Introduction
Schistosomiasis – a parasitic illness caused by trematodes from the genus Schistosoma – is a neglected disease linked with poverty, due to its association with the lack of sanitation and the domestic use of contaminated water. There are approximately 258 million people infected with schistosomiasis in 78 countries, accounting for 200 million deaths annually. Moreover, 800 million people are estimated to be at risk of this infection (Ferreira et al., Reference Ferreira, Oliva and Andricopulo2015; Neves et al., Reference Neves, Muratov, Machado, Andrade and Cravo2016; World Health Organization, 2016). Given the lack of a vaccine against schistosomiasis, treatment is based on chemotherapy with praziquantel (PZQ). According to Wang et al. (Reference Wang, Wang and Liang2012), S. mansoni strains with reduced susceptibility to PZQ have been widely found in many endemic foci, such as Egypt and Senegal. The increasing drug resistance and infection rates are clear concerns in the field of schistosomiasis research, and have driven the pursuit for new drugs and leads to treat schistosomiasis (Mantawy et al., Reference Mantawy, Ali and Rizk2011; Abdel-Hafeez et al., Reference Abdel-Hafeez, Ahmad, Abdulla, Aabdel-Wahab and Mosalem2012).
The important schistosomiasis agent S. mansoni remains viable in the host for up to 10 years, and its intravascular habitat promotes close contact with the host's immune system. Thus, the parasite has developed mechanisms of immune response evasion, which include a fast renewal rate of the structure of the tegument (especially in damaged areas) and the ability to mimic host antigens and to secrete/expose complexes that suppress or modulate host immune responses (Mclaren & Hockley, Reference Mclaren and Hockley1977; Hong et al., Reference Hong, Kosman, Thakur, Rekosh and LoVerde1992; Van Hellemond et al., Reference Van Hellemond, Retra, Brouwers, van Balkom, Yazdanbakhsh, Shoemaker and Tielens2006).
The host immune response to schistosomiasis involves Th1 and Th2 cytokine effectors that act in synergy with reactive nitrogen (RNS) and oxygen (ROS) species, respectively. In the chronic phase of infection, the Th2 response predominates, with abundant secretion of the superoxide anion (O2−) and of hydrogen peroxide (H2O2) by immune system cells (Pearce & MacDonald, Reference Pearce and MacDonald2002; Huang, Reference Huang2012). The accumulation of both of these reactive species (in the presence of ions of transition metals such as Fe (II/III)) leads to the formation of the hydroxyl radical, a highly reactive species that damages diverse macromolecule types, including lipids, proteins and DNA (Huang, Reference Huang2012).
Schistosoma mansoni superoxide dismutase enzymes (SmSODs) are fundamental in the detoxification of O2−, the main ROS species that affects the parasite (Abath & Werkhauser, Reference Abath and Werkhauser1996; Pearce & MacDonald, Reference Pearce and MacDonald2002; Van Hellemond et al., Reference Van Hellemond, Retra, Brouwers, van Balkom, Yazdanbakhsh, Shoemaker and Tielens2006), in the chronic phase of the disease. SODs – ubiquitous metallo-enzymes that convert O2− into H2O2 – are the only enzymes present in the parasite that can neutralize the superoxide anion. Schistosoma mansoni has three different types of superoxide dismutases: the mitochondrial Mn-SOD, which represents 5% of the total SOD activity in cells (Hong et al., Reference Hong, Kosman, Thakur, Rekosh and LoVerde1992), and is responsible for removing O2− produced as a result of mitochondrial metabolism; and Cu/Zn-SOD, which can be found as the signal-peptide-containing SmSPSOD, or the cytosolic SmCtSOD. Cu/Zn-SODs are found in the gut epithelium and the tegument of adult worms, and are likely to have a protective role against host Th2 responses. Decreased S. mansoni Cu/Zn-SOD activity reduces superoxide detoxification and increases the production of the hydroxyl radical (Huang, Reference Huang2012). Importantly, the use of Cu/Zn-SODs as vaccine antigens resulted in significant protection against schistosomiasis in a murine model, with reduction in worm burden (Shalaby et al., Reference Shalaby, Yin, Thakur, Christen, Niles and LoVerde2003). Moreover, only half of the total SOD activity observed in adult worms is found in the schistosomula, and Cu/Zn-SODs can be immunolocalized in the adult tegument only, emphasizing the importance of superoxide detoxification in the avoidance of tegument damage in adult worms (Hong et al., Reference Hong, Kosman, Thakur, Rekosh and LoVerde1992; Mei & LoVerde, Reference Mei and LoVerde1997). These data provide strong evidence that Cu/Zn-SODs have a key role in protecting S. mansoni against the activity of reactive species produced by host immune cells (during the chronic-phase Th2 response).
In a previous study, our group showed that 7-epiclusianone (7-epi), a benzophenone obtained from ‘bacupari’ – the fruit of the Garcinia brasiliensis tree (also known as Rheedia brasiliensis) – has potent schistosomicidal activity, in the low micromolar range. Although the mechanism of action of this compound remains unknown, and its molecular target has not been identified, 7-epi treatment produces abnormalities in the parasite tegument structure (Castro et al., Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015) that resemble the effects of SOD activity inhibition (Callahan et al., Reference Callahan, Crouch and James1988; Huang, Reference Huang2012). In the present study, we explored the possibility that SOD enzymes are the target for 7-epi, since SODs are the only parasite enzymes responsible for O2− detoxification. As a starting point, we performed an in silico analysis of the interaction between SmCtSOD and 7-epi, to evaluate the potential of 7-epi to inhibit this enzyme. Then, we analysed the impact of 7-epi on S. mansoni immune evasion and protection mechanisms in vitro, by biochemical analysis of both lipid peroxidation (as a surrogate for oxidative stress) and Cu/Zn-SOD activity, after incubation of adult worms with 7-epi. We also quantified Cu/Zn-SOD expression levels (mRNA) in the parasite, after incubation with 7-epi.
