A fundamental parameter that can influence cheese ripening, and that is not commonly taken into consideration, is oxidation-reduction (redox) potential (E h). Therefore, monitoring and control of E h during manufacture and ripening of cheese could be important to understand the effect of this parameter on growth and survival of microorganisms and on its organoleptic characteristics.
Redox potential can affect the levels of volatile flavour compounds in dairy products produced by the growth and activity of starter and non-starter lactic acid bacteria (Boucher et al. Reference Boucher, Brothersen and Broadbent2006; Kieronczyk et al. Reference Kieronczyk, Cachon, Feron and Yvon2006) and could influence their metabolic pathways of flavour generation (Martin et al. Reference Martin, Cachon, Pernin, De Coninck, Gervais, Guichard and Cayot2011). In a study by Kieronczyk et al. (Reference Kieronczyk, Cachon, Feron and Yvon2006) on the effect of extracellular E h on in vitro amino acid catabolism by two strains of Lactococcus lactis, differences were found in the flavour compounds produced under different redox potentials.
The development of a characteristic cheese aroma is influenced by redox potential (Davis, Reference Davis1932; Kristoffersen, Reference Kristoffersen1967; Green & Manning, Reference Green and Manning1982) and stable aroma is thought to be due to a negative E h (−150 to −300 mV) (Beresford et al. Reference Beresford, Fitzsimons, Brennan and Cogan2001). Cheddar cheese has an E h of about −120 mV and this negative value (Kristoffersen et al. Reference Kristoffersen, Gould and Purvis1964; Green & Manning, Reference Green and Manning1982; Urbach, Reference Urbach1993) is associated with the production of volatile sulphur compounds (Green & Manning, Reference Green and Manning1982; Kristoffersen, Reference Kristoffersen1985).
In the literature, addition of chemical compounds like dithiotreitol, potassium ferricyanide, sodium borohydride, cysteine (Bolduc et al. Reference Bolduc, Raymond, Fustier, Champagne and Vuillemard2006b; Kieronczyk et al. Reference Kieronczyk, Cachon, Feron and Yvon2006; Ignatova et al. Reference Ignatova, Prévost, Leguerinel and Guillou2009) or the use of gasses like oxygen, nitrogen and hydrogen (Ignatova et al. Reference Ignatova, Prévost, Leguerinel and Guillou2009; Jeanson et al. Reference Jeanson, Hilgert, Coquillard, Seukpanya, Faiveley, Neveu, Abraham, Georgescu, Fourcassié and Beuvier2009; Martin et al. Reference Martin, Cayot, Vergoignan, Journaux, Gervais and Cachon2010, Reference Martin, Cachon, Pernin, De Coninck, Gervais, Guichard and Cayot2011; Ebel et al. Reference Ebel, Martin, Le, Gervais and Cachon2011) or the application of electro-reduction (Bolduc et al. Reference Bolduc, Bazinet, Lessard, Chapuzet and Vuillemard2006a; Schreyer et al. Reference Schreyer, Bazinet, Chapuzet, Lessard and Britten2006, Reference Schreyer, Britten, Chapuzet, Lessard and Bazinet2008; Haratifar et al. Reference Haratifar, Bazinet, Manoury, Britten and Angers2011) have been used to control the redox potential of dairy products.
However, only a few studies have been conducted on the addition of redox agents to cheese (Galesloot, Reference Galesloot1961a; Green & Manning, Reference Green and Manning1982). Oxidising agents, like nitrate, nitrite or chlorate, were added to milk destined to the production of Edam cheese to prevent butyric acid fermentation and, as a consequence, the decrease in E h was delayed (Vos, Reference Vos1948; Galesloot, Reference Galesloot1961a); however, in the absence of butyric acid bacteria, nitrate had no effect on the redox potential of Edam cheese (Galesloot, Reference Galesloot1960).
Only in one study (Green & Manning, Reference Green and Manning1982) reducing agents (dithiothreitol, glutathione or cysteine) were added to cheese curd before the pressing stage of the manufacture of Cheddar cheese. The addition of reducing compounds caused a decrease in redox potential to values lower than the control cheese and led to the production of higher concentrations of hydrogen sulphide and methanethiol at 3 months of ripening.
