to death of males and females was also recorded at 24 h interval.
Newly emerged adult females were separately examined on each cultivar of green gram leaves, on which larvae were reared, to record the fecundity of S. obliqua through lifetime. The adult pre-oviposition period (APOP: the period between the emergence of an adult female and her first oviposition), total pre-oviposition period (TPOP: the time interval from birth to the beginning of oviposition), oviposition days, daily fecundity and total fecundity (number of eggs produced during the reproductive period) were recorded on each cultivar of green gram leaves (Chi, Reference Chi1988; Chi & Su, Reference Chi and Su2006).
Life table analysis
Raw data on the survival, development and oviposition of all individuals were analyzed based on age-stage, two-sex life table theory (Chi & Liu, Reference Chi and Liu1985; Chi, Reference Chi1988) using the computer program TWOSEX-MSChart (Chi, Reference Chi2017a). The calculated population parameters were the age-stage-specific survival rate (s xj, x is the age and j is the stage); the age-specific survival rate (l x, the probability of a newly laid egg surviving to age x); age-stage specific fecundity (f xj, the daily number of eggs laid by an individual at age x and stage j); the age-specific fecundity (m x, the mean fecundity of individuals at age x); the age-stage life expectancy (e xj, the time that an individual of age x and stage j is expected to live) and the age-stage reproductive value (v xj, the contribution of an individual of age x and stage j to the future population). The net reproductive rate (R 0: the total number of offspring that an individual can produce during its lifetime), intrinsic rate of increase (r), finite rate of increase (λ) and mean generation time (T) were also calculated using the computer program TWOSEX-MSChart (Chi, Reference Chi2017a).
The variances and standard errors of the population parameters were estimated using the bootstrap technique (Efron & Tibshirani, Reference Efron and Tibshirani1993), which is contained in the TWOSEX-MS Chart program (Chi, Reference Chi2017a). We used 100,000 replications in this study to get less variable results. The paired bootstrap test (Efron & Tibshirani, Reference Efron and Tibshirani1993; Reddy & Chi, Reference Reddy and Chi2015) was used to compare differences in development time, adult longevity, adult preoviposition period (APOP), total preoviposition period (TPOP), oviposition period and fecundity among three green gram cultivars. The differences in population parameters (r, λ, R 0 and T) among three green gram cultivars were also compared using the paired bootstrap test at the 5% significance level (Reddy & Chi, Reference Reddy and Chi2015).
Population projection
The potential population growth of S. obliqua with an initial population of ten eggs on three green gram cultivars (PDM 54 or PUSA BAISAKHI or SAMRAT) was projected according to Chi & Liu (Reference Chi and Liu1985) and Chi (Reference Chi1990) to predict the future population size and age-stage structure, by using the TIMING-MSChart program (Chi, Reference Chi2017b).
Food utilization indices
This experiment was conducted by taking the fourth generation newly emerged first instar larvae that had been reared for three generations on the same cultivars in the laboratory. Larvae were weighed initially and were placed in a glass jar (8 cm diameter × 10 cm length) containing leaves of a particular green gram cultivar. Three replicates (each replicate contained ten larvae) were performed for a particular cultivar of green gram leaves. The larvae were allowed to feed on the weighed quantity of leaves from each cultivar for 24 h interval, and remaining leaves after 24 h of feeding were reweighed. Sample leaves from each cultivar of green gram plants before feeding and after 24 h of feeding by the larvae were weighed, oven dried and reweighed to estimate percent dry weight conversion to allow estimation of the dry weight of the diet supplied to the larvae. The quantity of the food consumed by the larvae was estimated by determining the difference between the dry weight of diet remaining after 24 h interval and total dry weight of diet initially provided. The fresh weight gain of the larvae on each cultivar of green gram plant during the period of study was estimated by determining the differences in the weight of larvae at 24 h interval. The weight gain of the larvae was recorded at 24 h interval. Feces were collected at 24 h interval and weighed, and then placed in a hot air oven and reweighed to find the dry weight of excreta.
Food utilization indices (GR – growth rate, CR – consumption rate, RGR – relative growth rate, CI – consumption index, ER – egestion rate, HCR – host consumption rate, AD – approximate digestibility, ECI – efficiency of conversion of ingested food, ECD – efficiency of conversion of digested food and HUE – host utilization efficiency, all based on dry weight) were calculated based on the formulas of Waldbauer (Reference Waldbauer1968) to assess the feeding efficiencies of S. obliqua.
Biochemical analysis of leaves
The variation in nutritional quality of the leaves of three green gram cultivars were estimated by subjecting 1 g fresh leaves of respective type collected from non-infected plants to various biochemical analysis such as total carbohydrates (Dubois et al., Reference Dubois, Gilles, Hamilton, Rebers and Smith1956), total proteins (Lowry et al., Reference Lowry, Rosebrough, Farr and Randall1951), total lipids (Folch et al., Reference Folch, Lees and Sloane-Stanley1957), total amino acids (Moore & Stein, Reference Moore and Stein1948), total phenols (Bray & Thorpe, Reference Bray and Thorpe1954) and total flavonols (Howell et al., Reference Howell, Bell and Stipanovic1976). Dried leaves were used for determination of total tannins (Scalbert, Reference Scalbert, Hemingway and Laks1992) and total nitrogen (Vogel, Reference Vogel1958). The determination of each biochemical analysis was repeated five times.
