INTRODUCTION
Trypanosoma cruzi is a flagellate protozoan that causes Chagas' disease. This parasite exhibits a complex life-cycle, alternating between the insect vector and the mammalian host. The differentiation process appears to be highly regulated, and includes morphological alterations, modifications in gene expression and significant transformations in biological properties (Burleigh and Andrews, 1995).
Microtubules are the major components of the cytoskeleton in trypanosomatidae and play an important role in the morphological changes associated with the parasite life-cycle (Gull, 2001). Microtubules are polymers of globular tubulin subunits, which are organized in a cylindrical tube composed of protomers of α and β tubulin heterodimers. Using genetic and biochemical approaches, 4 new members of the tubulin superfamily, γ, δ, ε, and ζ, have also been described in Trypanosoma brucei (Vaughan et al. 2000). A number of post-translational modifications of tubulin are known, including acetylation, tyrosination, phosphorylation, polyglutamylation and polyglycylation (Chapin and Bulinski, 1991; MacRae, 1997). Post-translationally modified tubulin has been shown to be associated with specific populations of microtubules (Serrano et al. 1989; Kohl and Gull, 1998). However, the physiological significance of these modifications remains to be elucidated. In addition, several microtubule-associated proteins appear to be involved in interconnecting microtubules to each other, as well as to the plasma membrane (Díaz-Nido, Armas-Portela and Avila, 1992).
Various serine/threonine protein kinases have been shown to associate with and phosphorylate tubulin. These include protein kinase CK2 (Serrano et al. 1989; Díaz-Nido et al. 1992), a casein kinase-like enzyme (Crute and Van Buskirk, 1992), a Ca2+/calmodulin-dependent protein kinase (Goldenring, Casanova and Delorenzo, 1984) and the proto-oncogene protein kinase pp39mos (Zhou et al. 1991). CK2 has also been shown to phosphorylate brain tubulin in vitro and in vivo (Serrano et al. 1989) and to specifically bind tubulin and microtubules (Faust, Schuster and Montenarh, 1999). More recently, Lim et al. (2004) have shown that CK2 is a microtubule-associated protein that induces microtubule assembly and bundling in a phosphorylation-independent manner. CK2 is a serine/threonine protein kinase formerly referred to as casein kinase 2, which recognizes acidic rather than basic residues in its substrates. Mammalian CK2s are α2β2, α′2β2, or αα′β2 tetramers, where α and α′ correspond to catalytic subunits (35–44 kDa) and β is the regulatory subunit (24–30 kDa). CK2 is a pleiotropic kinase that can phosphorylate more than 160 proteins (Pinna and Meggio, 1997; Litchfield, 2003). It is ubiquitously expressed in nearly every tissue of eukaryotic organisms and is found in nearly every compartment of eukaryotic cells (Faust and Montenarch, 2000). CK2 is unusual among protein kinases in that it is capable of using both ATP and GTP as the phosphoryl donor. Additionally, it can be inhibited by various compounds such as heparin, emodin, 2,3 bisphosphoglycerate, polyglutamic acid and random polymers of glutamic acid and tyrosine, and activated by basic compounds such as polyamines and polylysine (Pinna and Meggio, 1997).
We have been examining the protein phosphorylation pattern of T. cruzi. The cloning of CK2 α and α′ subunits from Leishmania chagasi (Bhatia et al. 1998) and T. brucei (Park et al. 2002), and the existence of CK2-like activities in Trypanosoma evansi (Galán-Caridad et al. 2004) suggested that this type of enzyme would also be present in T. cruzi. Two protein kinase activities that use casein as a substrate were recently identified in T. cruzi epimastigotes (Calabokis et al. 2002). One of these enzymatic activities was purified to homogeneity and characterized as a protein kinase CK1 (Calabokis et al. 2003). In addition, Casas et al. (2002) demonstrated the in vitro phosphorylation of tubulin by a CK2 enzyme in T. cruzi epimastigotes. The fact that CK2 co-purified with this cytoskeletal component suggested an association between both proteins (Casas et al. 2002). Here, we established the tight binding of a CK2 enzyme to a pool of tubulin in T. cruzi epimastigotes. Interestingly, the genome sequence of T. cruzi was published very recently (El-Sayed et al. 2005), and putative sequences for CK2 catalytic and regulatory subunits have been reported in the NCBI protein sequence data bank (Accession numbers EAN91490 and EAN84040, respectively).
MATERIALS AND METHODS
Materials
Reagents were purchased from the following sources: [γ-32P] ATP (3000 Ci/mmol) from New England Nuclear or Amersham. Dephosphorylated casein, phenyl methyl sulfonyl fluoride (PMSF), leupeptin, benzamidine, L-trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64), gel filtration molecular weight protein standards, phosphocellulose, heparin, emodin, GTP, monoclonal anti-α tubulin antibodies, monoclonal anti-β tubulin antibodies, anti-rabbit IgG coupled to horseradish peroxidase, protein-A Sepharose, and bovine serum albumin (BSA) were from Sigma. Human recombinant CK2, polyclonal anti-human CK2α antibodies directed against the conserved sequence delimited by amino acids 70–89, and monoclonal anti-human CK2β antibodies were from Calbiochem. Diethylaminoethyl (DEAE)-Sepharose, and Sephacryl S-300 were from Pharmacia. P81 phosphocellulose chromatography paper was from Whatman. OptiPhase Hisafe II (liquid scintillation counting solution) from LKB. Nitrocellulose sheets, and West Pico substrate for luminography were from Pierce. Protein G-agarose was from Gibco BRL. Polyvinylidene difluoride (PVDF) microporous membranes were from Millipore. Protein Assay reagent was from Bio-Rad. Dr Susan S. Taylor (University of California, San Diego, USA) generously donated the synthetic CK2-specific peptide (Pep2=RRRADDSDDDDD). All other chemicals were of the highest quality grade available.