Materials and methods
Purification of 7-epiclusianone (7-epi)
Garcinia brasiliensis plants were obtained from the herbarium of the Federal University of Viçosa (MG, Brazil), after identification using the specimen voucher number VIC2604. 7-Epiclusianone (molecular weight (Mw): 502.695 g/mol) was purified from G. brasiliensis pericarp following the methodology described by Castro et al. (Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015), using only the hexane-soluble fraction of the ethanol extract. Crystals of 7-epi were obtained by two rounds of crystallization in hot methanol. Crystal purity (>99%) was confirmed by high performance liquid chromatography (HPLC) in an Aqua® C18 column (150.0 × 4.6 mm internal diameter, 125 Å pore size, 5 μm particle size; Phenomenex, Torrance, California, USA), with the mobile phase consisting of methanol with 0.2% acetic acid (95:5). The following HPLC conditions were used: 100 μl injection volume, 1.2 ml/min flow rate, 94 kgf/cm2 pressure, with detection in ultraviolet light (245 nm). The runs lasted approximately 16 min and the 7-epi peak was detected between the 10th and 11th minutes. A total of 16.75 g of pure 7-epi were obtained from all four columns. The 7-epi structure was confirmed by 1H nuclear magnetic resonance (NMR) and 13C NMR, and the data were comparable to those reported in previous studies (Santos et al., Reference Santos, Speziali, Nagem and Oliveira1998, 1999; Derogis et al., Reference Derogis, Martins, De Souza, De Moreira, Souza Filho, Doriguetto, De Souza, Veloso and Santos2008).
Animals and parasites
The S. mansoni LE strain used in this study was maintained by serial passage in Biomphalaria glabrata and Swiss mice, kept at the René Rachou Research Center/Oswaldo Cruz Foundation (Belo Horizonte, MG, Brazil).
To obtain adult parasites, mice were inoculated by sub-cutaneous injection on the back, according to the technique described by Katz et al. (Reference Katz, Pellegrino and Pereira1968). After 40 days of infection, mice were sacrificed by 10% ketamine (Syntec) overdose, administered intraperitoneally (~0.2 ml). Adult trematodes were obtained by retrograde liver perfusion with RPMI 1640 (pH 7.4), performed according to the method described by Castro et al. (Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015).
7-Epi incubation
Kolliphor® EL (Sigma, Darmstadt, Germany) (KEL) was used as a vehicle for 7-epi (stock solution at 19.89 mm), and treatment with praziquantel (Cestox, Merck, Kenilworth, New Jersey, USA) (PZQ) (Mw: 312.413 g/mol) was used as a tegument detachment (positive) control. For each group, 40 couples of adult parasites were placed in six-well plates (4 couples/well, 10 wells/group) in RPMI 1640 medium supplemented with 5.0% heat-inactivated foetal bovine serum and 1.0% penicillin (10,000 IU/ml) and streptomycin (10.0 mg/ml)(Sigma), acclimatized to the culture for 2 h in an incubator at 37°C (with 5% CO2). Parasites were incubated with 24.87, 49.74 or 99.48 μm of 7-epi in 0.01% KEL for 24 h. Control parasites were incubated in RPMI 1640 medium or with 0.01% KEL vehicle only (negative control, untreated), or with 6.4 μm praziquantel in KEL (positive control).
Preparation of parasite extracts and light microscopy imaging
After 24 h of contact with 7-epi, parasites were rinsed thoroughly in 0.1 m phosphate-buffered saline (PBS, pH 7.4, at 4°C) and then transferred to Falcon tubes with 2 ml of PBS containing 10 μl of a protease inhibitor cocktail (5 TIU/ml aprotinin, 1 mm pepstatin, 10 mm leupeptin and 2 mm E-64; Sigma-Aldrich) and 1% glycerol (v/v), and maintained at 4°C. Parasite suspensions were sonicated in a VCX 130PB ultrasonic processor (Sonics & Materials Inc., Newtown, Connecticut, USA), using a CV 188 probe, at 50% amplitude, in 4 pulses of 20 s each (with 1-min intervals on ice between pulses). Then, samples were spun for 3 min at 11,200 g rpm (4°C) and the pellet was resuspended in PBS. Both supernatants and pellets were stored at −80°C.
Total protein concentrations in parasite samples were determined by the method of Bradford (Reference Bradford1976), with modifications, or according to Zor & Selinger (Reference Zor and Selinger1996), using bovine serum albumin (BSA) as a standard. Absorbance was read at 590 and 450 nm, in a 200 RT spectrophotometer (Anthos Zenyth, Cambridge, UK).
Seven days after the end of 7-epi incubation (i.e. 8 days after the start of contact with the compound), parasites were imaged in a TS100 inverted microscope (Nikon, Tokyo, Japan) (fig. 1)

Fig. 1. Light microscopy images of Schistosoma mansoni male (a) and female (b) adult worms incubated with 24.87 μm 7-epiclusianone for 24 h, and then observed 7 days after incubation. Control parasites were incubated in medium only (c). In the male, the tegument is in the process of becoming detached (arrows in a), while the tegument is visibly detached in the female worm that was exposed to 7-epi (arrow in b).
Lipid peroxidation assay
Lipid peroxidation levels in parasite extracts (pellets and supernatants) were evaluated by malondialdehyde (MDA) quantitation, according to Draper & Hadley (Reference Draper, Hadley, Wilson and Sonnenberg1990), with the molecular complex formation detected by reading in a Cary Eclipse fluorescence spectrophotometer (Agilent, Santa Clara, California, USA), at excitation and emission wavelengths of 515 and 553 nm, respectively, using MDA as a standard. Results were normalized by protein concentration values.
Quantitative RT-PCR (qRT-PCR)
Parasites were incubated (in 24-well plates, in triplicate) with 198.96, 99.48, 49.74, 24.87 or 12.44 μm 7-epi in 0.01% KEL, for 24 h. Control parasites were incubated with KEL only (vehicle control), with 6.4 μm PZQ in PBS (positive control), or kept untreated (negative control). After 24 h of incubation, total RNA was extracted from frozen and macerated parasite samples (in 2-mercaptoethanol lysis buffer) using the Ambion PureLink RNA Mini Kit (Ambion, Carlsbad, California, USA).
For cDNA preparation, 10 μl of the total RNA from each sample was mixed with 1 μl of deoxyribonucleoside triphosphates (dNTPs) (Invitrogen, Carlsbad, California, USA), 1 μl of random primers (Invitrogen, Code 48190-011) and placed in a GeneAmp PCR System 9700 thermocycler (Applied Biosystems, Carlsbad, California, USA) for 5 min at 65°C. Then 2 μl of dithiothreitol (DTT), 4 μl of reaction buffer and 1 μl of Moloney murine leukaemia virus reverse transcriptase (MMLV-RT) (Invitrogen) were added to each sample, and the cycle was allowed to proceed for 50 min at 37°C, with a final step of 5 min at 95°C. cDNA samples were stored at −20° until further analysis.