Estimation of moisture content
One gram of each cultivar of green gram leaves was placed separately in a hot-air oven at 50 ± 1°C for 72 h and weighed in a monopan balance (± 0.01 mg). Differences in the fresh and dry weights of leaves were used to calculate the percent water content. The moisture content was repeated five times for each type of leaf.
Statistical analysis
Data obtained on different feeding indices of S. obliqua and biochemical analyses of leaves of the three green gram cultivars (PDM 54, PUSA BAISAKHI and SAMRAT) were subjected to one-way analysis of variance (ANOVA) (Zar, Reference Zar1999). Means of different feeding indices and biochemical parameters were compared by Tukey's test (HSD) when significant values were obtained (Zar, Reference Zar1999). All the statistical analysis was performed by using SPSS (version 13.0) software.
Results
Population parameters of S. obliqua on three green gram cultivars
The development time of each stage (i.e., egg, six larval instars, pre-pupa, pupa and male and female longevity) of S. obliqua on three green gram cultivars (PDM 54, PUSA BAISAKHI and SAMRAT) was presented in table 1, while adults APOP, TPOP, oviposition period and fecundity were listed in table 2. The development time of eggs was significantly (P < 0.05) longest on SAMRAT cultivar followed by PUSA BAISAKHI and shortest on PDM 54 (table 1), while the development times of first four instars were significantly (P < 0.05) shorter on PDM 54 and PUSA BAISAKHI compared to SAMRAT (table 1). The pupal stage was significantly (P < 0.05) longest on SAMRAT followed by PUSA BAISAKHI and shortest on PDM 54 (table 1). The pre-adult stage was significantly (P < 0.05) lowest on PDM 54 followed by PUSA BAISAKHI and highest on SAMRAT (table 1). Longevity of males and females did not show significant (P > 0.05) differences among three green gram cultivars (table 1). There are no significant differences in the APOP of S. obliqua among three green gram cultivars (table 2). The APOP does not take into account the total pre-adult stage and should be used with caution. The TPOP of S. obliqua was significantly (P < 0.05) shorter on PDM 54 (36.76 ± 0.25 days) compared to PUSA BAISAKHI (39.14 ± 0.38 days) and SAMRAT (39.80 ± 0.45 days) (table 2). The TPOP considers the total pre-adult stage and is the true preoviposition period. It can be employed to demonstrate the effect of the first reproducing age on the reproductive value. However, it does not essentially correspond with the maximum reproductive value. The total fecundity of S. obliqua was significantly (P < 0.05) lowest on SAMRAT (250.20 ± 4.74), intermediate on PUSA BAISAKHI (289.90 ± 4.36) and highest on PDM 54 (318.32 ± 2.96).
Table 1. Development time and adult longevity (mean ± SE) of Spilosoma obliqua on three green gram cultivars.

Standard errors were estimated using 100,000 bootstrap resampling. Data followed by the same lower-case letter within the row were not significantly different based on a paired bootstrap test at 5% significance level.
Table 2. Mean (±SE) of adult preoviposition period (APOP), total preoviposition period (TPOP), oviposition period and fecundity of Spilosoma obliqua fed on three green gram cultivars.

Standard errors were estimated using 100,000 bootstrap resampling. A paired bootstrap test at 5% significance level was used to detect differences between treatments.
The survivorship and stage differentiation of individuals fed on three green gram cultivars are presented in age-stage specific survival rate (s xj) (fig. 1). The overlap in survival curves of S. obliqua on three-gram cultivars is due to variable development rate among individuals (fig. 1). In comparison to others, s xj curve started to drop earlier on PDM 54 cultivar. A significant (P < 0.05) higher preadult mortality rate was observed in SAMRAT (0.69 ± 0.05) than those reared on PDM 54 (0.54 ± 0.05), reflecting higher preadult survival on PDM 54. The female curves emerged at age 34, 36 and 36 day on PDM 54, PUSA BAISAKHI and SAMRAT cultivars, respectively; whereas male curves emerged at age 33, 34 and 38 day on PDM 54, PUSA BAISAKHI and SAMRAT cultivars, respectively (fig. 1a–c).

Figure 1. Age-stage-specific survival value (s xj) of Spilosoma obliqua fed on three green gram cultivars.
The net reproductive rate (R 0) of S. obliqua was highest on PDM 54 and lowest on SAMRAT, indicating that more offspring would be produced by S. obliqua within one generation on PDM 54 cultivar (table 3). The intrinsic rate of increase (r) and finite rate of increase (λ) of S. obliqua were higher on PDM 54 and PUSA BAISAKHI compared to SAMRAT, suggesting that S. obliqua population will increase faster on PDM 54 and PUSA BAISAKHI compared to SAMRAT (table 3). The mean generation time (T, time required for a population to increase to R 0 fold of its population size at the stable age distribution) of S. obliqua was longer on PUSA BAISAKHI and SAMRAT compared to PDM 54, implicating that S. obliqua population will increase faster on PDM 54 compared to PUSA BAISAKHI and SAMRAT (table 3).