Parasites and culture conditions
A virulent Venezuelan strain of T. cruzi epimastigote forms (EPm strain) was used as the source of parasites, and epimastigotes were grown at 28 °C in liver infusion tryptose medium (Camargo, 1964). Epimastigotes were collected at various phases of the growth curve and washed twice with Phosphate Buffered-Saline (PBS) (57 mM Na2HPO4, 18 mM KH2PO4, 76·9 mM NaCl, pH (7·2)). During the final wash, an aliquot was used to count the number of parasites using a haemocytometer. The final cell pellet was kept frozen at −80 °C until further use.
Homogenization of parasites and in vitro kinase assay
T. cruzi epimastigotes (5×108) were extracted on ice by sonication in 3 ml of Buffer A (50 mM Tris-HCl (pH 8·0), 5 mM MgCl2, 5 mM CaCl2) or Buffer B (50 mM Tris-HCl (pH 8·0), 10 mM EGTA, 10 mM EDTA). Parasites were also homogenized with 50 mM Tris-HCl (pH 8·0) containing only 5 mM MgCl2, 5 mM CaCl2, 10 mM EGTA or 10 mM EDTA. All extraction buffers contained 50 μM PMSF, 10 μM leupeptin, 10 μM E-64 and 1 mM benzamidine as protease inhibitors. The homogenates (H) were centrifuged at 100000 g, for 1 h at 4 °C, producing supernatant (S) and pellet (P) fractions. In vitro kinase assays were then performed in a final volume of 70 μl. In all cases, the reaction mixtures contained 50 mM Tris-HCl (pH 8·0), 20 mM KF, 15 mM [γ-32P] ATP (specific activity ≅6000 cpm/pmol), and a concentration of MgCl2 that varied depending on the buffer used during extraction (12 or 50 mM MgCl2). Following addition of the samples, reactions were incubated for 15 min, at 30 °C, and terminated by spotting a 20 μl aliquot on Whatman P81 phosphocellulose papers (2 cm×2 cm). The papers were washed with 50 mM phosphoric acid 3 times (15 min per wash), dried and analysed for radioactivity by liquid-scintillation counting. The remaining 50 μl of the various samples was boiled at 100 °C for 5 min with sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970). The [32P]-labelled phosphopolypeptides were separated by SDS-PAGE and qualitatively analysed by autoradiography. Casein kinase and protein kinase CK2 activities were measured by including 1 mg/ml of dephosphorylated casein or 120 μM of the synthetic Pep2 peptide, respectively, in the reaction mixtures. Pep2 kinase activity was only measured on phosphocellulose papers.
Reversion assay
Parasites (5×108) were homogenized in 3 ml of Buffer A or Buffer B as described above, and centrifuged to yield the corresponding soluble and particulate fractions. The particulate fraction obtained in the presence of Buffer A (PA) was resuspended in 50 mM Tris-HCl (pH 8·0) containing 10 mM EDTA, 10 mM EGTA, and protease inhibitors. Likewise, CaCl2 (20 mM) and MgCl2 (20 mM) were added to the soluble fraction obtained following lysis with Buffer B (SB). Both reactions were then centrifuged at 100000 g, for 1 h, at 4 °C, to generate the resulting soluble and particulate fractions.
Separation of tubulin and the kinase responsible for its phosphorylation from T. cruzi epimastigotes
All steps were performed at 4 °C. Protein profiles were measured at 280 nm, and all fractions were analysed by SDS-PAGE. Tubulin elution was monitored by immunoblotting. Kinase activity was carried out in the absence or presence of casein or Pep2 as described above. Additionally, the effects of various CK2 inhibitors (0–100 μg/ml heparin, 0–100 μM emodin and 0–150 μM GTP) were evaluated.
Cation-exchange chromatography
A phosphocellulose column (30 ml) was equilibrated in 20 mM Tris-HCl (pH 6·6), 2 mM EGTA, 1 mM MgSO4. Epimastigotes (9·7×1010) were resuspended in 10 ml of buffer B containing protease inhibitors, and homogenized on ice by sonication. The resulting extract was centrifuged at 100000 g, for 1 h, and the soluble fraction was loaded onto the column. The flow-through fraction (FT) was collected, the column was extensively washed, and then a linear gradient from 0 to 1 M NaCl in 20 mM Tris-HCl (pH 6·6), 2 mM EGTA, 1 mM MgSO4 was applied and fractions of 1·5 ml were collected.
Anion-exchange chromatography
Epimastigotes (2·5×1011) were extracted by sonication using 20 ml of Buffer B with protease inhibitors. The homogenate was centrifuged at 100 000 g, for 1 h, and the resulting supernatant was loaded onto a DEAE-Sepharose column (60 ml) that had been equilibrated with 50 mM Tris-HCl (pH 8·0), 0·1 mM EDTA, 2 mM EGTA, 0·2 M NaCl, 50 μM PMSF, and 1 mM benzamidine. The flow-through fraction (FT) was collected and the column was washed. A step gradient of 0·6 M NaCl, in the same buffer, was successively applied and fractions of 5 ml were collected.
Gel filtration chromatography
The tubulin- and tubulin kinase-enriched fractions eluting from the DEAE-sepharose were pooled (6 mg) and applied to a Sephacryl S-300 size-exclusion column [total volume (Vt)=36 ml] previously equilibrated with 50 mM Tris (pH 8·0), 150 mM NaCl, 5 mM β-mercaptoethanol. Protein standards were used to calibrate the column. The excluded (Vo) and included volumes were determined by applying blue dextran and potassium dichromate, respectively. The column was run at a flow rate of 150 μl/min and the eluting proteins were monitored at 280 nm. The elution volume (Ve) was measured for each protein and Kav was calculated from Equation 1. In particular, the elution volumes of the T. cruzi tubulin and tubulin kinase were determined by Western blot and kinase activity assays, respectively.