Studies from our laboratory have measured redox potential during Cheddar cheesemaking and ripening (Topcu et al. Reference Topcu, McKinnon and McSweeney2008; McSweeney et al. Reference McSweeney, Caldeo, Topcu and Cooke2010; Caldeo & McSweeney, Reference Caldeo and McSweeney2012). We reported that during Cheddar cheesemaking a significant drop in redox potential (E h around −120 mV) occurs at the whey drainage stage until the milling stage (Caldeo & McSweeney, Reference Caldeo and McSweeney2012); the salting stage causes an increase in redox potential due to oxygen penetration. After salting, the redox potential decreases again to negative value within the first hours of pressing (McSweeney et al. Reference McSweeney, Caldeo, Topcu and Cooke2010) and this value is maintained during ripening (Topcu et al. Reference Topcu, McKinnon and McSweeney2008; McSweeney et al. Reference McSweeney, Caldeo, Topcu and Cooke2010).
The objective of this work was to control the redox potential during Cheddar ripening through the addition of oxidising or reducing agents to the salted curd before pressing. Cheeses were analysed to study the effect of the addition of redox agents on the ripening of Cheddar cheese and the development of flavour compounds.
Materials and methods
Cheddar cheese manufacture
Cheddar cheeses were made in the food processing facilities at University College Cork, Ireland, according to a standard Cheddar cheese-making protocol (Kosikowski & Mistry, Reference Kosikowski, Mistry and Kosikowski1997) utilising four open vats were filled with approximately 100 l of HTST-pasteurised (73·5 °C, 15 s) milk. The curd pieces obtained from the four vats were mixed and then separated into batches of 8 kg. To each batch 2·5% (w/w) of NaCl and oxidising or reducing agents were added. Four trials were manufactured. In the first trial, potassium iodate (KIO3; Sigma-Aldrich, Steinheim, Germany) at 1% and 0·1% (w/w) were used as the oxidising agents while sodium hydrosulfite (Na2S2O4; Sigma-Aldrich) at 0·05% (w/w) was used as the reducing agent. In the second and third trials, KIO3 at 0·1% and 0·05% (w/w) were used as the oxidising agent while Na2S2O4 at 0·1% (w/w) and cysteine (Cys, C3H7NO2S; Sigma-Aldrich) at 2% (w/w) were used as reducing agents. In the fourth trial, KIO3 at 0·1% (w/w) was used as the oxidising agent while Na2S2O4 at 0·1% (w/w) and Cys at 2% (w/w) were used as reducing agents. In each trial, a control cheese was made without the addition of redox agents. After 20 min, the curd was moulded and pressed at 490 kPa overnight at room temperature. Cheese blocks were vacuum packed and ripened at 8 °C for up to 6 months.
Measurement of oxidation-reduction potential during ripening
Oxidation-reduction potential was measured following the method of Topcu et al. (Reference Topcu, McKinnon and McSweeney2008) and Caldeo & McSweeney (Reference Caldeo and McSweeney2012). The accuracy of electrodes was checked against a 3 m KCl solution (Topcu et al. Reference Topcu, McKinnon and McSweeney2008) and tap water (Jeanson et al. Reference Jeanson, Hilgert, Coquillard, Seukpanya, Faiveley, Neveu, Abraham, Georgescu, Fourcassié and Beuvier2009; Martin et al. Reference Martin, Cachon, Pernin, De Coninck, Gervais, Guichard and Cayot2011) at 25 °C.
The Pt-electrode was inserted directly into a cheese block samples of 10 × 10 cm to a depth of 5 cm and the reference electrode was placed 2·5 cm apart in a hole of 4 cm deep and 1·5 cm wide filled with 3 m KCl solution as described by Topcu et al. (Reference Topcu, McKinnon and McSweeney2008).
The electrodes were connected to a data logger (Squirrel Data Logger 2040-2F16 Series, Grant, Data Acquisition, Cambridge, UK) through an amplifier (PHTX-21, Omega, USA) for data acquisition. The measured data were recorded every 5 min.
The redox potential data recorded (without reference to a hydrogen reference electrode) were converted to E h according to Caldeo & McSweeney (Reference Caldeo and McSweeney2012) and Abraham et al. (Reference Abraham, Cachon, Jeanson, Ebel, Michelon, Aubert, Rojas, Feron, Beuvier, Gervais and De Coninck2013) with temperature compensation.