Table 3. Mean (± SE) of the intrinsic rate of increase (r), finite rate (λ), net reproductive rate (R 0) and mean generation time (T) of Spilosoma obliqua fed on three green gram cultivars.

Standard errors were estimated using 100,000 bootstrap resampling. A paired bootstrap test at 5% significance level was used to detect differences between treatments.
The eggs produced by the female S. obliqua at age x and stage j is shown with f xj in fig. 2. The curves of age-stage specific fecundity (f xj) showed that oviposition pattern varied among green gram cultivars (fig. 2). The female age-stage specific fecundity and age-specific fecundity (m x) on PDM 54 started at 35 day (fig. 2a), and 2 days later on PUSA BAISAKHI and SAMRAT (37 day) (fig. 2b and c). The maximal daily oviposition rates were higher on PDM 54 followed by PUSA BAISAKHI and lower on SAMRAT. The females began to oviposit on 35 day and continued up to 41 day on PDM 54; whereas females started to oviposit on 37 day and ended at 44 day on PUSA BAISAKHI and SAMRAT. The highest f xj peaks and age-specific fecundity of total population (m x) on PDM 54 were 36 and 37 day, respectively, while highest f xj peaks and age-specific fecundity on PUSA BAISAKHI were 38 and 43 day, respectively, and the highest f xj peaks and age-specific fecundity on SAMRAT were 37 and 41 day, respectively (fig. 2a–c). The l x explained variations in the survival rate of S. obliqua with age. However, due to lower survivability at this age, the highest age-specific maternity (l xmx) was recorded on 37, 39 and 41 day on the PDM 54, PUSA BAISAKHI and SAMRAT, respectively (fig. 2a–c).
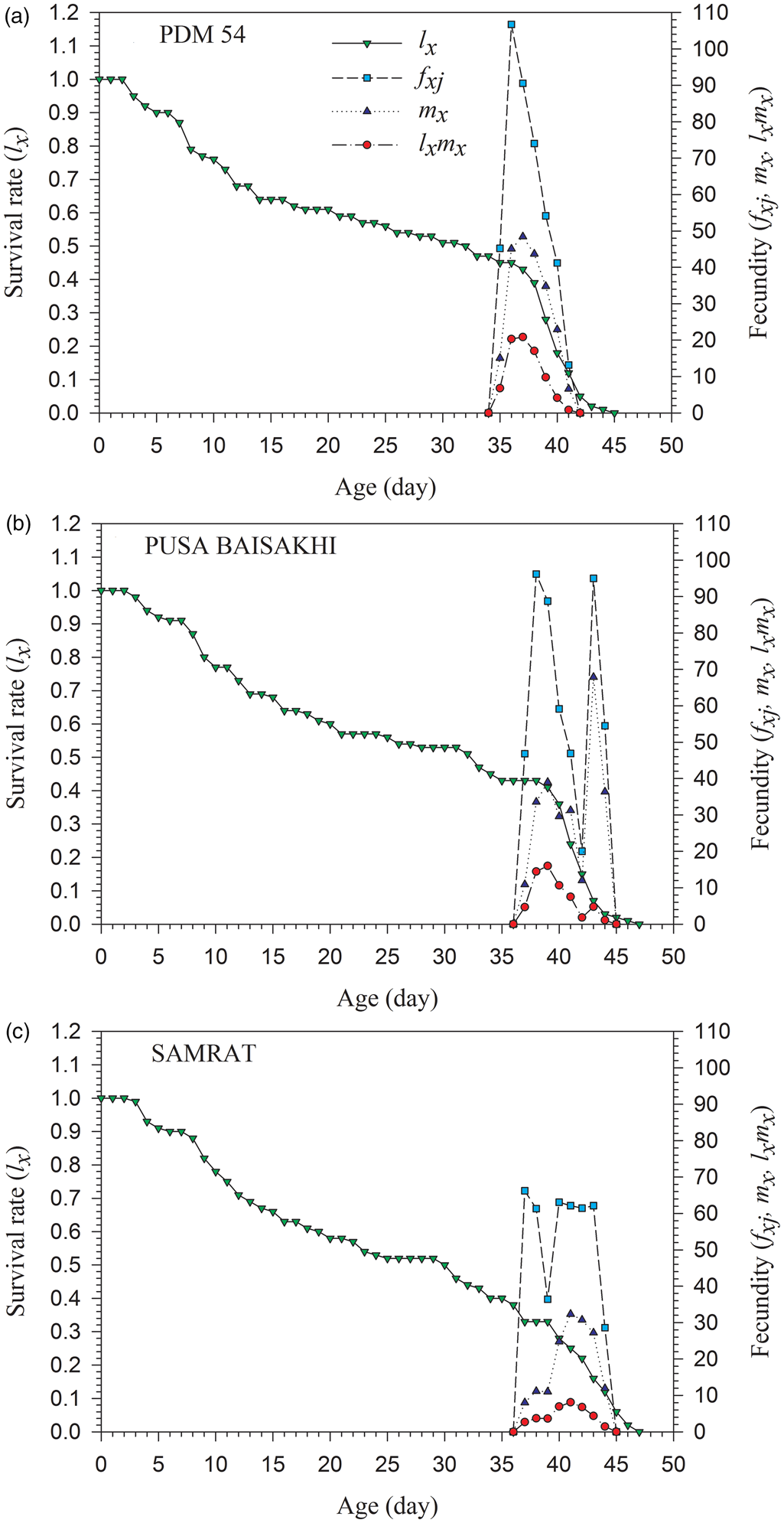
Figure 2. Age-specific survival rate (l x), age-stage-specific fecundity (f xj), age-specific fecundity (m x) and age-stage-specific maternity (l xmx) of Spilosoma obliqua fed on three green gram cultivars.