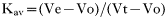
The molecular weight of the parasite proteins was empirically determined plotting the logarithm of the molecular weight of each standard versus its corresponding Kav value. Additionally, a linear relationship was obtained by plotting the Stokes radius versus (−log Kav)1/2 (Siegel and Monty, 1966).
Sucrose gradient centrifugation
Linear 5–20% sucrose gradients (4·2 ml) were prepared in 50 mM Tris-HCl (pH 8·0), 0·1 mM EDTA, 0·2 mM dithiothreitol, 5 mM MgCl2, 0·15 M NH4Cl. Protein markers with known sedimentation coefficients were used to calibrate the gradients. The soluble form of a variant surface glycoprotein (VSG) purified from the TEVA1 Trypanosoma evansi Venezuelan isolate was also included as a marker (Uzcanga et al. 2002, 2004). Samples containing the T. cruzi proteins, and all protein markers, were layered on top of the sucrose gradients, in a total volume of 300 μl. The samples were spun for 18 h, at 200000 g, in a Beckman SW60Ti rotor, at 4 °C. Fractions were collected through the bottom of the tubes, and aliquots were analysed by SDS-PAGE. Additionally, aliquots of each fraction were evaluated by immunoblots using anti-α and anti-β tubulin monoclonal antibodies, and were assayed for kinase activity. The sedimentation coefficient of each standard was plotted against its corresponding distance of migration, and the resulting linear curve was utilized to calculate the sedimentation coefficient of the parasite proteins (Martin and Ames, 1961).
Co-immunoprecipitation
A sample of clarified epimastigotes extracted with Buffer B, and containing 500 μg of total protein, was pre-incubated with 100 μl of protein G-agarose for 1 h, on ice, and centrifuged at 14000 rpm Eppendorf centrifuge 5415C for 10 min, at 4 °C, to reduce non-specific binding. Then, the resulting supernatant was incubated with anti-α tubulin monoclonal antibody (1/100 dilution) for 1 h at 4 °C. Protein G-agarose (60 μl) was added and incubated for 1 h, on ice, and the immune complexes were collected by centrifugation at 14 000 rpm Eppendorf centrifuge 5415C, for 20 min, at 4 °C. Subsequently, kinase activity assays were performed on the immunoprecipitated complexes. In addition, negative controls were carried out in parallel using normal mouse serum or a polyclonal antibody directed against the soluble form of a VSG purified from the TEVA1 T. evansi isolate (Uzcanga et al. 2002, 2004).
Phosphorylation followed by immunoprecipitation
Three identical samples of epimastigote extracts prepared in Buffer B, containing 30 μg of protein each, were phosphorylated as described, except that 100 mM EDTA and 0·1 mM EGTA were added to terminate the reaction. One of the samples received no further treatment. In order to reduce non-specific binding, the remaining samples were immunoprecipitated using normal rabbit antisera (1/20 dilution), for 30 min, and centrifuged. This step was repeated and the resulting supernatants were immunoprecipitated using either polyclonal anti-CK2α antibodies (1/10 dilution) or anti-epimastigote serum (1/10 dilution), for 2 h, at room temperature. Protein-A Sepharose was added and incubated for 30 min. The immune complexes were collected by centrifugation and were washed 4 times. The pellets were resuspended with sample buffer, separated by SDS-PAGE (Laemmli, 1970), and analysed by autoradiography.
Other procedures
The catalytic subunit of the cAMP-dependent protein kinase (PKA) was purified from porcine heart muscle (Nelson and Taylor, 1981). Protein concentration was determined using BSA as protein standard (Bradford, 1976). SDS-PAGE was carried out on 1·5-mm thick slab gels containing 10 or 12% polyacrylamide (Laemmli, 1970). Non-denaturing gel electrophoresis was performed according to the method described by Schagger, Cramer and Von Jagow (1994) on 0·75-mm thick, linear 2–15% polyacrylamide gradient slab gels. Coomassie blue R-250 or silver staining was used for protein visualization on gels. In some cases, gels were stained with a Coomassie blue G-250-silver double staining (De Moreno, Smith and Smith, 1986). For Western blot analyses, proteins separated by SDS-PAGE or native electrophoresis were electro-transferred from the gels to nitrocellulose or PVDF sheets (Towbin, Staehelin and Gordon, 1979).
RESULTS
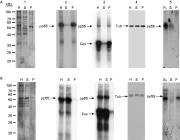
We have shown previously that tubulin was phosphorylated in vitro in whole-cell homogenates prepared from epimastigotes, and that GTP, heparin, and 2,3-bisphosphoglycerate inhibited the phosphorylation of both tubulin and exogenously added casein in a dose-dependent manner, indicating the involvement of a CK2 enzyme (Casas et al. 2002). When epimastigotes were homogenized in the presence of both 5 mM Mg2+ and 5 mM Ca2+, the CK2 apparently responsible for phosphorylating tubulin, as well as the phosphorylated tubulin (pp55), remained associated with the parasite particulate fraction (Fig. 1. A2). However, the CK2 enzyme and its phosphorylated substrate were released into the parasite cytosolic fraction when epimastigotes were extracted in the presence of 10 mM EDTA and 10 mM EGTA (Fig. 1. B2). Similar results were obtained when casein was included in the reaction mixtures (Fig. 1. A3 and B3). In these experiments, the relative protein concentration was maintained during fractionation, and all gels were loaded so that the sum of the amount of protein present in the soluble (S) and pellet (P) fractions was equal to that of the whole cell homogenate (H). Immunoblots using anti-tubulin monoclonal antibodies showed that tubulin was present in all parasite fractions (H, S and P), independently of the buffer used during the initial extraction (Fig. 1. A4 and B4). Thus, these results demonstrated a divalent cation-dependent differential partitioning of a pool of tubulin and the CK2 responsible for its phosphorylation, as previously reported by Uzcanga et al. (2003). Equivalent results were attained throughout the entire epimastigote growth curve (4–16 days).
