INTRODUCTION
Besnoitia besnoiti is a cyst-forming apicomplexan parasite in the Toxoplasmatinae subfamily along with other members of medical and veterinary importance, such as Toxoplasma gondii and Neospora caninum. Besnoitia besnoiti is the aetiological agent of bovine besnoitiosis, a disease that causes important economical losses in many tropical and subtropical regions, especially in sub-Saharan Africa (Pols, Reference Pols1960). Moreover, a re-emergence of the disease has been reported in Europe (European Food Safety Authority, 2010; Mutinelli et al. Reference Mutinelli, Schiavon, Ceglie, Fasolato, Natale, Rampin and Carminato2011), as disease cases have increased in the last 20 years in Portugal (Cortes et al. Reference Cortes, Reis, Waap, Vidal, Soares, Marques, Pereira da Fonseca, Fazendeiro, Ferreira, Caeiro, Shkap, Hemphill and Leitao2006), Spain (Fernández-García et al. Reference Fernández-García, Risco-Castillo, Pedraza-Díaz, Aguado-Martínez, Alvarez-García, Gómez-Bautista, Collantes-Fernández and Ortega-Mora2009b), France (Alzieu, Reference Alzieu2007; Liénard et al. Reference Liénard, Salem, Grisez, Prévot, Bergeaud, Franc, Gottstein, Alzieu, Lagalisse and Jacquiet2011), Italy (Agosti et al. Reference Agosti, Belloni, Morini and Vacirca1994; Gentile et al. Reference Gentile, Militerno, Schares, Nanni, Testoni, Bassi and Gollnick2012) and Germany (Schares et al. Reference Schares, Basso, Majzoub, Cortes, Rostaher, Selmair, Hermanns, Conraths and Gollnick2009), mainly associated with infected animal trade from endemic regions.
The complete life cycle of B. besnoiti is still unknown, and a definitive host, which sheds oocysts after the ingestion of infected tissues, has not yet been identified (Basso et al. Reference Basso, Schares, Gollnick, Rutten and Deplazes2011). Nonetheless, in intermediate hosts (cattle, blue wildebeest and impala), tachyzoites (endozoites) rapidly multiply in blood vessels and are responsible for the acute stage of the disease, which is characterized by fever, lymphadenitis, subcutaneous oedema, loss of body condition and testicular inflammation. In chronically infected cattle, tachyzoites switch into low-dividing bradyzoites, which are contained in thick-walled tissue cysts mainly found in the cutis, subcutis, fascia, conjunctiva, and the genital and respiratory mucosa.
During this stage, the skin can become severely lichenified and alopecic, and bulls can develop necrotizing orchitis, which may result in permanent infertility (Bigalke, Reference Bigalke1968) with the subsequent economic losses. Unfortunately, an effective vaccine or treatment is not available, and the control measures are based on management practices based on previous clinical and serological diagnosis (García-Lunar et al. Reference García-Lunar, Ortega-Mora, Schares, Gollnick, Jacquiet, Grisez, Prevot, Frey, Gottstein and Alvarez-García2013).
In this context, the use of proteomic tools may help to elucidate unknown aspects of parasite biology and identify diagnostic, drug and/or vaccine targets, as previously performed for other important apicomplexan parasites (reviewed by Weiss et al. Reference Weiss, Fiser, Angeletti and Kim2009). In this sense, recent pioneering proteomic work on the tachyzoite stage of B. besnoiti showed the utility of a classical proteomic approach, 2-dimensional electrophoresis coupled to mass spectrometry (MS), because 27 protein spots were successfully identified. These proteins are involved in energy metabolism, stress, host cell invasion processes, and cell redox homeostasis. Moreover, some of them proved to be immunogenic (García-Lunar et al. in press). An essential event in the pathogenesis of B. besnoiti is the tachyzoite-to-bradyzoite conversion, which permits the persistence of the parasite. However, the molecular mechanisms that govern this process remain unknown although, according to previous studies, differential protein expression is suspected because a distinct antigenic pattern has been described between both parasite developmental stages (Fernández-García et al. Reference Fernández-García, Alvarez-García, Risco-Castillo, Aguado-Martínez, Marugán-Hernández and Ortega-Mora2009a; Schares et al. Reference Schares, Basso, Majzoub, Rostaher, Scharr, Langenmayer, Selmair, Dubey, Cortes, Conraths and Gollnick2010). Currently, proteomic approaches are essential to identify novel targets because, unfortunately, a reduced number of Besnoitia ESTs are available in databases, and the entire genome has not yet been sequenced. However, the availability of complete genomes of T. gondii and N. caninum and the marked conservation of their genomes and gene expression (Reid et al. Reference Reid, Vermont, Cotton, Harris, Hill-Cawthorne, Konen-Waisman, Latham, Mourier, Norton, Quail, Sanders, Shanmugam, Sohal, Wasmuth, Brunk, Grigg, Howard, Parkinson, Roos, Trees, Berriman, Pain and Wastling2012) allow for the use of these genomes to identify orthologues. Moreover, difference gel electrophoresis (DIGE) technology has proved to be a straightforward approach to identify differentially developmental stage-expressed proteins in N. caninum tachyzoite and bradyzoite stages in a study performed in members of the family Sarcocystidae (Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010).