The age-stage specific life expectancy of S. obliqua on three green gram cultivars can be observed in fig. 3, which gives the probability that an individual of age x and stage j is expected to live. The life expectancies of S. obliqua at age zero (e 01) were 26.18, 27.26 and 26.57 days on PDM 54, PUSA BAISAKHI and SAMRAT cultivars, respectively. This is because individuals of the same age can develop to different stages, and these individuals will show different life expectancies. The maximum life expectancy of female S. obliqua on PDM 54 cultivar was 43 days and to that of males were 44 days (fig. 3a). When reared on PUSA BAISAKHI, the maximum life expectancies of female and male were 46 and 45 days, respectively (fig. 3b). The maximum life expectancies of female and male on SAMRAT were recorded as 45 and 46 days, respectively (fig. 3c).

Figure 3. Age-stage-specific life expectancy (e xj) of Spilosoma obliqua fed on three green gram cultivars.
Age-stage reproductive value (v xj), the scale of population forecasting, of S. obliqua on three green gram cultivars is shown in fig. 4. The reproductive value of a newborn individual (v 01) is the finite rate of increase on three green gram cultivars, i.e., 1.122 day−1 on PDM 54, 1.107 day−1 on PUSA BAISAKHI and 1.091 day−1 on SAMRAT (fig. 4a–c). However, when old adults did not lay eggs, the reproductive value becomes zero at age 42, 45 and 45 day on PDM 54, PUSA BAISAKHI and SAMRAT, respectively. The reproductive values (v xj) of S. obliqua on PDM 54 and PUSA BAISAKHI increased to 263.44 day−1 at 34 day and 262.13 day−1 at 36 day, respectively, when the female adults emerged (fig. 4a and b); whereas, the v xj value increased to 251.32 day−1 at 36 day on SAMRAT when female adults emerged (fig. 4c). The highest reproductive value of S. obliqua on PDM 54, PUSA BAISAKHI and SAMRAT was 295.49, 290.20 and 274.31 day−1 at 35, 37 and 37 day, respectively. These findings suggest that, as opposed to other ages, female individuals of the above ages had the highest contribution to the future population on the tested green gram cultivars, respectively.

Figure 4. Age-stage-specific reproductive value (v xj) of Spilosoma obliqua fed on three green gram cultivars.
Population projection of S. obliqua on three green gram cultivars
The population growth and age-stage structure of the different stages of S. obliqua simulated from a starting population of ten eggs using the TIMING-MSChart program are shown in fig. 5. The increased population of S. obliqua showed that the population growth was the fastest on PDM 54, intermediate on PUSA BAISAKHI and slowest on SAMRAT. After 80 days, the numbers of preadults on PDM 54 were 58,053 and adults were 66 (37 females and 29 males), while the numbers of preadults on PUSA BAISAKHI were 17,083 and adults were 169 (89 females and 80 males); whereas the numbers of preadults on SAMRAT were 2369 and adults were 44 (27 females and 17 males).