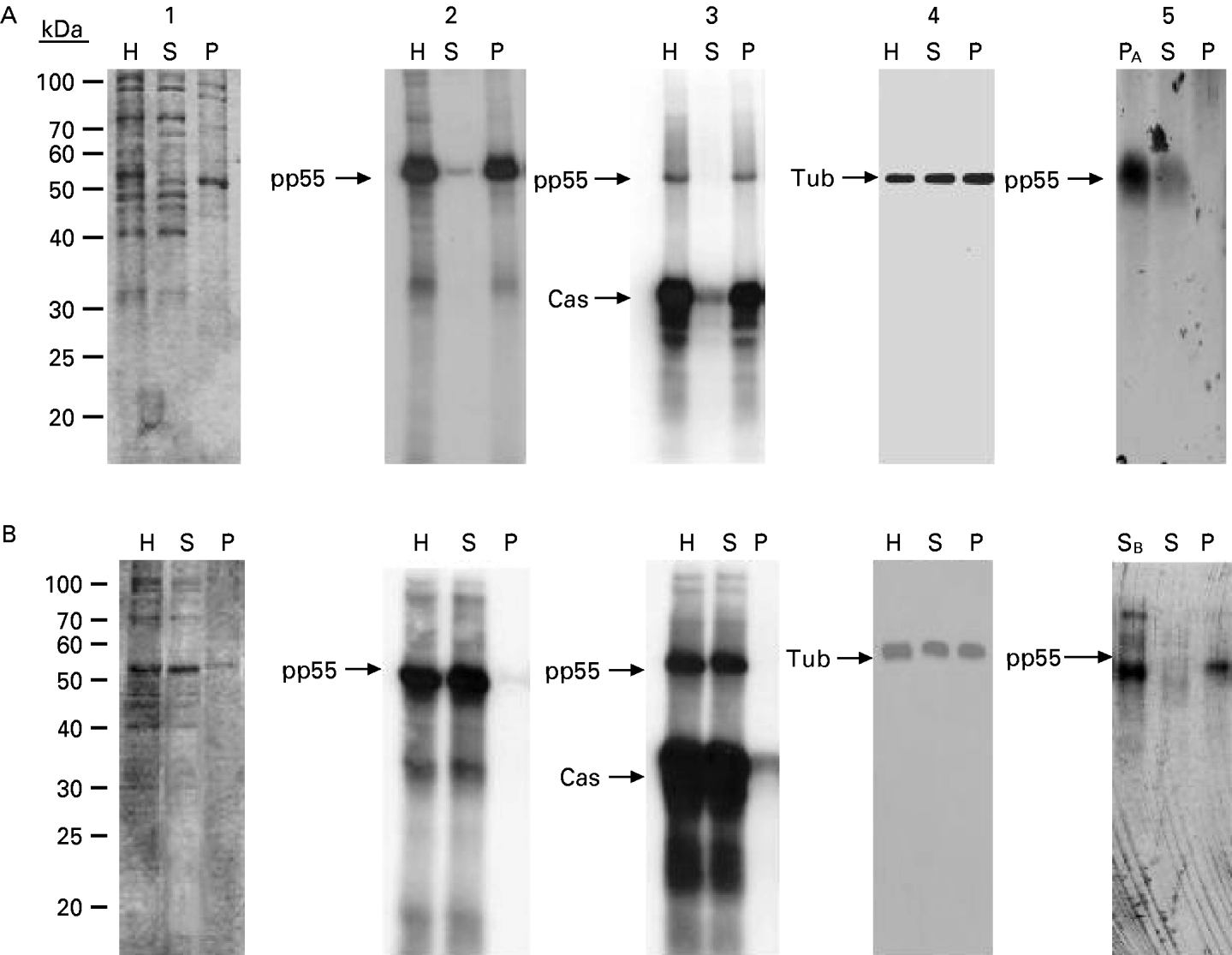
Fig. 1. Phosphorylated tubulin solubility and casein kinase activity respond similarly to divalent cations in Trypanosoma cruzi epimastigotes. Whole-cell homogenates (H), soluble (S), and particulate (P) fractions were obtained from epimastigotes extracted with a solution containing either 5 mM MgCl2 and 5 mM CaCl2 (A) or 10 mM EDTA and 10 mM EGTA (B), and separated by SDS-PAGE. (1) Polypeptide profile. Phosphorylation assays were visualized by autoradiography and were performed in the absence (2) or presence (3) of casein (Cas). (4) Western blots employing anti-tubulin antibodies. In A5, the particulate fraction obtained following homogenization with 5 mM Ca2+ and 5 mM Mg2+ (PA) was re-extracted with a buffer containing 10 mM EGTA and 10 mM EDTA. In B5, the soluble fraction obtained following extraction with 10 mM EGTA and EDTA (SB) was re-homogenized with a solution containing 5 mM Ca2+ and Mg2+. pp55, phosphorylated tubulin; Tub, tubulin.
When the particulate fraction that resulted from the extraction of the parasites with Buffer A (PA) was re-homogenized with buffer containing 10 mM EDTA and 10 mM EGTA and then centrifuged, the phosphorylated tubulin and the kinase responsible for its phosphorylation were predominantly released into the supernatant (Fig. 1. A5). Likewise, both proteins were sedimented with the pellet when the soluble fraction that resulted from the homogenization of epimastigotes with Buffer B (SB) was incubated with 5 mM Mg2+ and 5 mM Ca2+ and centrifuged, (Fig. 1. B5). Therefore, simply changing the divalent cation concentration in the homogenization buffer altered the solubilization properties of this CK2 and its tubulin substrate; the distribution of these proteins to the parasite particulate fraction was conditioned by the presence of divalent cations and was independent of other membrane components.