Therefore, in the present study, a proteomic approach based on DIGE technology followed by MS analysis was applied to compare B. besnoiti tachyzoite and bradyzoite extracts to identify stage-regulated proteins.
MATERIALS AND METHODS
Cell culture and tachyzoite and bradyzoite purification
Besnoitia besnoiti tachyzoites from the Bb-Spain1 isolate (Fernández-García et al. Reference Fernández-García, Risco-Castillo, Pedraza-Díaz, Aguado-Martínez, Alvarez-García, Gómez-Bautista, Collantes-Fernández and Ortega-Mora2009b) were grown by continuous passage in a MARC-145 cell culture following standard procedures. Tachyzoites were harvested 3·5 days post-infection, when the majority of the tachyzoites were still intracellular, grown exponentially for 4 days, and purified with disposable PD-10 desalting columns (GE Healthcare). Tachyzoite viability was confirmed by counting in a Neubauer chamber. Microscopic observations of the purified tachyzoites were carefully performed to detect and discard parasite batches with host cell contamination. This method of purification has proved to be appropriate to reduce host cell contamination in previous DIGE studies carried out in N. caninum (Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010; Regidor-Cerrillo et al. Reference Regidor-Cerrillo, Alvarez-García, Pastor-Fernández, Marugán-Hernández, Gómez-Bautista and Ortega-Mora2012).
Bradyzoites were released by trypsinization of a skin biopsy from the vulvar region containing numerous macroscopic tissue cysts from the chronically infected cow from which the B. besnoiti Bb-Spain1 isolate was obtained (Fernández-García et al. Reference Fernández-García, Risco-Castillo, Pedraza-Díaz, Aguado-Martínez, Alvarez-García, Gómez-Bautista, Collantes-Fernández and Ortega-Mora2009b) using a previously described method, with a few modifications (Shkap et al. Reference Shkap, Pipano and Greenblatt1987). Bradyzoites were separated from tissue debris with a 40 μm sieve, collected by centrifugation and counted in a Neubauer chamber. Microscopy showed a 2% contamination with bovine erythrocytes.
Tachyzoites and bradyzoites were grouped into 4 different batches of production of approximately 2×108 zoites each, which is the required number of replicates to perform DIGE. Then the parasites were pelleted by centrifugation at 1350 g for 10 min and stored at −80 °C until use.
Protein extracts from B. besnoiti tachyzoites and bradyzoites and bovine erythrocytes
To avoid interference from protein contamination by bovine erythrocytes in the identification of abundant bradyzoite proteins in the tachyzoite-bradyzoite proteomic study, an initial DIGE analysis was performed with bovine erythrocytes and purified bradyzoites. Thus, a blood sample was collected from a cow into an EDTA tube by coccygeal venipuncture, and the erythrocytes were purified using a Percoll gradient (Pertoft et al. Reference Pertoft, Johnsson, Wärmegård and Seljelid1980).
The number of erythrocytes included in the DIGE study corresponded to the 2% erythrocyte contamination found in the purified bradyzoite samples. Protein extraction was performed as described previously (Regidor-Cerrillo et al. Reference Regidor-Cerrillo, Alvarez-García, Pastor-Fernández, Marugán-Hernández, Gómez-Bautista and Ortega-Mora2012), with limited modifications. Frozen tachyzoites, bradyzoites and erythrocytes were disrupted in 150 μL of lysis buffer containing 6 m urea, 2 m thiourea, 4% (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 65 mm 1,4-dithioerythreitol (DTE), 10 mm Tris-HCl, pH 7, and 1 mm phenylmethanesulfonyl fluoride (PMSF) (added fresh) and subjected to 3 cycles of rapid freezing and thawing using liquid nitrogen. Tachyzoites, bradyzoites and erythrocytes were further disrupted by sonication for 15 min at 4 °C in a water bath sonicator (Ultrasonics Ltd). Solubilization was aided by the subsequent addition of 150 μL of rehydration buffer containing 8 m urea, 2 m thiourea, 2% (w/v) CHAPS, 65 mm DTE, and 1% ampholyte. Insoluble material was removed by centrifugation at 13 000 g for 30 min at 4 °C. The protein concentrations of the resulting supernatants were determined by the Bradford method using bovine serum albumin as a standard.