The following primers were used for quantitative polymerase chain reaction (qPCR): for actin (ACT; GenBank: U19945.1), 5′-ACG TCG GTG ATG AAG CAC AA-3′ (forward) and 5′-CAT CGG GTC GTA CAA CTG GT-3′ (reverse); for the S. mansoni Cu/Zn-SOD (GenBank: Q01137.1, Cu/Zn-SOD mRNA) 5′-GAC TGG TAC AGC TGG CGT AA-3′ (forward) and 5′-GAT TTA GGC CGT GGT GGT CA-3′ (reverse). Primers were designed using the Primer BLAST (NCBI) tool, and were synthesized by Exxtend (Campinas, SP, Brazil).
PCR reactions contained 5 μl of SYBR Green I (Applied Biosystems), 2 μl of H2O (RNase-free), 1 μl of each primer (from 2 μm stocks) and 1 μl of cDNA (or RNase-free H2O, for negative controls). Reactions were run in a StepOne Real Time PCR System (Life Technologies, Carlsbad, California, USA), using the following conditions: a holding stage of 10 min at 95°C, and a cycling stage of 40 cycles of 95°C for 15 s and 60°C for 60 s. Data were analysed by comparative Ct (ΔCt), based on melting curves fitted to each run (from 60 to 95°C, with 0.3°C steps).
In silico molecular docking analysis
Molecular docking analysis of the interaction between 7-epi and the S. mansoni SOD was performed using the AutoDock4 and AutoGrid4 programs, based on data prepared using MGLTools (Morris et al., Reference Morris, Huey, Lindstrom, Sanner, Belew, Goodsell and Olson2009) (software and tools were developed by The Scripps Research Institute, San Diego, California, USA). The following structures were used in the analysis: for the S. mansoni cytosolic SOD (SmCtSOD), PDB (Protein Data Bank) (Berman et al., Reference Berman, Henrick and Nakamura2003) entry 1TO4 (resolution: 1.55 Å) (Cardoso et al., Reference Cardoso, Silva, De Araújo, Tanaka, Tanaka and Garratt2004); and for 7-epiclusianone, PubChem CID: 5471610 (Alves et al., Reference Alves, Alves, Romanha, Zani, Santos and Nagem1999; Santos et al., Reference Santos, Nagem, Oliveira and Braz-Filho1999).
To validate the results generated by AutoDock4, the analysis was repeated using the induced fit docking (IFD) 2015-2 protocol from the Glide software (version 6.4; Schrödinger, Cambridge, Massachusetts, USA).
Molecular structures and interaction maps were generated using VMD (Humphrey et al., Reference Humphrey, Dalke and Schulten1996) and LIGPLOT (Wallace et al., Reference Wallace, Laskowski and Thornton1995), respectively.
Superoxide dismutase (SOD) activity assay
Total SOD activity in the supernatant of parasite extracts was measured using the SOD assay kit (Sigma-Aldrich, code 19160), which follows the method described by Peskin & Winterbourn (Reference Peskin and Winterbourn2000). Mn-SOD activity was determined in the presence of 1 mm potassium cyanide (KCN). One SOD activity unit was defined as the enzyme activity necessary to inhibit 50% of water-soluble tetrazan-1 (WST-1) reduction to WST-formazan, quantified by colorimetry at 450 nm, in a 200 RT spectrophotometer (Anthos Zenyth). Results were normalized by protein concentration values.
Statistical analysis
Lipid peroxidations and SOD activity were analysed in parasite extracts, where each group was made from 40 adult couples. For PCR, the RNA for each group was extracted from three adult couples. The analytical readings for MDA and qPCR were performed in quintuplicate, and those for SOD activity in triplicate.
Data were analysed by one-way analysis of variance (ANOVA) followed by post-hoc Tukey's test. Results were considered to be statistically significant when P < 0.001. Statistical analysis was performed using Prism software (version 5.0; GraphPad Software, Inc., La Jolla, California, USA).
Results
Lipid peroxidation levels increased after incubation with 7-epi
Oxidative stress favours lipid peroxidation, the levels of which can be used as a surrogate for oxidative stress levels (Sayed et al., Reference Sayed, Cook and Williams2006; El-Lakkany et al., Reference El-Lakkany, El-Din and Ebeid2011; Li et al., Reference Li, Chen, Wang, Xu, Nie, Qin, Chen and Gong2013; Aziz et al., Reference Aziz, Yacoub, Rashid and Solieman2015). Thus, we analysed lipid peroxidation levels in extracts of parasites incubated with 7-epi for 24 h. While Castro et al. (Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015) used 1% methanol as a vehicle for 7-epi, we preferred to use Kolliphor® EL (KEL, at 0.01%) in the present study, due to the higher solubility of 7-epi in this solvent, which has a lower impact on SOD activity and lower parasite toxicity (data not shown). Importantly, lipid peroxidation values increased in S. mansoni trematodes incubated with 7-epi at doses of 99.48 μm (fig. 2), suggesting that higher concentrations of 7-epi induced oxidative stress in S. mansoni. In contrast, incubation with 24.87 μm of 7-epi significantly reduced lipid peroxidation levels (fig. 2). Our data also revealed that the vehicle, KEL, increased the basal lipid peroxidation levels found in parasite supernatants, because the concentration of the indirect lipid peroxidation marker MDA almost doubled in the vehicle-treated control (P < 0.001).