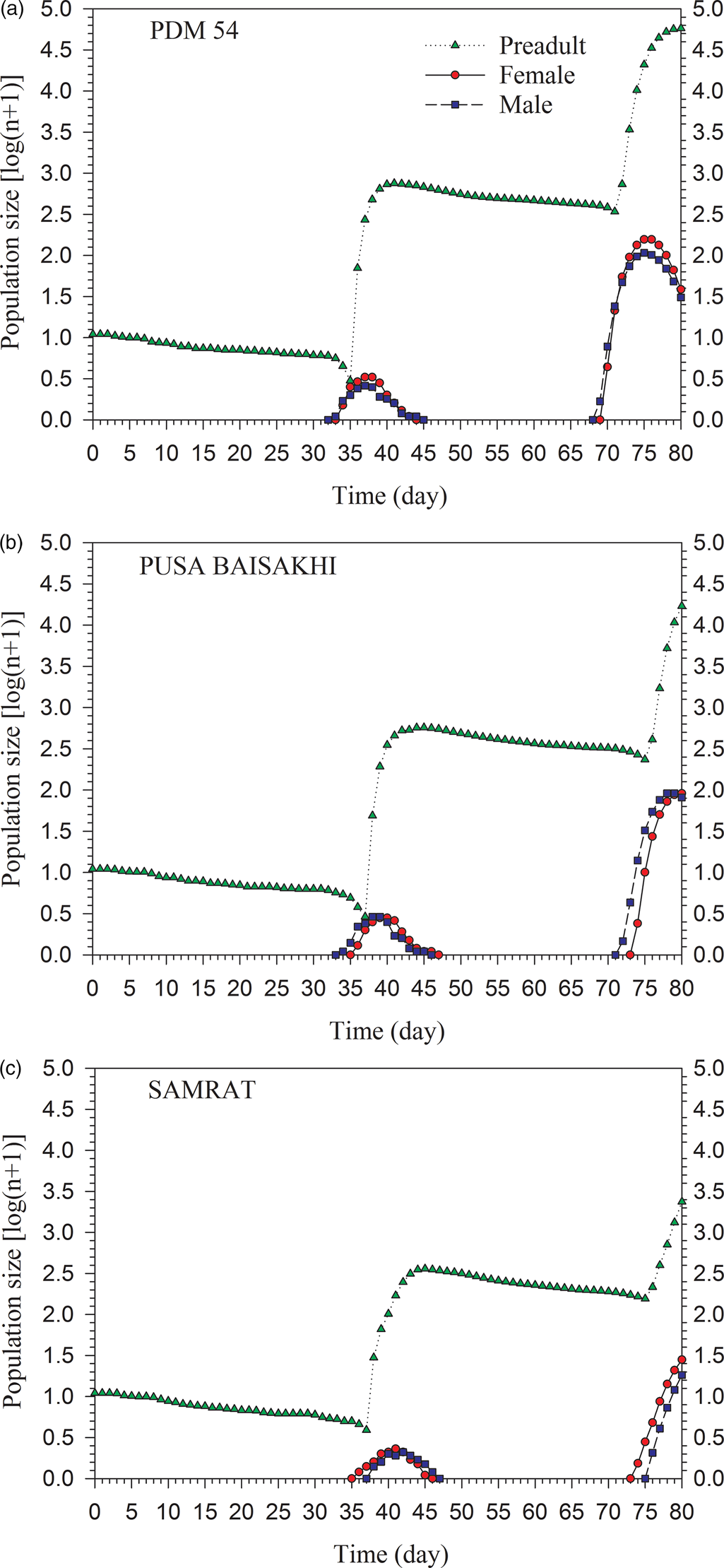
Figure 5. Population projection of Spilosoma obliqua fed on three green gram cultivars.
Food utilization efficiency measurement
The food utilization efficiency measures for the sixth instar larvae of S. obliqua on leaves of the three green gram cultivars (PDM 54, PUSA BAISAKHI and SAMRAT) were presented in table 4 (food utilization efficiency measures from the first to fifth instar larvae of S. obliqua were presented in Supplementary table 1–5). Higher GR and RGR values were noted for insects fed on PDM 54 followed by PUSA BAISAKHI and SAMRAT (P < 0.0001), while higher CR value was recorded on PDM 54 compared to PUSA BAISAKHI and SAMRAT (P < 0.006) (table 4). CI was higher on PDM 54 cultivar (P < 0.001) compared to the other two green gram cultivars (table 4). Similar to CI, ER and HCR were greater on PDM 54 compared to PUSA BAISAKHI and SAMRAT (P < 0.0001) (table 4). A higher value of AD was recorded on PDM 54 and SAMRAT compared to PUSA BAISAKHI (P < 0.005) (table 4). Both ECI and ECD values were highest on PUSA BAISAKHI followed by SAMRAT and lowest on PDM 54 (ECI: P < 0.005; ECD: P < 0.004) (table 4). HUE value was the best on PDM 54 compared to PUSA BAISAKHI and SAMRAT (P < 0.025) (table 4).
Table 4. Food utilization efficiency measurement (mean ± SE) of sixth instar larvae (n = 30) of Spilosoma obliqua fed on three green gram cultivars (PDM 54, PUSA BAISAKHI and SAMRAT).

Within the row means followed by the same letter(s) are not significantly different by Tukey's test.
Food utilization efficiency measures: GR, growth rate; CR, consumption rate; RGR, relative growth rate; CI, consumption index; ER, egestion rate; HCR, host consumption rate; AD, approximate digestibility; ECI, efficiency of conversion of ingested food; ECD, efficiency of conversion of digested food; HUE, host utilization efficiency.
Biochemical analysis of leaves
Total carbohydrates, proteins, lipids and amino acids were highest in PDM 54 followed by PUSA BAISAKHI and least in SAMRAT (table 5). The nitrogen content was also highest in PDM 54 followed by PUSA BAISAKHI and lowest in SAMRAT (table 5). Total phenols were higher in SAMRAT compared to PDM 54 and PUSA BAISAKHI; whereas total flavonols were highest in SAMRAT followed by PUSA BAISAKHI and least in PDM 54 (table 5). Tannins were higher in SAMRAT and PUSA BAISAKHI compared to PDM 54 (table 5). PDM 54 leaves had the highest water content, followed by PUSA BAISAKHI and lowest in SAMRAT (table 5).