For each cheese, single measurement of redox potential was taken at 1, 14, 30, 60, 120 and 180 d of ripening.
Measurement of microbial growth, compositional analysis and pH
Starter and non-starter lactic acid bacteria (LAB) counts were performed on 14, 30, 60, 120 and 180 d-old cheeses as described by Ciocia et al. (Reference Ciocia, McSweeney, Piraino and Parente2013).
The composition (pH, protein, salt, moisture and fat) of 14-d-old cheeses was determined in triplicate. The protein content of the cheeses was determined by the macro-Kjeldahl method (IDF, 1986), salt by a potentiometric titration (Fox, Reference Fox1963), moisture by oven drying at 103 ± 1 °C (IDF, 1982) and fat by the Gerber method (IIRS, 1955).
The pH was measured in triplicate at 1, 14, 30, 60, 120 and 180 d of ripening. pH of the cheeses were measured by probing the cheese directly with a combined glass electrode (PHC3001-8, Radiometer Analytical) connected to a pH metre (PHM210 Standard pH Metre, Radiometer Analytical).
Determination of volatile compounds by SPME-GC-MS
Cheese samples at 2 and 6 months of ripening were wrapped in aluminium foil, vacuum packed and stored at −20 °C until analysed. Volatile compounds were analysed by solid phase microextraction coupled to gas chromatography-mass spectrometry (SPME GC-MS) at Teagasc Food Research Centre as described by Hou et al. (Reference Hou, Hannon, McSweeney, Beresford and Guinee2014).
Flow cytometry
Flow cytometry (FCM) was measured as described by Kilcawley et al. (Reference Kilcawley, Nongonierma, Hannon, Doolan and Wilkinson2012). Reference control populations of live, permeabilised/damaged, or dead cells were identified as described by Sheehan et al. (Reference Sheehan, O'Loughlin, O'Cuinn, FitzGerald and Wilkinson2005) and Doolan & Wilkinson (Reference Doolan and Wilkinson2009).
Statistical analysis
Analysis of variance (one-way ANOVA) of redox potential measurements, microbiological counts, composition and pH of the cheeses were conducted using SPSS Version 20·0 for Mac OS X (SPSS Inc., Chicago, IL, USA). When differences were significant (P < 0·05), the means were analysed using Tukey's test.
The data for the volatile compounds were analysed by principal component analysis (PCA) by Unscrambler V 6·1 (CAMO AS, N-70421 Trondheim, Norway).
Results and discussion
Cheeses were analysed for composition at 14 d of ripening. Redox potential, microbial growth and pH were monitored at 1, 14, 30, 60, 120 and 180 d. The data reported for E h and microbial growth are averaged values of three independent trials for all the cheeses, except for cheeses made with the addition of 1% KIO3 and 0·05% Na2S2O4 that were made only in Trial 1. Furthermore, cell viability of control cheeses and cheeses made with the addition of 0·1% KIO3 was measured at 60 d of ripening.
Chemical composition, pH and microbiological growth of the cheeses
The values of moisture, salt, fat and protein contents of the cheeses were within the range of those typical of Cheddar cheese (data not shown) (Lawrence et al. Reference Lawrence, Gilles, Creamer, Crow, Heap, Honoré, Johnston, Samal, Fox, McSweeney, Cogan and Guinee2004).
Changes in pH of control and experimental cheeses were measured during ripening (data not shown). The pH of the cheese made with the addition of 1% KIO3 significantly differed from the control cheese and the other experimental cheeses and had values around 5·8 throughout ripening. During ripening, the pH of control cheese and cheese made with the addition of 0·05% KIO3 was 5·2–5·3 and the other experimental cheeses had a pH significantly slightly higher (pH around 5·4) than the control. At the end of ripening, the pH of all the cheeses, except the cheese made with the addition of 1% KIO3, had values between 5·3 and 5·4.