CyDye labelling and DIGE
CyDye labelling was performed according to the manufacturer's protocols (GE Healthcare). Briefly, 50 mg of protein per sample was labelled with 400 pmol of Cy2, Cy3 or Cy5 fluorochromes dissolved in dimethylformamide (DMF) (99·8%) for 30 min at 41 °C in the dark. Then, reactions were quenched with 1 mL of lysine (10 mm/50 mg protein) for 15 min at 41 °C in the dark. All DIGE gels included the internal standard, which was prepared by pooling equal amounts of protein from each biological sample in the experiment and labelling them with Cy2 dye; thus, all proteins from all samples were represented in the internal standard (Alban et al. Reference Alban, David, Bjorkesten, Andersson, Sloge, Lewis and Currie2003).
First, erythrocytes and bradyzoites were compared by DIGE (DIGE-1, Table 1). After erythrocyte spots were identified (Fig. 1), tachyzoites and bradyzoites were also compared by DIGE (DIGE-2, Table 1; Fig. 2).

Fig. 1. Difference gel electrophoresis (DIGE) gels of Besnoitia besnoiti bradyzoite and bovine erythrocyte protein extracts. Silver-stained 2D-PAGE gel with bradyzoite extract (A) and bovine erythrocytes (B). Bovine erythrocyte spots are marked with circles. (C) Overlay of images of bradyzoite and erythrocyte protein extracts showing Cy3-labelled (bradyzoite extract) and Cy5-labelled (erythrocytes) proteins. Proteins were separated in the first dimension along a non-linear pH gradient (pH 3–11, 24 cm) and on a 12% polyacrylamide gel in the second dimension.

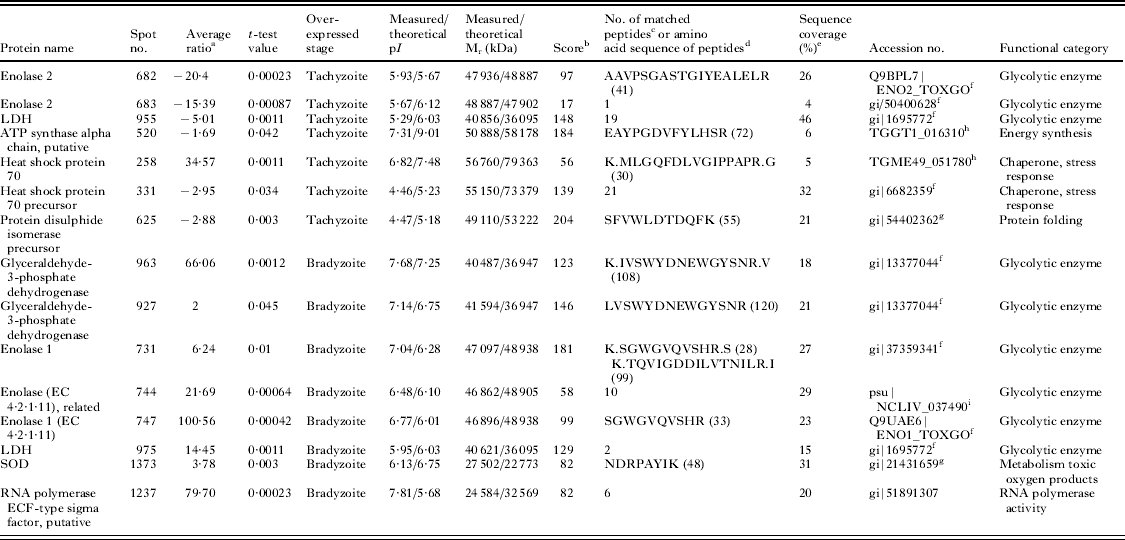
Fig. 2. Difference gel electrophoresis (DIGE) gels of Besnoitia besnoiti tachyzoite and bradyzoite proteins. (A) Overlay of images of gel 1 showing Cy3-labelled (bradyzoite extract) and Cy5-labelled (tachyzoite extract) parasite proteins showing changes in the proteomes of B. besnoiti bradyzoites and/or tachyzoites. Haemoglobin spots are shown in white. (B) Silver-stained DIGE gel with internal standard. Proteins were separated in the first dimension along a non-linear pH gradient (pH 3–11, 24 cm) and on a 12% polyacrylamide gel in the second dimension. Identified proteins (Table 2) are indicated with arrows in red for spots that were overexpressed in the tachyzoite stage and green for spots overexpressed in the bradyzoite stage. Haemoglobin (α and β Hb) proteins are shown in black. Proteins are annotated with abbreviated names and spot numbers are also indicated to distinguish isoforms: ENO2: enolase 2; LDH: lactate dehydrogenase; ATPs: ATP synthase alpha chain; HSP70: heat shock protein 70; HSP70p: heat shock protein 70 precursor and PDIp: protein disulphide isomerase precursor for tachyzoites. GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ENO1: enolase 1; LDH lactate dehydrogenase; SOD: superoxide dismutase and RNA pol: RNA polymerase ECL type sigma factor for bradyzoites.