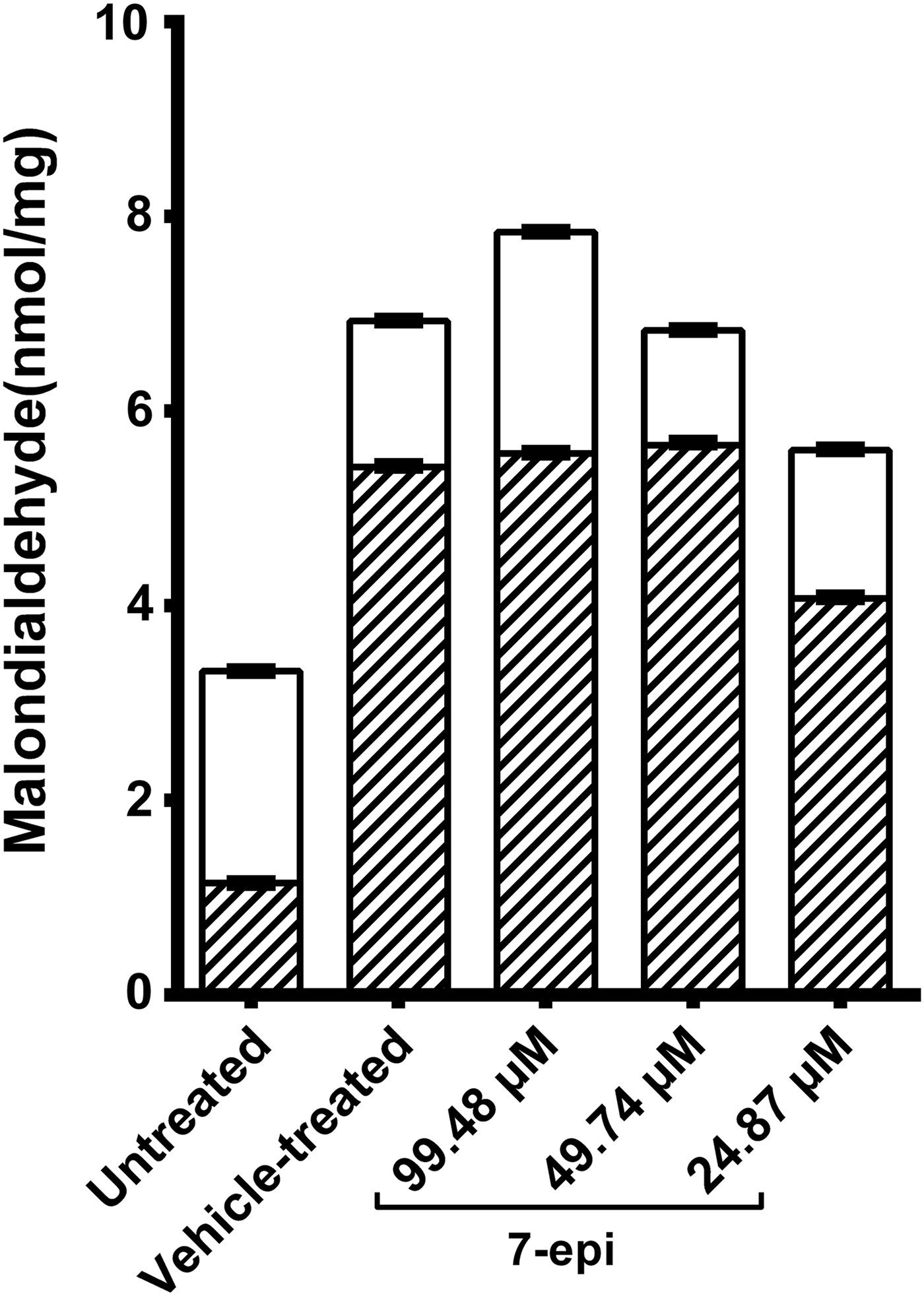
Fig. 2. Effect of 7-epiclusianone (7-epi) on the lipid peroxidation activity of Schistosoma mansoni adult worms. Parasites were incubated with different doses of 7-epi for 24 h at 37°C, and lipid peroxidation was estimated by malondialdehyde (MDA) quantification, in pellets (hatched) and supernatants of parasite extracts (unshaded), prepared 24 h after the end of the incubation period. Kolliphor® EL (KEL) was used as the vehicle for 7-epi. Data represent mean ± SD of five independent experiments. With the exception of the 24.87 μm supernatant samples, all groups yielded significantly different results when compared with the vehicle-treated group (P < 0.001, by one-way ANOVA followed by Tukey's test).
Incubation with 7-epi does not alter S. mansoni SOD expression
To assess whether 7-epi incubation alters the expression of the S. mansoni Cu/Zn-SOD enzyme, adult worms were incubated with 12.44, 24.87, 49.74, 99.48 or 198.96 μm 7-epi for 24 h, or with PZQ, KEL (vehicle) or medium only (RPMI) as controls, and then subjected to SOD quantitative real-time PCR (qRT-PCR) analysis, using actin (ACT) as a constitutive reference gene. As estimated by qRT-PCR, SOD expression did not vary significantly between parasite groups incubated with 7-epi, or between treated and untreated (or vehicle control) groups (fig. 3).
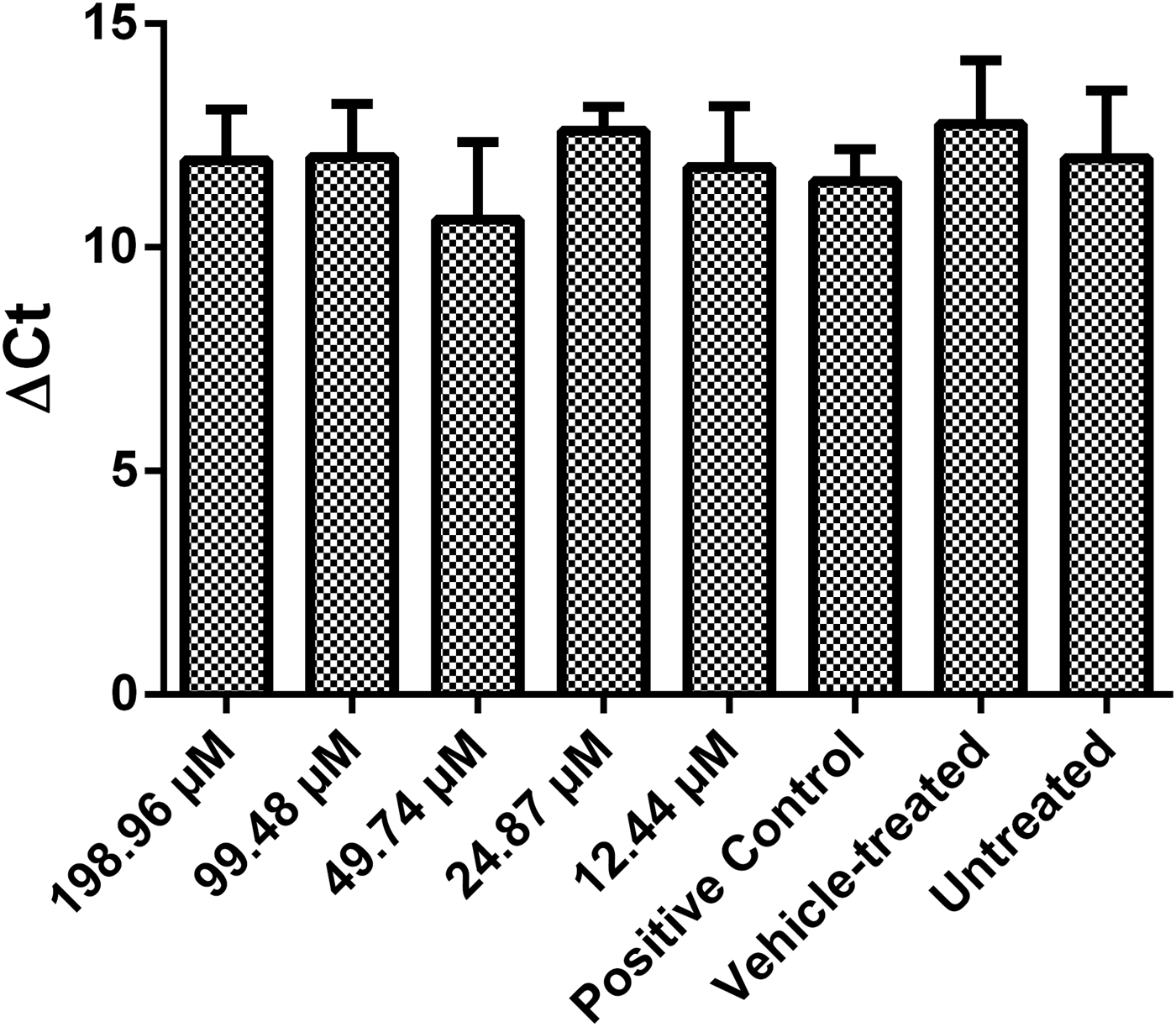
Fig. 3. Effect of incubation with 7-epiclusianone (7-epi) on Schistosoma mansoni superoxide dismutase (Cu/Zn-SOD) expression. Adult worms were incubated with different doses of 7-epi for 24 h at 37°C, and SOD expression was analysed (from total RNA samples of frozen and macerated worms) by quantitative real-time PCR (qRT-PCR), using actin as a constitutively expressed reference gene. Kolliphor® EL (KEL) was used as vehicle for 7-epi, and praziquantel (PZQ) as a tegument detachment control. Data represent mean ± SEM of five independent experiments (n = 3 adult worm couples/sample). No statistically significant differences were observed between samples (by one-way ANOVA followed by Tukey's test).
SOD inhibition by 7-epi may rely on allosteric effects
Reduction or loss of Cu/Zn-SOD activity may lead to tegument disruption (Callahan et al., Reference Callahan, Crouch and James1988; Huang, Reference Huang2012), which is observed after incubation with 7-epi (Castro et al., Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015; and in the present work). Therefore, we investigated the possibility that 7-epi interacts with SmCtSOD, by performing an in silico analysis of the molecular docking of 7-epi to SmCtSOD, since molecular docking analysis has low costs and may be used effectively to guide further analyses.