Table 5. Biochemical analyses (mean ± SE) of leaves of three green gram cultivars.

DWL, dry weight of leaves; FWL, fresh weight of leaves.
Within the row means followed by the same letter(s) are not significantly different by Tukey's test.
Discussion
The present study demonstrated that the three different green gram cultivars (PDM 54, PUSA BAISAKHI and SAMRAT) significantly influenced fecundity, development, survival and reproduction of S. obliqua (tables 1 and 2). Three green gram cultivars also influenced life table parameters such as net reproductive rate (R 0), the intrinsic rate of natural increase (r), mean generation time (T) and finite rate of increase (λ) of S. obliqua (table 3). A number of biological studies have been reported for S. obliqua on sunflower (Varatharajan et al., Reference Varatharajan, Singh, Keisa, Singh and Selvasundaram1998), sesame (Biswas, Reference Biswas2006), soybean (Chaudhary, Reference Chaudhary2009), jute (Selvaraj et al., Reference Selvaraj, Gotyal, Satpathy and Meena2015), black gram and cowpea (Haribhai, Reference Haribhai2015). But, none of the studies have been performed on S. obliqua using age-stage, two sex life table, and further, the biology of S. obliqua is reported for the first time.
In the current investigation, we provided excised leaves of three green gram cultivars to study the age-stage, two-sex life tables and food utilization efficiency measures of S. obliqua (table 4). We also studied nutritional factors (total carbohydrates, proteins, lipids, amino acids and nitrogen content) and antinutritional factors (total phenols, flavonols and tannins) from excised leaves of three green gram cultivars to relate the development and fecundity of S. obliqua by feeding on leaves of three green gram cultivars (table 5). Host plant resistance may be altered when leaves are excised; however, we believe that it should be exactly investigated on test host plants, and the possible differences must be compared between excised leaves and leaves attached with the plants. When antixenosis or antibiosis is one of the most important components of plant resistance, the excised leaf disc assays could be employed effectively for quick screening of germplasm, breeding materials and mapping populations in a short time span with minimum cost and under uniform insect infestations (Sams et al., Reference Sams, Lauer and Radcliffe1975; Sharma et al., Reference Sharma, Pampapathy, Dhillon and Ridsdill-Smith2005; Roy & Barik, Reference Roy and Barik2013; Alami et al., Reference Alami, Naseri, Golizadeh and Razmjou2014).
The data on biochemical analyses of leaves of the three green gram cultivars revealed that the leaves of SAMRAT cultivar were of poor nutritional quality compared to other two cultivars of green gram plants (PDM 54 and PUSA BAISAKHI) because nutritional factors such as total carbohydrates, proteins, lipids, amino acids and nitrogen content were higher in PDM 54 and PUSA BAISAKHI compared to SAMRAT; while antinutritional factors such as total phenols, flavonols and tannins were higher in SAMRAT compared to PDM 54 (table 5). Phenols act as protective agents and inhibitors against invading organisms, i.e., herbivores, nematodes, phytophagous insects including fungal and bacterial pathogens (Harborne, Reference Harborne2003). Tannins serve as an anti-herbivore component by reducing the digestibility of substances (Harborne, Reference Harborne2003). Flavonols serve an important role by protecting plants from various stresses as well as insect attack by influencing their behavior, and growth and development (Treutter, Reference Treutter2006; War et al., Reference War, Paulraj, Ahmad, Buhroo, Hussain, Ignacimuthu and Sharma2012). This might be a possible explanation for the lower growth of S. obliqua by feeding on SAMRAT compared to PDM 54 and PUSA BAISAKHI. We recorded higher amount of water content in PDM 54 and PUSA BAISAKHI compared to SAMRAT leaves as lower water content causes a reduction in survival of phytophagous insects (Mattson & Scriber, Reference Mattson, Scriber, Slansky and Rodriguez1987; Roy & Barik, Reference Roy and Barik2012, Reference Roy and Barik2013). This might be another explanation for the higher growth of S. obliqua by feeding on PDM 54 compared to SAMRAT. The results of biochemical analyses suggest that leaves of PDM 54 and PUSA BAISAKHI are of better nutritional quality compared to SAMRAT leaves, which influenced higher longevity along with growth and development of S. obliqua on PDM 54 and PUSA BAISAKHI compared to SAMRAT. Further, females reared on PDM 54 showed the highest fecundity rate, indicating that the nutritional contents of PDM 54 were more appropriate for S. obliqua compared to the other two green gram cultivars. Feeding by the larvae on nutritional poor host plants could cause lower female fecundity on a plant (Sarfraz et al., Reference Sarfraz, Dosdall and Keddie2007; Golizadeh et al., Reference Golizadeh, Ghavidel, Razmjou, Fathi and Hassanpour2017a, Reference Golizadeh, Jafari-Behi, Razmjou, Naseri and Hassanpour2017b). Hence, the differences in fecundity of S. obliqua on the three green gram cultivars might be due to variation in nutritional quality and quantity including secondary compounds in host cultivars.