Table 1. Difference gel electrophoresis (DIGE) experimental design

a Fluorochrome labelling extracts.
DIGE was performed as previously described (Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010). Briefly, a total of 150 mg of protein containing the internal standard and the extracts to be compared were mixed, and an equivalent volume of loading buffer was added (8 m urea, 4% CHAPS, 130 mm DTT and 2% immobilized pH gradient (IPG) buffer, pH 3–10). Samples were loaded into 24-cm non-linear pH 3–11 IPG strips (GE Healthcare), and isoelectric focusing (IEF) was performed with an IPGphor II unit (GE Healthcare) to a total of 50–55 kVh. Subsequently, the strips were equilibrated twice in 10 mL of equilibration buffer (6 m urea, 30% glycerol v/v, 2% SDS, and 100 mm Tris-HCl, pH 6·8), first adding DTE (0·5%) and then adding iodoacetamide (4·5%), and then the strips were sealed with 1% agarose to 12% polyacrylamide gels. Proteins were separated (17 h, 2 w/gel) in an Ettan Dalt Six unit (GE Healthcare).
Image visualization and the analyses of the different abundances of spots from different stages were performed as previously described (Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010).
Mass spectrometry analysis
For protein identification, preparative 2D gels were run with a total of 300 μg protein and visualized with mass spectrometry (MS)-compatible Coomassie blue staining (Gorg et al. Reference Gorg, Obermaier, Boguth, Harder, Scheibe, Wildgruber and Weiss2000).
Differentially expressed spots that were excised for MS analysis were selected based on the following criteria: (i) increase or decrease in their relative abundance (high ratios); (ii) spot visualization in DIGE or Coomassie-stained gels in order to have enough quantity of protein for MS. Next the proteins selected were in-gel reduced, alkylated, digested with trypsin (Sechi and Chait, Reference Sechi and Chait1998) and prepared for MALDI-TOF MS fingerprinting. MALDI-TOF MS was performed in a MALDI-TOF/TOF mass spectrometer (4700 Proteomics Analyzer; PerSeptive Biosystems) operating in reflector mode with an accelerating voltage of 20 000 V. All mass spectra were calibrated externally using a standard peptide mixture (Sigma).
Monoisotopic peptide masses were compared with NCBI non-redundant (NCBI nr) (2367365 sequences; 802797248 residues), Swiss-PROT/TrEMBL (250296 sequences; 91444238 residues) and ToxoDB 6.2 (N. caninum (Nc-Liverpool isolate): 270109 (5587 sequences; 5496774 residues); T. gondii: 290109 (23941 sequences; 16960681 residues)) databases using the MASCOT algorithm v2.2 (www.matrixscience.com) (Gajria et al. Reference Gajria, Bahl, Brestelli, Dommer, Fischer, Gao, Heiges, Iodice, Kissinger, Mackey, Pinney, Roos, Stoeckert, Wang and Brunk2008). For tandem mass spectrometry (MS–MS) sequencing analyses, suitable precursors were selected, and fragmentation was carried out using CID. De novo sequencing from fragmentation spectra of peptides was performed using the DeNovo software tool (Applied Biosystems), and database homology searches of the sequences were carried out by BLAST.
RESULTS AND DISCUSSION
Tachyzoite-to-bradyzoite conversion seems to be critical for B. besnoiti persistence in the host, and it has been described for other cyst-forming coccidian parasites (Innes, Reference Innes2007) despite their different cell tropism. Moreover, evasion of the host immune system, the conversion process and the establishment of chronic infection might involve similar and/or different pathways in B. besnoiti. From a practical point of view, serological and molecular assays based on antigenic stage-specific proteins may offer additional information on the determination of the phase of infection, with subsequent improvement in the diagnosis of acute and subclinical cases, which is the main handicap of the serological diagnosis of bovine besnoitiosis for control purposes (García-Lunar et al. Reference García-Lunar, Ortega-Mora, Schares, Gollnick, Jacquiet, Grisez, Prevot, Frey, Gottstein and Alvarez-García2013). This issue is of particular interest because a different pattern of antigen recognition has been observed between tachyzoite and bradyzoite extracts (Fernández-García et al. Reference Fernández-García, Alvarez-García, Risco-Castillo, Aguado-Martínez, Marugán-Hernández and Ortega-Mora2009a; Schares et al. Reference Schares, Basso, Majzoub, Rostaher, Scharr, Langenmayer, Selmair, Dubey, Cortes, Conraths and Gollnick2010).