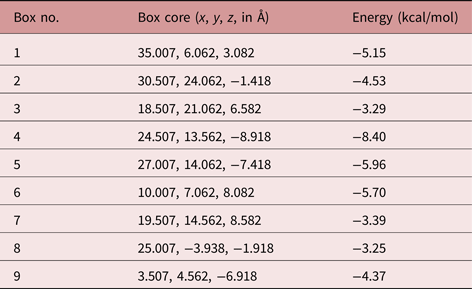
The SmCtSOD structure chosen for docking experiments consists of four protein chains (A, B, C and D) arranged in dimers (one dimer formed by A and B chains, and another by C and D strands). Each monomer has in its active site a zinc atom with a 2+ formal charge, and a copper atom in either of two alternative (x, y and z) locations, corresponding to the copper atom with a 1+ or 2+ formal charge. The first four molecular docking models were performed at the catalytic site, using the monomer (A chain) with Cu+ or Cu2+ (table 1 and fig. 4). Moreover, new potential allosteric binding sites were identified by the AutoLigand program (Harris et al., Reference Harris, Olson and Goodsell2007), which uses predicted maps generated by AutoGrid4 to engage atoms in the receptor's cavities. Using this software, ten ‘boxes’ representing alternative binding sites were created for each dimer and monomer, as a list of box midpoints, or cores (tables 2 and 3). To set the docking region, grid dot spacing was set to default (0.375 Å) and 60 grid dots were applied for each axis.
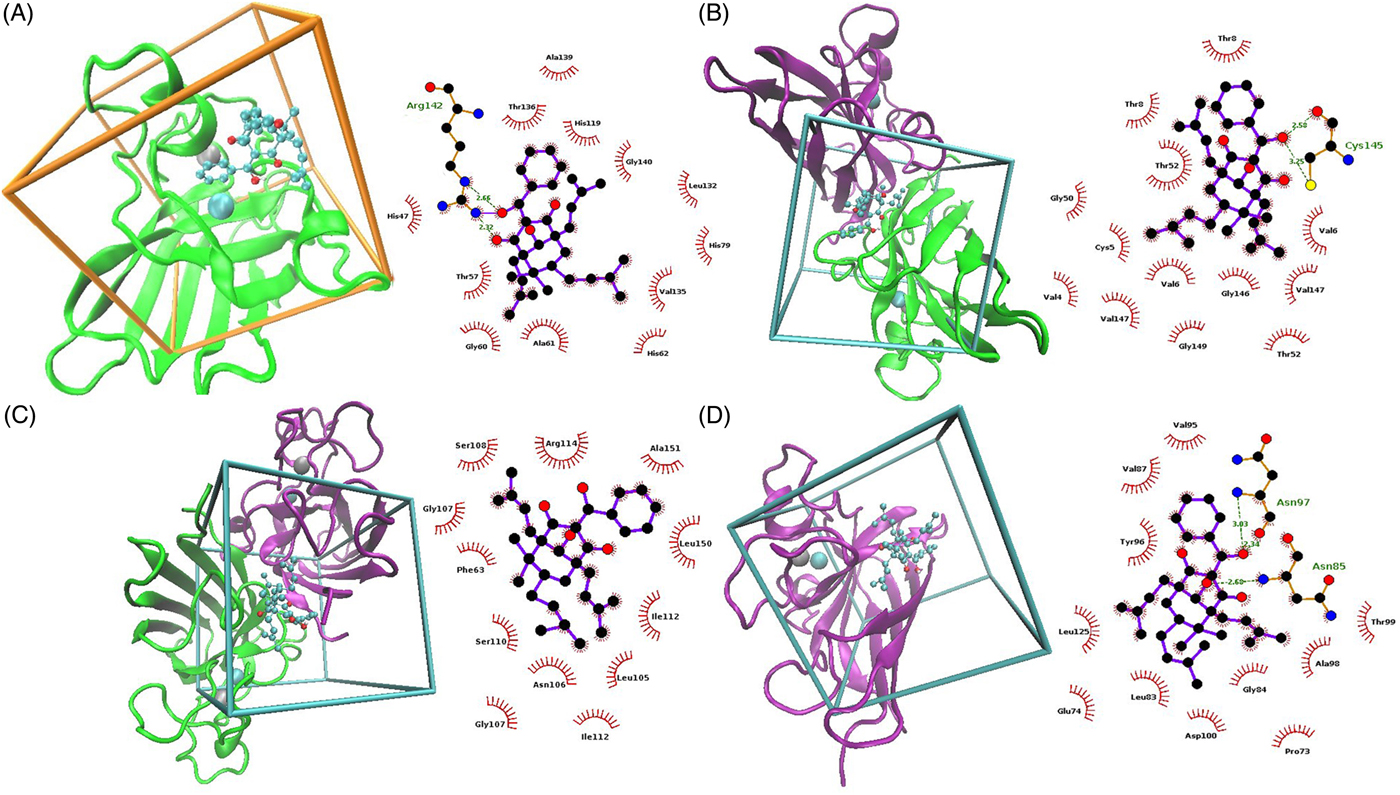
Fig. 4. Molecular docking analysis models showing the interaction between 7-epiclusianone (7-epi) and the entire Schistosoma mansoni cytosolic superoxide dismutase (SmCtSOD) structure (on the left), and graphic representations of the specific residues involved in docking (on the right), where red arches and dashed green lines represent hydrophobic interactions and hydrogen bonds, respectively. (A) 7-epi (cyan) and SmCtSOD monomer (green) with copper ion + 1 (cyan). (B) 7-epi (cyan) and dimeric SmCtSOD (green and pink) in box 3. (C) 7-epi (cyan) and dimeric SOD (green and pink) in box 6. (D) 7-epi (cyan) and monomeric SmCtSOD (pink) in box 4.
Table 1. Binding energies between 7-epiclusianone (7-epi) and the active site of the S. mansoni cytosolic SOD (SmCtSOD), calculated using AutoDock4.
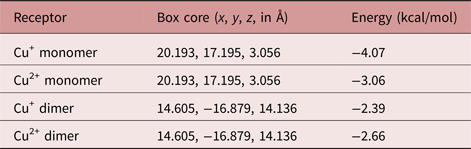
Table 2. Energy of interaction between 7-epiclusianone (7-epi) and the dimeric S. mansoni cytosolic superoxide dismutase (SmCtSOD), using allosteric binding sites (calculated using AutoDock4).
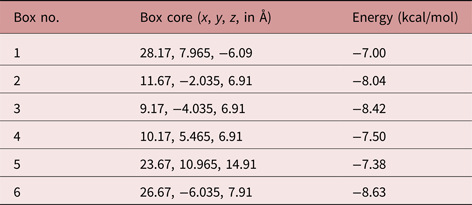
Table 3. Energy of interaction between 7-epiclusianone (7-epi) and the monomeric S. mansoni cytosolic superoxide dismutase (SmCtSOD), at binding sites different from the active site (calculated using AutoDock4).