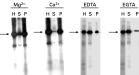
The presence of only 1 divalent cation in the extraction buffer, 5 mM Mg2+ or 5 mM Ca2+, was sufficient to induce the recruitment of both phosphorylated tubulin and CK2 to the epimastigote particulate fraction (Fig. 2), and the presence of 1 chelating agent, either 10 mM EDTA or 10 mM EGTA, was enough to solubilize both proteins (Fig. 2). However, Ca2+ was more effective than Mg2+, and EGTA was more effective than EDTA when employed in the corresponding extraction buffers.

Fig. 2. Effect of divalent cations on the solubilization of a pool of tubulin and the CK2 responsible for its phosphorylation. Epimastigotes were extracted with a buffer containing 5 mM Mg2+, 5 mM Ca2+, 10 mM EDTA, or 10 mM EGTA. Aliquots of the parasite homogenates (H), soluble (S), and particulate (P) fractions were phosphorylated in vitro, separated by SDS-PAGE, and evaluated by autoradiography. Phosphorylated tubulin is indicated by the arrow.
Column chromatography was utilized in an effort to separate the parasite tubulin from its kinase. Epimastigotes on the late-stationary phase of their growth curve were extracted in the presence of 10 mM EDTA and 10 mM EGTA. Following ultracentrifugation, the clarified fraction was chromatographed on a phosphocellulose cation-exchange column. A predominant 55 kDa-phosphopolypeptide band corresponding to phosphorylated tubulin was seen on autoradiography of the non-adhering material (Fractions 10–33), and exogenously added casein was also phosphorylated by the same fractions (Fig. 3A). Three well-known CK2 inhibitors, emodin, heparin and GTP, were capable of inhibiting the endogenous phosphorylation of tubulin by its kinase in a dose-dependent manner (Fig. 3A, inset). The ability of this kinase to phosphorylate casein and Pep2, a selective peptide substrate for CK2, was also inhibited by increasing amounts of emodin, heparin and GTP (data not included). Thus, the T. cruzi tubulin kinase belongs to the CK2 family of enzymes and co-purifies with tubulin in the phosphocellulose flow-through. Following elution with the linear salt gradient, 2 additional T. cruzi casein kinase activity peaks were separated at 0·5 and 0·66 M NaCl, respectively (Fig. 3A). Both peaks recognize exogenously added Pep2 with a high specific activity (305 and 750 nmol of Pi incorporated/mg/min for fractions 63 and 68, respectively). However, no phosphorylated tubulin was observed in these fractions.

Fig. 3. (A) Phosphocellulose chromatography. Left panel, the clarified soluble fraction from Trypanosoma cruzi epimastigotes was applied to a phosphocellulose column, and the flow-through material, as well as the fractions obtained after applying the salt gradient, were collected. Endogenous protein kinase ([bull ]) and casein kinase (□) activities were determined by spotting an aliquot of the reaction mixture on P81 phosphocellulose filters. Inset, autoradiographies showing the inhibition of tubulin phosphorylation by increasing concentrations of heparin (Hep, 0–100 μg/ml), emodin (Em, 0–25 μM) and GTP (0–50 μM). Right panel, protein kinase activity of the resulting fractions in the presence of dephosphorylated casein (Cas), visualized by autoradiography. (B) DEAE-Sepharose chromatography. Left panel, the parasite soluble fraction was applied to a DEAE-Sepharose column and, after extensive washing, the proteins were eluted with 0·6 M NaCl. Fractions were collected and spectrophotometrically assayed for protein ([bull ]). Endogenous protein kinase (□) and casein kinase (○) activities were determined using P81 phosphocellulose papers. Inset, autoradiographies showing the effect of increasing concentrations of emodin (Em, 0–100 μM) and GTP (0–150 μM) on the phosphorylation of tubulin. Right panel, autoradiography of the phosphorylated fractions. (C) Sephacryl S-300 gel filtration chromatography. Left panel, the tubulin-enriched fraction from the DEAE-sepharose column was concentrated and subjected to size-exclusion chromatography on a Sephacryl S-300 column. Fractions were collected and assayed for protein ([squf ]), endogenous protein kinase (○) and casein kinase (□). Right panel, protein kinase activity of the eluting fractions, visualized by autoradiography. pp55, phosphorylated tubulin.
The epimastigote clarified fraction was also chromatographed on a DEAE-Sepharose column. As seen in Fig. 3B, the absorbance profile showed a sharp protein peak eluting when the ionic strength was increased to 0·6 M NaCl, which overlapped with the peak of endogenous phosphorylation (Fractions 13–20). Phosphorylation of tubulin and casein coincided with this protein peak (Fig. 3B). As seen in Fig. 3B (inset), emodin and GTP, were capable of inhibiting the endogenous phosphorylation of tubulin in a concentration- dependent manner.
The DEAE-Sepharose fractions containing the co-eluting tubulin and the CK2 responsible for its phosphorylation were combined, concentrated, and chromatographed using a Sephacryl S-300 size-exclusion column. As illustrated in Fig. 3C, both phosphorylated tubulin and CK2 again co-purified in the same protein peak (Fractions 25–40) after gel filtration.
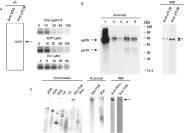
Various immunoprecipitation assays were then carried out to effectively demonstrate that pools of the parasite CK2 and tubulin were tightly associated. Initially, crude epimastigote extracts were immunoprecipitated using anti-α tubulin monoclonal antibodies, normal mouse serum, or an unrelated polyclonal antibody directed against a VSG isolated from T. evansi (Uzcanga et al. 2004). The immunoprecipitated samples were incubated in the presence of [γ-32P] ATP, and the endogenous tubulin kinase activity was examined by autoradiography following SDS-PAGE separation. As seen in Fig. 4A, anti-α tubulin antibodies co-immunoprecipitated tubulin and the kinase responsible for its phosphorylation. No signal was detected in the control assays using normal mouse serum (data not included) or anti-VSG antibodies (Fig. 4A). The immunoprecipitated protein kinase was clearly identified as a CK2-like enzyme because emodin, heparin and GTP appropriately inhibited the phosphorylation of the co-precipitated tubulin in a dose-dependent mode (Fig. 4A).