In cyst-forming coccidian parasites, preliminary comparative studies on the differences between tachyzoite and bradyzoite stages have been carried out by genomic approaches (Cleary et al. Reference Cleary, Singh, Blader, Brewer and Boothroyd2002; Radke et al. Reference Radke, Behnke, Mackey, Radke, Roos and White2005). Proteomic tools have also been employed to compare different stages in apicomplexan parasites (Florens et al. Reference Florens, Washburn, Raine, Anthony, Grainger, Haynes, Moch, Muster, Sacci, Tabb, Witney, Wolters, Wu, Gardner, Holder, Sinden, Yates and Carucci2002; Lal et al. Reference Lal, Bromley, Oakes, Prieto, Sanderson, Kurian, Hunt, Yates, Wastling, Sinden and Tomley2009; Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010). Currently, only 1 proteomic study has been carried out in B. besnoiti, in which the proteome and immunome of the tachyzoite stage was characterized. Indeed, 20 proteins were identified, and 9 of them proved to be immunogenic (García-Lunar et al. in press). This study showed the feasibility of applying proteomic approaches in Besnoitia spp. with the availability of the whole genome sequences of closely related T. gondii and N. caninum. Indeed, a previous study (Reid et al. Reference Reid, Vermont, Cotton, Harris, Hill-Cawthorne, Konen-Waisman, Latham, Mourier, Norton, Quail, Sanders, Shanmugam, Sohal, Wasmuth, Brunk, Grigg, Howard, Parkinson, Roos, Trees, Berriman, Pain and Wastling2012) recently reported conserved genome sequences between these two species. Accordingly, a comparative proteomic study between tachyzoite and bradyzoite stages of B. besnoiti is challenging, and an appropriate experimental design is essential for DIGE analysis considering the nature of the samples, which may be contaminated with red blood cells (bradyzoites from vulva biopsies) and monolayer cells (tachyzoites from Marc-145 cell culture).
Recognition of erythrocyte protein spots by DIGE
The proteomic study performed here required a high amount of both zoite extracts for the recommended number of 2D gel replicates. Besnoitia besnoiti tachyzoites are easily obtained in cellular culture (Fernández-García et al. Reference Fernández-García, Alvarez-García, Risco-Castillo, Aguado-Martínez, Marugán-Hernández and Ortega-Mora2009a). However, an in vitro methodology to obtain B. besnoiti bradyzoites has not been developed, in contrast to T. gondii (Weiss et al. Reference Weiss, Laplace, Takvorian, Tanowitz, Cali and Wittner1995) and N. caninum (Risco-Castillo et al. Reference Risco-Castillo, Fernández-García and Ortega-Mora2004; Vonlaufen et al. Reference Vonlaufen, Guetg, Naguleswaran, Muller, Bjorkman, Schares, von Blumroeder, Ellis and Hemphill2004). Thus, bradyzoites must be obtained and purified from tissue cysts present in the subcutaneous tissue or mucosa of B. besnoiti naturally infected cows (Shkap et al. Reference Shkap, Pipano and Greenblatt1987). Following this procedure, erythrocyte contamination remained in the collected samples. Consequently, to exclude erythrocyte protein spots in the tachyzoite-bradyzoite comparison, an initial DIGE analysis was performed with bovine erythrocyte and B. besnoiti bradyzoite extracts, and matched spots were removed from the bioinformatics analysis when DIGE gels with bradyzoite and tachyzoite extracts were compared.
Over 1600 spots were detected when erythrocyte and bradyzoite extracts were resolved in a DIGE gel (Fig. 1). However, only 82 spots in the bradyzoite extract matched spots detected in the erythrocyte extract, and most of them exhibited a low abundance; the latter spots were excluded in the subsequent bioinformatic tachyzoite-bradyzoite extract comparative analysis by DIGE to avoid false identification of abundant bradyzoite proteins. Indeed, one of the spots that showed a significant increase in the relative abundance (±1·5-fold, P<0·05, t-test) was selected for MS analysis and proved to be haemoglobin (Fig. 2).