Docking simulation at the active site suggests that 7-epi interacts with the His47, Thr57, Gly60, Ala61, His62, His79, His119, Leu132, Val135, Thr136, Ala139, Gly140 and Arg142 residues of SmCtSOD (fig. 4A). However, the low empirical variation suggests that 7-epi has a volume larger than that of the active site; thus, it cannot enter therein, and may exert allosteric inhibition instead. Also, the active site has some level of hydrophilicity, due to the presence of residues such as histidine and aspartic acid (Cardoso et al., Reference Cardoso, Silva, De Araújo, Tanaka, Tanaka and Garratt2004), whereas 7-epi has stronger non-polar features, because of the aromatic rings that constitute its structure and can contribute to its low affinity to active-site residues. The presence of the copper atom, in 1+ or 2+ forms, had little effect on interaction intensity results (table 1), compared with the active site without metallic ions, since the ligand does not interact with these ions, and the small fluctuations in power values may be due to random effects of the search algorithm employed here.
The in silico analysis predicted stronger interactions between 7-epi and sites found at boxes 3 and 6 for the SOD dimer (fig. 4B and C, respectively), and box 4 for the monomer (fig. 4D).
The results obtained by IFD are in agreement with those generated using AutoDock4, showing a poor interaction between 7-epi and the SmCtSOD active site (table 4). Also, the IFD analysis reinforced the presence of strong allosteric interactions at the sites represented in boxes 3 and 6 (dimeric template) and in box 4 (monomeric template), with lower IFD scores – indicative of stronger interactions - for the dimeric template (table 4).
Table 4. Energy of interaction between 7-epiclusianone (7-epi) and the S. mansoni cytosolic superoxide dismutase (SmCtSOD), at the active site (Cu+ and Cu2+) and at selected allosteric binding sites (boxes 3 and 6 from the dimer and box 4 from the monomer), calculated using the Glide software.
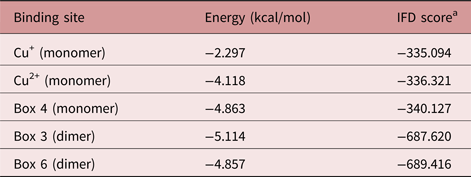
a IFD, induced fit docking.
7-epi effectively inhibits S. mansoni SOD activity
To evaluate the effect of 7-epi on S. mansoni SOD, parasites were incubated with 7-epi for 24 h, and SOD activity was measured in the supernatant of parasite extracts. We observed no SOD activity in assays performed after incubation of extracts with KCN, which inhibits Cu/Zn-SOD. Given the relatively low activities of total SOD found in the extracts (fig. 5), and considering that Mn-SOD accounts for only ~5% of the total S. mansoni SOD activity (Hong et al., Reference Hong, Kosman, Thakur, Rekosh and LoVerde1992), our data using KCN indicate that Mn-SOD activity was not detectable in our assays. Therefore, the SOD activity data shown here is likely to represent the action of Cu/Zn-SOD molecules only.