The present study revealed that the preadult durations of S. obliqua on PDM 54, PUSA BAISAKHI and SAMRAT cultivars of green gram were 35.5, 37.3 and 39.3 days, respectively, while males and females lived for 3.94–4.39 and 4.67–4.92 days on three green gram cultivars, respectively. Varatharajan et al. (Reference Varatharajan, Singh, Keisa, Singh and Selvasundaram1998) reported that the preadult duration of S. obliqua on sunflower was 44 days (total larval development time and pupal duration were 28.8 and 11.2 days, respectively), while adults lived for a minimum period of 6 days. The preadult duration of S. obliqua on cowpea was within 29–40 days (total larval development time and pupal duration were 15–22 and 8–10 days, respectively), while adult males and females survived for 4–6 and 6–8 days, respectively (Haribhai, Reference Haribhai2015). According to Jashvantbhai (Reference Jashvantbhai2015), the preadult duration of S. obliqua on castor was within 37–48 days (total larval development time was 23–28 days and pupal duration was 8–10 days), while adult males and females survived for 5–7 and 9–12 days, respectively. Further, an average of 29.84 days was required to complete all six instars of S. obliqua on jute, while males and females lived for 4.06 and 7.34 days, respectively (Selvaraj et al., Reference Selvaraj, Gotyal, Satpathy and Meena2015). These differences in the development time of S. obliqua might be due to differences in the nutritional quality of host plants (Golizadeh & Razmjou, Reference Golizadeh and Razmjou2010; Golizadeh et al., Reference Golizadeh, Jafari-Behi, Razmjou, Naseri and Hassanpour2017b). Spilosoma obliqua showed longest development time on SAMRAT cultivar; whereas the population reared on PDM 54 cultivar had a faster development time, indicating that PDM 54 cultivar might allow a short life cycle and rapid population growth, which would be subsequently reflected in final population size.
According to Gabre et al. (Reference Gabre, Adham and Chi2005) and Liu et al. (Reference Liu, Li, Yang, Chi and Chen2018), the peak value of the reproductive value (v xj) is close to the TPOP. In this study, the female adults emerged at age 34, 36 and 36 day on PDM 54, PUSA BAISAKHI and SAMRAT cultivars, respectively (fig. 1), while the first reproductive age was 35, 37 and 37 days on PDM 54, PUSA BAISAKHI and SAMRAT cultivars, respectively (fig. 2). The maximum life expectancies of S. obliqua females on PDM 54, PUSA BAISAKHI and SAMRAT cultivars were 43, 46 and 46 days, respectively (fig. 3). Here, the peak values of v xj of S. obliqua were recorded at the same age as the first reproductive age (i.e., 35, 37 and 37 days on PDM 54, PUSA BAISAKHI and SAMRAT cultivars, respectively) (fig. 4). The peak value of v xj is at the same age of first reproduction when insects reproduce by laying egg masses. The reproductive value increases to a high value with emergence of adults, and subsequently, the peak reproductive value occurs after the first reproductive age, which is close to the TPOP. The present findings were consistent with the observation of Liu et al. (Reference Liu, Li, Yang, Chi and Chen2018), and states that TPOP is more important than APOP.
According to Chi (Reference Chi1988), the relationship between the mean fecundity (F) and the net reproductive rate (R 0) is R 0 = F × (N f /N). All of the results in the present study were consistent with the relationship between F, R 0, N f and N as proved by Chi (Reference Chi1988). Intrinsic rate of population increase (r) is an important parameter among the life table parameters, which is used as an indicator of pest population performance to assess the level of plant resistance to herbivorous insect pests (Razmjou et al., Reference Razmjou, Moharramipour, Fathipour and Mirhoseini2006; Sedaratian et al., Reference Sedaratian, Fathipour and Moharramipour2011; Naseri et al., Reference Naseri, Golparvar, Razmjou and Golizadeh2014; Golizadeh et al., Reference Golizadeh, Jafari-Behi, Razmjou, Naseri and Hassanpour2017b). Further, r is influenced by several factors such as development time, survivorship and fecundity rate of an insect population, implicating that this parameter states the physiological qualities of an insect in relation to its capacity to increase (Das et al., Reference Das, Koner and Barik2018). Hence, r is the excellent parameter to evaluate the performance of an insect on different host plants including different cultivars of a host plant (Southwood & Henderson, Reference Southwood and Henderson2000). This study revealed lower r on SAMRAT cultivar (0.0875 day−1) compared to other two green gram cultivars [PDM 54 (0.1148 day−1) and PUSA BAISAKHI (0.1018 day−1)], suggesting that SAMRAT cultivar is the least suitable cultivar. Varatharajan et al. (Reference Varatharajan, Singh, Keisa, Singh and Selvasundaram1998) and Das & Chaudhuri (Reference Das and Chaudhuri2005) reported that r values of S. obliqua were 0.1295 day−1 and from 0.0982 to 0.1568 day−1 on sunflower and jute, respectively. The variation in r might be due to nutrients and secondary metabolites. In the current study, net reproductive rate is lower on SAMRAT, which is the major factor influencing lower r on SAMRAT cultivar compared to the other two cultivars. Moreover, the longer mean generation time on SAMRAT cultivar (41.42 ± 0.44 days) influenced a lower r. Therefore, the variations in life table parameters were due to feeding by the larvae of S. obliqua on different green gram cultivars. Hence, the lower performance of S. obliqua on SAMRAT cultivar of green gram plant might be due to the lower amount of essential primary nutrients compared to other two cultivars (PDM 54 and PUSA BAISAKHI) or the presence of secondary metabolites that deter the potential herbivore development and fecundity of S. obliqua on SAMRAT cultivar (Sedaratian et al., Reference Sedaratian, Fathipour and Moharramipour2011; Roy & Barik, Reference Roy and Barik2013; Alami et al., Reference Alami, Naseri, Golizadeh and Razmjou2014; Mukherjee et al., Reference Mukherjee, Karmakar and Barik2017; Golizadeh et al., Reference Golizadeh, Ghavidel, Razmjou, Fathi and Hassanpour2017a, Reference Golizadeh, Jafari-Behi, Razmjou, Naseri and Hassanpour2017b; Malik et al., Reference Malik, Das and Barik2018).