Comparison of tachyzoite and bradyzoite protein abundance by DIGE
Spots with a significant increase (or decrease) in relative abundance (±1·5-fold, P<0·05, t-test) were considered to be developmentally regulated proteins. After the bioinformatics analysis (in which putative erythrocyte spots were discarded), a total of up to 262 differentially expressed proteins were obtained: 130 were more abundant in the bradyzoite stage and 132 were more abundant in the tachyzoite stage. The bradyzoites obtained from in vivo cysts are primarily mature bradyzoites that, together with the different nature of the zoite origin (bradyzoites in an intermediate host vs tachyzoites in cell culture), explain the higher number (approximately 3 times more) of stage-regulated proteins detected compared with a similar proteomic study performed in N. caninum, in which only 72 differentially abundant spots were found (53 spots more abundant in bradyzoites vs 19 spots more abundant in tachyzoites) (Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010). Indeed, the in vitro methodology developed to obtain N. caninum bradyzoites is characterized by a low amount of bradyzoites that are obtained and later purified and by the fact that a mixture of tachyzoites and bradyzoites is obtained, and no mature cysts are produced (Risco-Castillo et al. Reference Risco-Castillo, Fernández-García and Ortega-Mora2004, Reference Risco-Castillo, Marugán-Hernández, Fernández-García, Aguado-Martínez, Jiménez-Ruiz, Rodríguez-Marco, Alvarez-García and Ortega-Mora2011). These limitations may explain the significant differences in the relative abundance of up-regulated bradyzoite proteins (± 5-fold in N. caninum, unpublished data, vs up to 100-fold for B. besnoiti ENO1, data not shown).
Protein identification
Finally, 25 differentially expressed spots were excised from gels for MS analysis. However, only 15 spots were identified (Fig. 2): 7 were more abundant in the tachyzoite stage, whereas 8 were more abundant in the bradyzoite stage (Table 2). Novel proteins were identified as T. gondii and N. caninum orthologues.
Table 2. Besnoitia besnoiti stage-expressed proteins identified by MS or MS/–MS
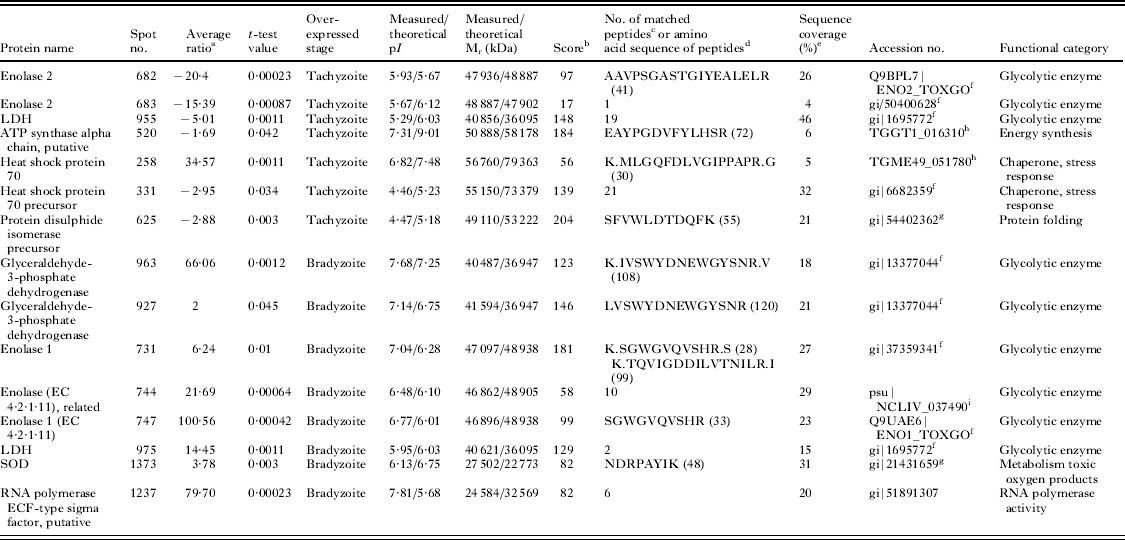
a Average volume ratio of the spots (bradyzoites vs tachyzoites) quantified by DeCyder biological variation analysis module. Only spots exhibiting over ±1·5-fold in their relative abundance with a P<0·05 in t-test between both stages were analysed by MS for identification.
b MASCOT MS protein score, obtained from MALDI TOF/TOF spectra. In all cases, the probability score was <0·05.
c Number of peptide masses values search.
d Amino acid sequence identified by MS–MS; the ion score is indicated in parentheses.
e Amino acid sequence coverage for the identified proteins by MS and MS–MS.
f Identified against NCBI nr database as T. gondii protein.
g Identified against NCBI nr database as N. caninum protein.
h Identified in ToxoDB 6.2 database as T. gondii protein.
i Identified in ToxoDB 6.2 database as N. caninum protein.
Several differences were observed between tachyzoites and bradyzoites. However, there were still hundreds of shared spots, most likely general metabolism and housekeeping proteins, which means that tachyzoites and bradyzoites still share several processes, as both are intracellular invasive forms. Moreover, although stage-specific differences have been previously observed between both stages by Western blot assay (Fernández-García et al. Reference Fernández-García, Alvarez-García, Risco-Castillo, Aguado-Martínez, Marugán-Hernández and Ortega-Mora2009a), DIGE only allows for differentiating abundance, not strictly stage-specific proteins.