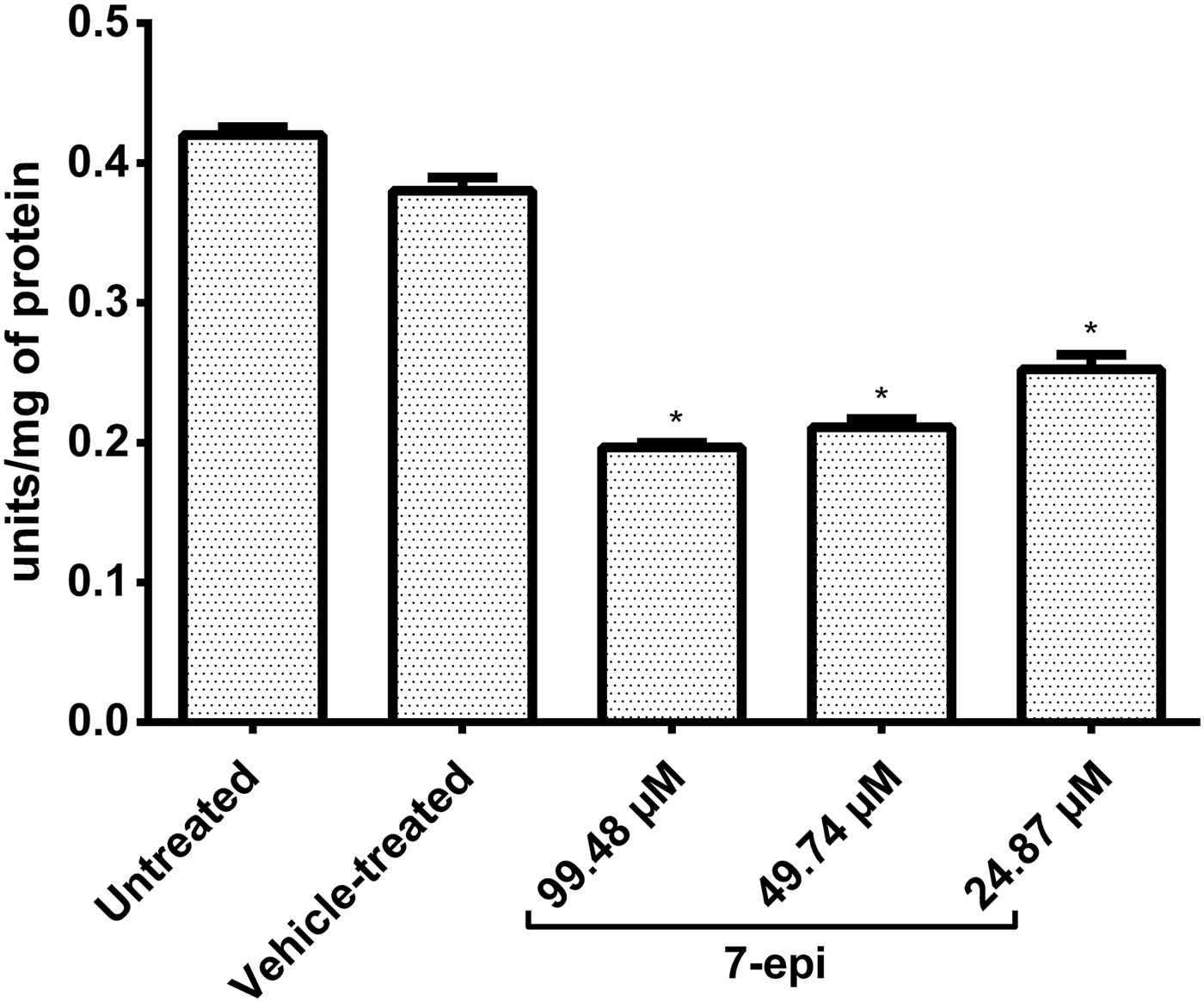
Fig. 5. Effect of incubation with 7-epiclusianone (7-epi) on the activity of Schistosoma mansoni Cu/Zn superoxide dismutase (Cu/Zn-SOD). Adult worms were incubated with different doses of 7-epi for 24 h at 37°C, and SOD activity was measured in the supernatants of parasite extracts (prepared 24 h after the end of the incubation period), with one unit representing an amount of enzyme capable of inhibiting 50% of water-soluble tetrazan-1 (WST-1) reduction. Kolliphor® EL (KEL) was used as the vehicle for 7-epi. Data represent mean ± SD of three independent experiments (n = 40 adult worm couples/sample). *P <0.001 vs. the vehicle-treated group (by one-way ANOVA followed by Tukey's test).
We observed a significant reduction in SOD activity in the groups incubated with 7-epi, compared with those in contact with KEL only (~30%; P < 0.001), and SOD activity did not vary significantly between the groups incubated with 7-epi (fig. 5). Overall, the SOD activity data show that 7-epi significantly decreases S. mansoni Cu/Zn-SOD activity, at concentrations ≥24.87 μm. At this dose, 7-epi induced clear tegument blistering and disruption, as observed by light microscopy imaging (fig. 1).
Discussion
The tegument of S. mansoni worms, which is affected by incubation with 7-epi, is a key structure in the defence against host immune mechanisms, by secreting and/or exposing substances that influence the immune response (Abath & Werkhauser, Reference Abath and Werkhauser1996; Van Hellemond et al., Reference Van Hellemond, Retra, Brouwers, van Balkom, Yazdanbakhsh, Shoemaker and Tielens2006). Nevertheless, the tegument barrier does not fully protect the parasite against damage from host defence mechanisms (Mclaren & Hockley, Reference Mclaren and Hockley1977; Hong et al., Reference Hong, Kosman, Thakur, Rekosh and LoVerde1992; Van Hellemond et al., Reference Van Hellemond, Retra, Brouwers, van Balkom, Yazdanbakhsh, Shoemaker and Tielens2006). In the presence of iron, the release of superoxide and hydrogen peroxide by host cells leads to the production of the hydroxyl radical, which is very harmful to parasite lipids, DNA and proteins.