Fig. 4. Association of a pool of tubulin and the CK2 responsible for its phosphorylation. (A) Left panel, a Trypanosoma cruzi epimastigote extract was immunoprecipitated with anti-α tubulin antibodies and subsequently phosphorylated with [γ-32P] ATP. The homogenate was also subjected to immunoprecipitation with anti-T. evansi VSG polyclonal antibodies. Phosphorylated tubulin is indicated by the arrow. IP, immunoprecipitation. Right panel, dose-dependent inhibition of the phosphorylation of the immunoprecipitated tubulin by heparin (Hep, 0–100 μg/ml), GTP (0–100 μM), and emodin (Em, 0–50 μM). (B) Left panel, the parasite lysate was phosphorylated in vitro and directly analysed (1) or immunoprecipitated once (2) or twice (3) with normal rabbit serum, and then with anti-CK2α antibodies (4) or anti-epimastigote serum (5). The samples were separated by SDS-PAGE followed by electro-transference to PVDF membranes and autoradiography. Arrows show pp55 and pp38. Right panel, the sample in lane 4 was developed using anti-α or anti-β tubulin antibodies (Anti α or β TUB). (C) Native electrophoresis of the partially purified CK2-tubulin complex previously phosphorylated with [γ-32P] ATP. The following standards were used: β-amylase (βAM), alcohol dehydrogenase (ADH), bovine serum albumin (BSA), carbonic anhydrase (CA), cytochrome C (CC), and the catalytic subunit of the cAMP-dependent protein kinase (PKA). Protein staining (Coomassie), autoradiography (Autorad), and immunoblot (WB) using anti-CK2α or a mixture of anti-α and anti-β tubulin antibodies (Anti TUB). Arrow shows the migration of the CK2-tubulin complex (CK2-TUB).
Whole-cell epimastigote extracts were also labelled with [γ-32P] ATP (Fig. 4B, lane 1), and aliquots of the original sample were immunoprecipitated once (lane 2) or twice (lane 3) with normal rabbit serum to eliminate non-specific precipitations, and then with anti-CK2α antibodies (lane 4). As shown in the figure, the anti-CK2α antibodies immunoprecipitated a phosphorylated 55 kDa polypeptide duplet. A positive control using rabbit antisera raised against total epimastigote homogenates from the same Venezuelan strain also immunoprecipitated the 55 kDa phosphorylated duplet (lane 5). The 55 kDa phosphopolypeptide bands were identified as the α and β subunits of tubulin when a sample identical to that electrophoresed in lane 4 reacted with anti-α and anti-β tubulin monoclonal antibodies in a Western blot (Fig. 4B, WB). Therefore, both tubulin subunits appeared to be phosphorylated by the associated CK2. Furthermore, the parasite tubulin appears to be highly immunogenic as α and β tubulins were immunoprecipitated with anti-epimastigote hyperimmune sera. A 38 kDa phosphopolypeptide band was also shown in Fig. 4B (lanes 4 and 5), which was not immunodetected by either anti-tubulin antibodies, or antibodies to human CK2α or human CK2β (data not shown).
When the purified tubulin-CK2 complex was electrophoresed in a non-denaturing polyacrylamide gel, and then Western blotted with anti-human CK2α antibodies, a protein band was clearly recognized (Fig. 4C, WB), which migrated with the same relative mobility as the band that contained the phosphorylated protein complex (Fig. 4C, Autorad). Incubation of the PVDF membrane with a mixture of anti-α and anti-β tubulin antibodies also identified the same slow-migrating protein band (Fig. 4C, WB). These results indicated that the band identified by autoradiography and immunoblotting contained both CK2α and tubulin, demonstrating again that it corresponds to a tubulin-CK2 complex. As a control, the autophosphorylated catalytic subunit of the PKA from porcine heart muscle was also electrophoresed and revealed a different migration than the phosphorylated tubulin-CK2 complex (Fig. 4C, Autorad). A series of protein standards were also included and showed a different migration than the CK2-tubulin complex by native electrophoresis (Fig. 4C, Coomassie). Interestingly, no signal was seen when anti-human CK2β antibodies were employed following electrophoresis under non-denaturing conditions (data not included). As positive controls, dot blots were performed using the human recombinant CK2 enzyme and anti-human CK2α and anti-human CK2β antibodies (data not shown).
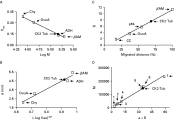
The native molecular weight of the protein complex conformed by the parasite tubulin and its kinase was estimated by gel permeation chromatography and sucrose gradient ultracentrifugation. An enriched fraction of the protein complex was obtained after DEAE-Sepharose or phosphocellulose chromatography. A Sephacryl S-300 molecular exclusion column was calibrated using proteins with known molecular weight and Stokes radii. By plotting the corresponding Kav value of each protein marker versus the logarithm of its molecular weight (log M), the native molecular mass of the parasite protein complex was estimated to be approximately 147500 Da (Fig. 5A). A calibration curve of (−log Kav)1/2 versus each Stokes radius was also obtained (Fig. 5B). The measured elution volume yielded the (−log Kav)1/2 for the complex and a Stokes radius of 46·5 Å was determined by interpolation.