Most of the identified proteins were glycolytic enzymes related to energy metabolism. A bias towards anaerobic glycolysis has been previously reported for both Toxoplasma and Neospora (Tomavo, Reference Tomavo2001; Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010). Thus, B. besnoiti seems to exhibit the same tendency, as 2 different isoforms of glyceraldehyde-3-phosphate dehydrogenase were found. Moreover, enolase (ENO) and lactate dehydrogenase (LDH) isoforms seem to be associated with different parasite stages, as previously noted for T. gondii (Yang and Parmey, Reference Yang and Parmey1997) and N. caninum (Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010). Here, the ENO isoform identified as more abundant in tachyzoites corresponded to T. gondii ENO2, which was previously described in T. gondii tachyzoites, and this finding was supported by the recent identification of BbENO2 in the B. besnoiti tachyzoite proteome and immunome (García-Lunar et al. in press). In addition, the ENO isoform that proved to be more abundant in bradyzoites corresponded to T. gondii ENO1, which was also previously described in the bradyzoite stage. Unexpectedly, the enzyme LDH was identified as the same protein in both stages by MS. Nonetheless, the existence of developmentally regulated LDH isoforms in B. besnoiti, as in T. gondii, should not be excluded due to the high gene homology between distinct isoforms. Moreover, an ATP synthase alpha chain directly related to the generation of energy (synthesizing ATP, the final product in the Krebs cycle) appeared with a higher abundance in tachyzoites. An ATP synthase has also been recently identified in the B. besnoiti tachyzoite proteome (García-Lunar et al. in press). This finding may be easily explained by the high energy requirement of the fast proliferative tachyzoite stage in contrast to low-dividing bradyzoites.
Both heat shock proteins (HSP) 70, precursor and mature, were more abundant in the tachyzoite stage. The HSP family, particularly HSP70, has been described to be expressed during stress situations, such as the differentiation process from tachyzoites to bradyzoites, in both T. gondii (Weiss et al. Reference Weiss, Ma, Takvorian, Tanowitz and Wittner1998) and N. caninum (Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010). HSP70 is a highly abundant protein in apicomplexan parasites, as it is usually detected in proteomic studies (Cohen et al. Reference Cohen, Rumpel, Coombs and Wastling2002; Shin et al. Reference Shin, Lee, Shin, Kim, Lee, Kim, Jang, Gershwin, Kim, Kim, Kim, Suh and Jung2004; Marugán-Hernández et al. Reference Marugán-Hernández, Alvarez-García, Risco-Castillo, Regidor-Cerrillo and Ortega-Mora2010, Reference Marugán-Hernandez, Alvarez-García, Tomley, Hemphill, Regidor-Cerrillo and Ortega-Mora2011; García-Lunar et al. in press), and has different isoforms (Lee et al. Reference Lee, Kim, Shin, Shin, Suh, Kim, Kim, Kim and Jung2003). This finding agrees with the recent identification of 4 isoforms of BbHSP70 in the proteome of the B. besnoiti tachyzoite stage (García-Lunar et al. in press). However, the use of a commercial monoclonal antibody recognizing HSP70 in an immunohistochemical assay showed reactivity in B. besnoiti tissue cysts, providing evidence for the presence of HSP70 in the bradyzoite stage (Irigoien et al. Reference Irigoien, Del Cacho, Gallego, Lopez-Bernad, Quilez and Sanchez-Acedo2000).
The protein disulphide isomerase (PDI) precursor was also found to be more abundant in the tachyzoite stage. Besnoitia besnoiti PDI has recently been identified and characterized (Marcelino et al. Reference Marcelino, Martins, Morais, Nolasco, Cortes, Hemphill, Leitão and Novo2011) and has been detected in both tachyzoites and bradyzoites. Moreover, a previous study (García-Lunar et al. in press) recently identified BbPDI in the tachyzoite proteome. PDI seems to be essential for the correct 3-dimensional structure of proteins and has also been described in N. caninum (Naguleswaran et al. Reference Naguleswaran, Alaeddine, Guionaud, Vonlaufen, Sonda, Jenoe, Mevissen and Hemphill2005) and T. gondii (Meek et al. Reference Meek, Back, Klaren, Speijer and Peek2002a, Reference Meek, Back, Klaren, Speijer and Peek2002b), in which PDI plays an important role in host cell invasion and is a relevant target for the host immune response (Shin et al. Reference Shin, Lee, Shin, Kim, Lee, Kim, Jang, Gershwin, Kim, Kim, Kim, Suh and Jung2004, Reference Shin, Shin, Kim, Lee, Yang, Palaksha, Youn, Kim, Kim, Marsh, Lakritz and Jung2005). Thus, the higher abundance found here in the tachyzoite stage is in agreement with its relevant role in parasite invasion. Moreover, PDI also seems to be an interesting target for vaccine development because mouse oral vaccination with recombinant N. caninum PDI conferred approximately 90% protection against disease (Debache et al. Reference Debache, Guionaud, Alaeddine and Hemphill2010).