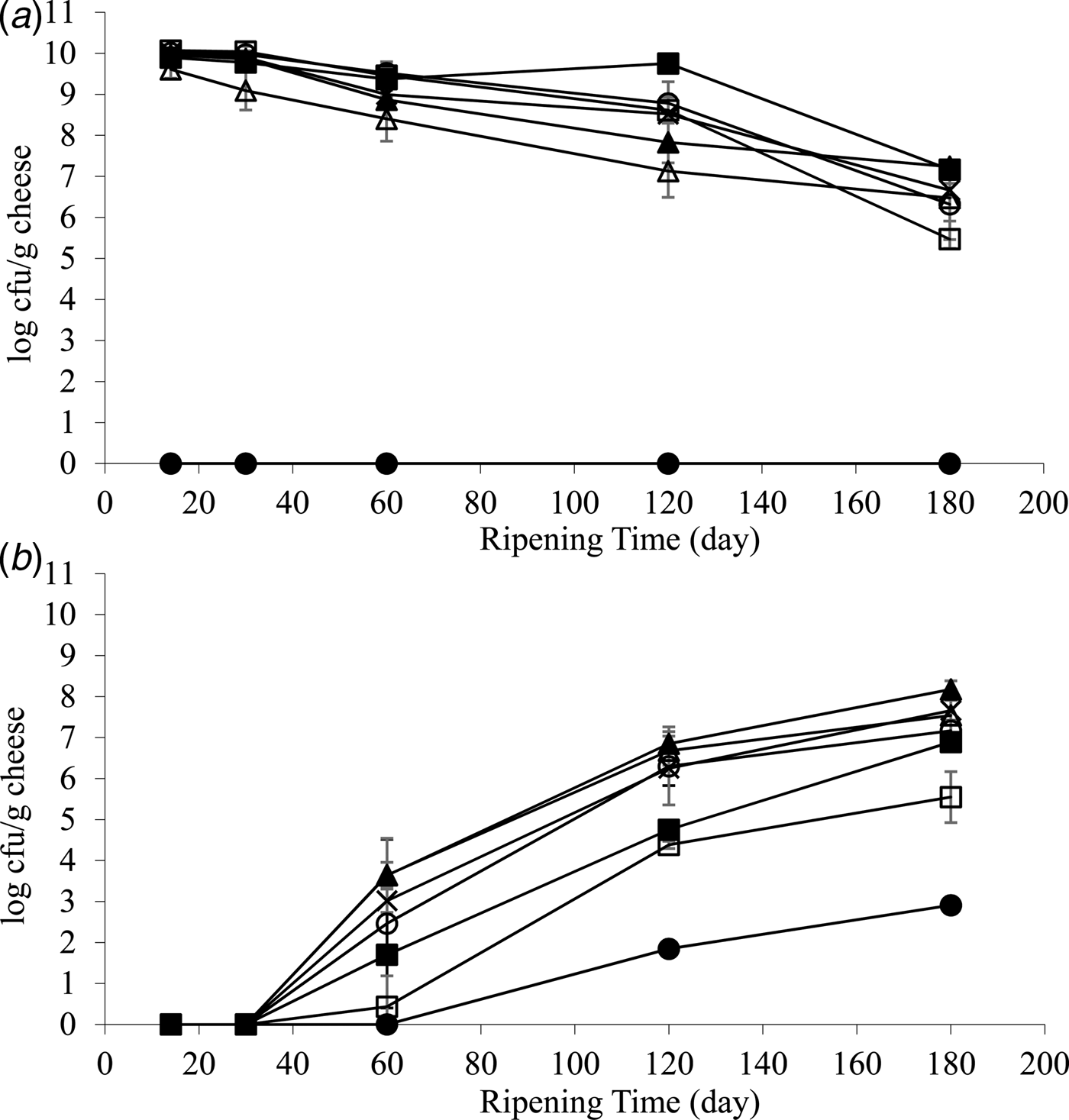
Growth of starter and non-starter LAB are shown in Fig. 1a, b, respectively. At 14 d of ripening, starter LAB grew to similar values (9–10 log cfu/g cheese) in control cheeses and cheese made with the addition of 0·1 and 0·05% KIO3, 0·1 and 0·05% Na2S2O4 and 2% Cys. During ripening, those cheeses showed a typical decline in starter LAB number. Cheese made with 1% KIO3 did not contain any viable starter LAB during ripening, suggesting that the quantity of KIO3 used might have been toxic for the bacteria. Therefore, the cheese made with the addition of KIO3 at 1% was produced only in the first trial.