Fig. 5. Determination of the hydrodynamic parameters of the CK2-tubulin complex. Molecular weight (A) and Stokes radius (B) were determined by gel filtration. The calibration curve was established with βAM (200 kDa, 54·0 Å), ADH (150 kDa, 45·5 Å), ovalbumin (ovoA, 43 kDa, 30·5 Å), and chymotrypsinogen A (Chy, 25 kDa, 20·9 Å). Kav was calculated as described in Equation 1. Sedimentation by ultracentrifugation (C). The calibration of the sucrose gradient was carried out with βAM (11·3 S), a Trypanosoma evansi VSG (5·67 S) (Uzcanga et al. 2002, 2004), ovoA (3·5 S), and CC (1·86 S). The elution and sedimentation position of the CK2-tubulin complex (CK2-Tub, [bull ]) were identified by Western blot using a mixture of anti-α and anti-β tubulin, and by assaying for kinase activity in the absence or presence of casein. The CK2-tubulin complex size was also established as described in Equation 2 (D), using the following markers: 1=CC; 2=Chy; 3=CA; 4=ovoA; 5=ADH; 6=aldolase; and 7=catalase. S, sedimentation coefficient; M, molecular weight; a, Stokes radius.
The tubulin-CK2 protein complex was also subjected to velocity sedimentation on a 5–20% sucrose gradient, in the presence of protein standards with known sedimentation coefficients. Tubulin was monitored by Western blot using anti-tubulin monoclonal antibodies and tubulin kinase activity assays were used to identify the migration of CK2. In agreement with all previous experiments, both tubulin and the CK2 responsible for its phosphorylation co-sedimented in the same fraction. The sedimentation coefficient for the T. cruzi protein complex was determined to be 7·5 S from the calibration curve (Fig. 5C). For spherical molecules, the molecular mass can be calculated from a combination of the measured Stokes radius and sedimentation coefficient using Equation 2 (Siegel and Monty, 1966)
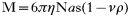
in which M is the molecular mass, a is the Stokes radius, s is the sedimentation coefficient, ν is the partial specific volume, η is the viscosity of the medium, ρ is the density of the medium, and N is Avogadro's number. A calibration curve of M versus the Stokes radius multiplied by the sedimentation coefficient was prepared using the values reported for various protein markers (Fig. 5D). A molecular weight of 145400 Da was estimated for the parasite protein complex.
The frictional coefficient ratio f/fo can also be determined from the molecular mass and the Stokes radius as seen in Equation 3.
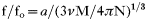
The calculated frictional ratio for the protein complex was 1·33. The f/fo ratio is a theoretical value that facilitates determination of the apparent protein shape. While globular proteins have f/fo values between 1·25 and 1·4, moderately elongated and fibrous proteins have values higher than 1·6 (Bloomfield, Dalton and Van Holde, 1967). From our results, the parasite tubulin-CK2 complex appeared to fold in a compact globular structure.
DISCUSION
Here we demonstrate that a pool of the heterodimeric α/β tubulin is tightly associated with a CK2 enzyme in T. cruzi epimastigotes. Divalent cations hindered the solubility of both parasite proteins, as previously reported by Uzcanga et al. (2003), and they co-eluted using various chromatographic separations on the basis of parameters as different as charge (anion and cation exchange) and size (gel filtration). Not even phosphocellulose, which has been reported as a very useful resin to purify casein kinases from mammalian cells (Hathaway and Traugh, 1979), was capable of separating these proteins. Serrano et al. (1987) have reported similar results using rat neuroblastoma cells. However, Crute and Van Buskirk (1992), using bovine brain cells, recovered tubulin completely free of any kinase activity in the phosphocellulose flow through material. The interaction between a CK2 and a pool of tubulin in this parasite was also substantiated by native polyacrylamide gel electrophoresis and co-immunoprecipitation experiments. As the parasite tubulin and CK2 remained tightly bound even in the presence of reducing agents (5 mM β-mercaptoethanol), as shown when molecular exclusion chromatography was employed, the association between these proteins is probably not mediated by the formation of disulfide bonds.
A divalent cation-dependent intracellular translocation has been reported for other protein kinases. While in the presence of Ca2+ PKC associates with the particulate fraction, this kinase is soluble in the presence of divalent cation-chelating compounds (Ikeda et al. 1996). In T. cruzi, the subcellular distribution of a Ca2+/calmodulin-dependent protein kinase (TcCaMK) depends on its phosphorylation state, which is also regulated by the levels of intracellular calcium (Ogueta, Intosch and Téllez-Iñon, 1996). When TcCaMK is activated by autophosphorylation (Ogueta, Macintosch and Téllez-Iñon, 1998), it is released into the parasite cytosol, but when it is dephosphorylated, it is inactivated and is maintained bound to T. cruzi cytoskeletal elements (Ogueta et al. 1996). An opposite mechanism may be hypothesized for the tubulin-CK2 complex reported here, which will probably bind to cytoskeletal components in the presence of increasing concentrations of calcium.
The binding of CK2 to tubulin could be mediated by anchoring proteins, as has been described for PKA in higher eukaryotes (Huang et al. 1997). For example, calmodulin (CaM) has been previously identified in T. cruzi in association with the parasite membranous fractions (Benaim et al. 1991). In general, the association of CaM to various target proteins is a direct consequence of its binding of calcium ions, which produces a conformational change in CaM characterized by an increase in α helix secondary structure and the exposure of hydrophobic amino acids (Chin and Means, 2000). Since CK2 is capable of phosphorylating CaM in mammalian cells (Meggio et al. 1994), it is possible that CaM serves as a mediator for the association of the parasite tubulin-CK2 complex. If this is the case, the increased hydrophobic character of calcium bound-CaM will favour the association of the tubulin-CK2 complex to the particulate fraction. Additionally, this will be consistent with our results showing that components from the parasite membrane were not required to relocate the tubulin-CK2 complex towards the particulate fraction. Further studies should reveal whether CaM functions as an anchoring protein participating together with tubulin and CK2 in the complex reported here. Furthermore, it is also necessary to demonstrate whether the parasite CaM can be phosphorylated by the CK2 present in the complex. Since CaM is a very small protein with a molecular mass of 15·8 kDa (Benaim et al. 1991), its molecular weight could also be easily accounted for in the total size of the protein complex.