Population projection based on age-stage, two sex life table help to understand the changes in stage structure of population growth in addition to trends and emergence of different stages of development as well as emergence of adult males and females (Chi, Reference Chi1990). We observed that growth of S. obliqua population would be fastest on PDM 54, intermediate on PUSA BAISAKHI and slowest on SAMRAT. This finding could help for implementing pest management programs such as application of pesticides and use of light traps.
Host-plant utilization is influenced by the ability of an insect to ingest, assimilate and convert food into body tissues (Awmack & Leather, Reference Awmack and Leather2002; Genc, Reference Genc2006). Thus, host plant quality influences development time, fecundity and survival of adults (Awmack & Leather, Reference Awmack and Leather2002, Shobana et al., Reference Shobana, Murugan and Naresh Kumar2010). In the current study, we observed variation in all nutritional indices when S. obliqua were fed on leaves of three green gram cultivars (PDM 54, PUSA BAISAKHI and SAMRAT). The growth rate (GR) of insects is dependent on the efficiency of conversion of digested food (ECD), while a reduction in ECD suggests higher metabolic maintenance cost (Xue et al., Reference Xue, Pang, Wang, Li and Liu2010, Sarkar et al., Reference Sarkar, Mukherjee and Barik2016). We recorded higher GR of sixth instar larvae on PDM 54 followed by PUSA BAISAKHI and SAMRAT, suggesting greater suitability of PDM 54 influenced shorter development time and higher fecundity of S. obliqua. But, we observed that the larvae of S. obliqua fed on SAMRAT were more efficiently converting these tissues in body mass than leaf tissues of PDM 54 as having higher efficiency of conversion of digested and ingested food on SAMRAT compared to PDM 54. This is due to homeostatic adjustment of consumption rates and efficiency parameters of an insect to achieve ideal growth rate even with foods of different quality (Xue et al., Reference Xue, Pang, Wang, Li and Liu2010), suggesting that when S. obliqua larvae were fed on SAMRAT then the larvae are able to compensate by more efficiently utilizing SAMRAT cultivar leaf tissue than the other two cultivar's leaf tissue (Zhu et al., Reference Zhu, Zhang and Ren2005).
In conclusion, the results of the current study revealed that food quality in respect of leaves of three green gram cultivars influenced S. obliqua development and fitness. We could suggest that SAMRAT was the least suitable cultivar of S. obliqua among three green gram cultivars. The longest development time and lowest fecundity of S. obliqua resulted in lower intrinsic rate of increase of S. obliqua on SAMRAT cultivar, indicating that lower population growth of S. obliqua could lead to lower subsequent infestations. On the contrary, PDM 54 was the most suitable cultivar for S. obliqua, indicating that this cultivar is less resistant due to greater nutritional quality in the leaf tissue. Thus, the use of partially resistant SAMRAT cultivar could enhance biological and chemical control methods. The obtained information regarding demographic parameters and feeding dynamics of S. obliqua could help to understand population dynamics and to develop time-based application of different control measures of this insect pest on green gram. However, further studies are needed to determine the quality and quantity of nutrients (carbohydrates, proteins, lipids and amino acids), as well as secondary metabolites (phenols, flavonols and tannins), which act as insect growth deterrent compounds of each cultivar for a comprehensive discussion.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485319000452
Acknowledgements
We thank anonymous reviewers for many helpful comments and suggestions of earlier versions of the manuscript. We are thankful to Dr Rahul Joshi, Scientist, Zoological Survey of India, Gangetic Plains Regional Centre, Bihar for identifying the insect. The financial assistance from the Govt. of India as CSIR-Junior Research Fellowship to Syed Husne Mobarak is gratefully acknowledged. We are also thankful to DST PURSE Phase-II for providing necessary instrumental facilities.