Schistosoma mansoni worms may be exposed to ROS via different routes. In the parasite gut, haemoglobin degradation releases Fe (II) protoporphyrin IX, which may be oxidized in the presence of oxygen, creating superoxide anions (Loria et al., Reference Loria, Miller, Foley and Tilley1999; Huang, Reference Huang2012). The latter are also formed during mitochondrial energy metabolism: up to 3% of the electrons that pass through the respiratory chain react with molecular oxygen, generating O2− (Sayed et al., Reference Sayed, Cook and Williams2006; Pal & Bandyopadhyay, Reference Pal and Bandyopadhyay2012; Williams et al., Reference Williams, Bonilla, Gladyshev and Salinas2013). Since parasite lysates have a mixture of ROS from these different sources, we chose to evaluate oxidative stress in this environment indirectly, by lipid peroxidation. Incubation with 24.87 μm 7-epi significantly reduced lipid peroxidation levels (i.e. reduced oxidative stress levels), especially in the pellet of S. mansoni extracts, compared with the vehicle-treated control (fig. 2). However, we observed some tegument detachment in adult worms, 7 days after contact with 7-epi (fig. 1), in agreement with the ED90 value of 26.66 μm reported for 7-epi (Castro et al., Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015). Additionally, Santa-Cecília et al. (Reference Santa-Cecília, Santos, Fuzissaki, Derogis, Freitas, Gontijo, Stringheta, Nagem, Brigagão and Santos2012) showed that 7-epi had no substantial antioxidant activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical-scavenging tests, or in oxidation–reduction and chelating potential tests, providing strong evidence that 7-epi has no direct antioxidant effect that could protect the parasite. Incubations with 99.48 μm 7-epi increased total lipid peroxidation levels – indicating a potential increase in ROS levels – in agreement with the high degree of tissue degeneration observed at this concentration.
The conflict between the tegument morphology data and ROS levels (as estimated by lipid peroxidation), at the lower concentrations of 7-epi, may be attributed to the different timing of data collection in each assay, relative to the time of contact with 7-epi. For lipid peroxidation assays, parasites were sonicated immediately after incubation with 7-epi, while for tegument detachment analysis, 7-epi was removed from the medium after incubation and parasites were observed 7 days later. If 7-epi reduces a protective antioxidant activity found in the tegument, instead of causing tissue damage directly, tissue degeneration may only be expressed after prolonged contact with ROS, and/or after ROS accumulation (as a result of the parasite's metabolism). This effect could explain why tissue damage was seen 7 days after incubation with 24.87 μm 7-epi, while this 7-epi concentration did not increase peroxidation levels in parasite extracts.
To test this hypothesis, we evaluated the relationship between 7-epi and S. mansoni Cu/Zn-SOD – the only enzyme responsible for exogenous superoxide detoxification in the parasite (Callahan et al., Reference Callahan, Crouch and James1988; Hong et al., Reference Hong, Loverde, Thakur, Hammarskjold and Rekosh1993; Maizels et al., Reference Maizels, Bundy, Selkirk, Smith and Anderson1993; Sayed et al., Reference Sayed, Cook and Williams2006). SOD expression analysis by qRT-PCR showed no statistically significant alterations in SOD mRNA levels after contact with 7-epi, compared with the controls (fig. 3), suggesting that 7-epi reduces enzyme activity, rather than enzyme production.
The in silico molecular docking analysis performed here estimated a low power of interaction between 7-epi and the SOD active site, suggesting that 7-epi has a larger volume than the active site. Therefore, it is unlikely that 7-epi inhibits SOD activity by entering the active site. However, 7-epi may exert allosteric inhibition by blocking the entrance to the active site. The higher power of interaction to alternative sites in the interface between the two monomers (boxes 3 and 6; table 2) suggests that 7-epi may inhibit SOD activity by lodging in the interface between the two monomers, thereby destabilizing the dimer. Another significant result was obtained using box 4 of the monomeric form, which showed a strong interaction energy in a cavity located in the posterior segment of the active site. This interaction with residues adjacent to (but not at) the catalytic site (such as Leu125) may result in a rearrangement in the active-site cavity. Despite the low level of sequence identity between SmCtSOD and human SODs, the residues responsible for maintaining active-site geometry (Gly43, Gly60, Pro65, Gly81, Gly137 and Gly140) and for stabilizing the β-barrel structure (Gly15, Leu37, Phe44, Leu105 and Gly146) are conserved (Cardoso et al., Reference Cardoso, Silva, De Araújo, Tanaka, Tanaka and Garratt2004). Our molecular docking analysis supports the notion that 7-epi toxicity is selective towards the parasite, since only the ligand in box 6 of the SOD dimer interacts directly with one of the residues conserved in human homologues (Leu105).
Importantly, we observed a significant reduction in the total SOD activity (likely to represent Cu/Zn-SOD activity only) in supernatants of parasites incubated with as little as 24.87 μm 7-epi, which is close to the ED90 value of 26.66 μm reported for in vitro testing (Castro et al., Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015). These data support the notion that partial inhibition of SOD contributes to the antiparasitic effect of 7-epi towards S. mansoni, although 7-epi does not completely inactivate the enzyme.
In conclusion, tegument blistering has been reported to occur in vitro at concentrations that did not reduce SOD activity significantly (<12.44 μm) (Castro et al., Reference Castro, De Mattos, Pereira, Anchieta, Silva, Dias, Silva, Barros, Souza, Santos and Marques2015), which suggests that SOD inhibition is an additional effect of 7-epi treatment, rather than the only cause of tegument detachment. Enzymes responsible for the detoxification of hydrogen peroxide, such as glutathione peroxidase and glutathione S-transferase, could be investigated further, since peroxide and superoxide detoxification pathways are synergic. It is possible that, upon partial SOD inhibition by 7-epi, the initial harm caused to the tegument could expose the deepest tissues of the parasite to the host immune system. Thus, even moderate levels of SOD inhibition would make the parasite more susceptible to antigen recognition, complement formation and granulocyte adherence, amplifying the effectiveness of the host immune response against S. mansoni (Hong et al., Reference Hong, Kosman, Thakur, Rekosh and LoVerde1992; Abath & Werkhauser, Reference Abath and Werkhauser1996; LoVerde et al., Reference LoVerde, Carvalho-Queiroz and Cook2004; Van Hellemond et al., Reference Van Hellemond, Retra, Brouwers, van Balkom, Yazdanbakhsh, Shoemaker and Tielens2006).
Acknowledgements
The authors thank Dr João Augusto Alves Meira Neto for proper plant identification, and Dr Paulo Marcos Zech Coelho for his help and support.
Financial support
Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP) are thanked for financial support and scholarships. This work was funded by Programa Nacional de Pós-Doutorado (PNPD)/CAPES, project number 2011/90.
Conflict of interest
None.
Ethical standards
The experimental protocols used here were approved by the Ethical Committee for Animal Care at the Federal University of Alfenas (approval number: 576/2014; 30 June 2014), and all animal work was performed according to CONCEA (Conselho Nacional de Experimentação Animal) and ARRIVE (Animal Research: Reporting of In Vivo Experiments) – NC3Rs (National Centre for the Replacement, Refinement & Reduction of Animals in Research) guidelines for animal use and care in the laboratory.