In addition to tubulin, a 38 kDa phosphopolypeptide band was also immunoprecipitated using anti-human CK2α antibodies. Since anti-tubulin antibodies did not recognize the 38 kDa polypeptide, it is improbable that this band corresponds to a tubulin proteolytic fragment. Plausibly, it might represent the T. cruzi CK2 catalytic subunit because subunits with similar sizes have been reported in yeast and mammals (Hanna, Rethinaswamy and Glower, 1995; Litchfield et al. 1990). The recently reported putative sequence for the T. cruzi CK2 catalytic subunit (El-Sayed et al. 2005) consists of 345 residues, and its approximate size is in good agreement with the apparent molecular mass of the immunoprecipitated 38 kDa band. A molecular weight of 145400–147500 was assessed for the parasite tubulin-CK2 complex by gel permeation chromatography and sucrose-gradient ultracentrifugation. After subtracting a molecular mass of about 110 kDa corresponding to the tubulin dimer constituted by α and β tubulin, a difference of approximately 35–38 kDa remained to be accounted for in the protein complex, which also agrees with the size of the 38 kDa phosphopolypeptide. No recognition of the 38 kDa polypeptide band was obtained when immunoblots using anti-CK2α antibodies were carried out under denaturing conditions. However, these antibodies also failed to recognize the parasite CK2 enzyme in whole-cell homogenates by Western blot following SDS-PAGE (data not shown). Since the motif recognized by the anti-CK2α antibodies in the human enzyme (70LKPVKKKKIKREIKILENL89R) is very homologous to the corresponding segment in the T. cruzi CK2 catalytic subunit (72LKPVKKKKILRELKILQNL91Q), these antibodies must be capable of binding to the parasite CK2α in its native conformation. An alternative possibility is that the 38 kDa band is not related to either tubulin or CK2 and simply constitutes another associated protein component that co-immunoprecipitated with the tubulin-CK2 complex. However, the molecular weight that was assessed here for the tubulin-CK2 complex would not be sufficient to account for both the CK2 and this additional protein.
The anti-CK2β monoclonal antibodies used here did not bind to parasite polypeptides blotted under native or denaturing conditions. These antibodies recognize the segment delimited by residues 123–127 from the human CK2β-subunit (GLSDI) (Nastainczyk et al. 1995). A comparative analysis between the human CK2β primary structure and the recently published T. cruzi CK2 regulatory subunit sequence (El-Sayed et al. 2005) showed that this linear epitope is absent in the parasite putative protein.
T. cruzi possesses a fascinating life-cycle during which the cells undergo a variety of modulations of shape and motility. The precise form of the cell, its division, and motility are a reflection of its highly organized internal microtubule cytoskeleton. One of the characteristic features of Trypanosomatidae protozoa is the presence of a layer of microtubules localized below the plasma membrane and designated as subpellicular microtubules. This regularly spaced helical array of subpellicular microtubules determines the shape of the cell and is present throughout the complete cell cycle, during which the cell inserts new microtubules and partitions the resulting cytoskeleton to the two daughter cells. Additionally, trypanosomatids also contain a well-defined array of highly cross-linked microtubules recognized as the flagellum-paraflagellar rod-basal body-axoneme complex. Both pellicular and flagellar microtubules are relatively stable structures; they are not disrupted by cold and are resistant to most of the common drugs that affect microtubular assembly and disassembly in mammalian systems. Interestingly, it has been reported that subpellicular microtubules in trypanosomatids are either stabilized in the presence of EGTA (Robinson et al. 1991), or depolymerized in the presence of calcium (Dolan, Reid and Voorheis, 1986). Concomitantly, a complete retention of the flagellar microtubules was observed when isolated plasma membrane-microtubule complexes were incubated with calcium (Dolan et al. 1986). As our results indicate that the intracellular concentration of divalent cations modulates the solubility of the parasite tubulin-CK2 complex, it can be postulated that a conformational change of tubulin, mediated by calcium ions, could be responsible for the differential solubilization of this protein complex. On the basis of the findings of Dolan et al. (1986), the pool of tubulin that is involved in the association with CK2 must originate from another source than the corset of subpellicular microtubules.
Various studies have suggested that CK2 could be involved in the regulation of microtubule cytoskeleton reorganization (Diaz-Nido et al. 1988; Serrano et al. 1987, 1989). CK2 was localized in microtubule structures such as the mitotic spindle of dividing cells and was found to associate with the cold-stable fraction of microtubules from the rat brain (Serrano et al. 1989; Diaz-Nido et al. 1992). Lim et al. (2004) have also shown that CK2 is a microtubule-associated protein that directly affects microtubule dynamics inducing their assembly and bundling in vitro. Additionally, knockdown of CK2α/α′ in cultured cells dramatically destabilized their microtubule networks, implying that CK2 also mediates microtubule integrity in vivo (Lim et al. 2004). Given that a CK2 activity was always associated with a pool of tubulin, the possible role of this enzyme on the regulation of microtubule stability in T. cruzi epimastigotes requires elucidation.
This research was supported by grants from FONACIT (N ° S1-99001075, S1-2001000683, and LAB-2000001639), and from CDCH-UC (N ° FCS-97018 and 2003005). R.M. is a recipient of a graduate research assistantship from Decanato de Investigación y Desarrollo, Universidad Simón Bolívar. We would like to thank Dr Susan S. Taylor for generously supplying the synthetic Pep2 peptide, and Dr Howard Takiff for his critical reading of this manuscript.