After parasite invasion, activated macrophages attempt to inhibit intracellular multiplication by producing a number of toxic products, including reactive oxygen intermediates. Enzymes such as superoxide dismutase (SOD) and catalase protect the parasite from oxygen toxicity and damage (Haas and Goebel, Reference Haas and Goebel1991; Miller and Britigan, Reference Miller and Britigan1997). In the present study, an SOD enzyme showed higher abundance in the bradyzoite stage. This protein has been detected in both T. gondii (Odberg-Ferragut et al. Reference Odberg-Ferragut, Renault, Viscogliosi, Toursel, Briche, Engels, Lepage, Morgenstern-Badarau, Camus, Tomavo and Dive2000) and N. caninum (Cho et al. Reference Cho, Na, Song, Cho, Kang, Lee, Song and Kim2004) bradyzoites and tachyzoites, suggesting that SOD might be essential for the intracellular growth of different developmental stages, protecting the parasite from oxidative killing (Michalski and Prowse, Reference Michalski and Prowse1991). The higher abundance of SOD in bradyzoites might be explained by the origin of parasite stages because the bradyzoites originated from an in vivo infection, whereas tachyzoites were propagated in an epithelial monkey-derived cell line.
Concerning RNA polymerase, which is directly involved in protein synthesis, it proved to be more abundant in bradyzoites than in tachyzoites. However this result can be explained by the fact that not only the tachyzoite fast-proliferative phase requires a high amount of proteins. Indeed, other situations such as stress or during bradyzoite to tachyzoite conversion may also require a high protein synthesis rate.
LDH, ENO2, ATP synthase, HSP70 and PDI were expected to be abundant in the tachyzoite proteome because they were previously localized in the B. besnoiti tachyzoite-stage proteome, showing approximately the same isoelectric point and molecular weight (García-Lunar et al. in press). Interestingly, the present study predicts them to be interesting diagnostic, vaccine or drug targets due to their developmentally specific regulation. However, ENO2 should be discarded for diagnostic purposes because it is antigenically cross-reactive between B. besnoiti and N. caninum (García-Lunar et al. in press). Other proteins, such as GAPDH and SOD, may be present in small amounts in the tachyzoite proteome because they were identified for the first time in the present study.
Surface proteins were not identified in the present study, most likely due to their hydrophobic nature. Thus, additional approaches should be conducted to identify the B. besnoiti glycoproteome (Che et al. Reference Che, Madrid-Aliste, Burd, Zhang, Nieves, Kim, Fiser, Angeletti and Weiss2011; Luo et al. Reference Luo, Upadhya, Zhang, Madrid-Aliste, Nieves, Kim, Angeletti and Weiss2011) because most stage-specific proteins described in T. gondii and N. caninum are surface proteins (Boothroyd et al. Reference Boothroyd, Hehl, Knoll and Manger1998; Hemphill and Gottstein, Reference Hemphill and Gottstein2006). Moreover, secreted proteins are also expected to be present in both invasive forms; thus, subcellular fractionation strategies (Bradley et al. Reference Bradley, Ward, Cheng, Alexander, Coller, Coombs, Dunn, Ferguson, Sanderson, Wastling and Boothroyd2005; Marugán-Hernández et al. Reference Marugán-Hernandez, Alvarez-García, Tomley, Hemphill, Regidor-Cerrillo and Ortega-Mora2011) could help to identify these minor proteins involved in invasion and proliferation.
In conclusion, 15 B. besnoiti spots were identified in tachyzoites and bradyzoites, which could be considered a successful number of identifications considering that the B. besnoiti genome has not yet been sequenced. Furthermore, the present proteomic study has significantly increased the number of known B. besnoiti proteins and has allowed for the identification of several B. besnoiti stage-regulated proteins, demonstrating that the differentiation process from tachyzoites to bradyzoites in this parasite involves pathways similar to the well-characterized T. gondii and N. caninum. These results set the basis for the development of improved diagnostic tools to differentiate between acute and chronic infection and the identification of new drugs or vaccine targets. Moreover, the role of these proteins in tachyzoite-to-bradyzoite conversion and the host cell environment should be the subject of further research. Finally, there is still an urgent need for sequencing the B. besnoiti genome for a progression in basic parasite knowledge.
ACKNOWLEDGEMENTS
We gratefully acknowledge Vanesa Navarro for her excellent technical assistance. The DIGE experiment was carried out in the Proteomics Facility UCM-PCM, a member of the ProteoRed-ISCIII network.
FINANCIAL SUPPORT
This work was supported by a research grant from the Santander-Universidad Complutense de Madrid.