Fig. 1. Numbers of starter (A) and non-starter (B) lactic acid bacteria in control Cheddar cheese (○) and cheeses containing different redox agents during 6 months of ripening. Potassium iodate (KIO3) at 1 (●), 0·1 (△) and 0·05% (▲) (w/w) were used as the oxidising agents while sodium hydrosulfite (Na2S2O4) at 0·1 (□) and 0·05% (■) (w/w) and cysteine (Cys) at 2% (×) (w/w) were added as the reducing agents. Values are average of microbial counts of three independent trials, except for cheeses made with the addition of 1% KIO3 and 0·05% Na2S2O4 that were made only in Trial 1. Counts were measured in duplicate in each cheese.
During ripening, non-starter LAB levels increased in all the cheeses. However, at 120 and 180 d of ripening, the cheeses made with the addition of 1% KIO3 and 0·1% Na2S2O4 had non-starter LAB numbers lower by five and three log cycles, respectively, compared to the control cheese. Cheese made with the addition of 0·05% Na2S2O4 had counts lower by three log cycles than the control cheese at 120 d of ripening. Other experimental cheeses showed a growth of non-starter LAB similar to the control cheese.
Cell viability
Since KIO3 at 1% appeared to be toxic for starter LAB growth in Trial 1, lower concentrations of KIO3 were used (0·1 and 0·05%) in subsequent trials. FCM was used to identify bacteria cells in various physiological states in control cheese and cheese made with 0·1% KIO3. Figure 2 shows the FCM plots of bacterial populations harvested. Lower left quadrant (LL) is mainly debris while lower right (LR) quadrant is normally live intact cells, upper left quadrant (UL) usually contains highly damaged/permeabilized/dead cells and upper right quadrant (UR) usually contains damaged/permeabilized cells which may also be viable (Sheehan et al. Reference Sheehan, O'Loughlin, O'Cuinn, FitzGerald and Wilkinson2005; Doolan & Wilkinson, Reference Doolan and Wilkinson2009).

Fig. 2. Flow cytometer mulitparameter dot plots of SYTO 9 fluorescence (FL1) vs. propidium iodide fluorescence (FL3) of cells harvested from control cheeses and from cheeses with 0·1% KIO3 of trial 1 (T1), 2 (T2) and 3 (T3).
In all cheeses distinct populations of permeabilized cells were observed in the UR regions of the dot plots. However, in the control cheeses there was also a distinct population of intact/live cells (LR), whereas fewer of these intact cells were evident in the cheeses made with 0·1% KIO3. This population of cells could be starter or non-starter LAB (Sheehan et al. Reference Sheehan, O'Loughlin, O'Cuinn, FitzGerald and Wilkinson2005).
FCM results indicate that the addition of 0·1% KIO3 caused a decrease in the population of intact cells and did not affect the damaged/permeabilized and perhaps viable cells.
Control of oxidation-reduction potential during ripening
Figure 3 shows the E h equilibrium values reached at each time point during cheese ripening.

Fig. 3. Equilibrium values of oxidation-reduction potential (E h) during ripening of control Cheddar cheese (○) and cheeses made with the addition of redox agents. Potassium iodate (KIO3) at 1 (●), 0·1 (△) and 0·05% (▲) (w/w) were used as the oxidising agents while sodium hydrosulfite (Na2S2O4) at 0·1 (□) and 0·05% (■) (w/w) and cysteine (Cys) at 2% (×) (w/w) were added as the reducing agents. Values are average of equilibrium Eh values of three independent trials, except for cheeses made with the addition of 1% KIO3 and 0·05% Na2S2O4 that were made only in Trial 1.
The redox potential of control cheese was around −130 mV during ripening. This value is in agreement with previous studies where E h of mature Cheddar cheese was measured (Kristoffersen et al. Reference Kristoffersen, Gould and Purvis1964; Topcu et al. Reference Topcu, McKinnon and McSweeney2008; McSweeney et al. Reference McSweeney, Caldeo, Topcu and Cooke2010). The E h of cheeses made with the addition of reducing agents did not differ significantly from the control cheese.
Cheese made with the addition of 1% KIO3 had positive redox potential values, around +400 mV, throughout ripening, probably due to the absence of LAB and the higher pH. Starter LAB were not able to survive during ripening and non-starter LAB only grew slowly at the end of ripening. In a study by Green & Manning (Reference Green and Manning1982), Cheddar cheese made under aseptic conditions and in the absence of starter LAB, had a positive redox potential (+315 mV) at 42 d of ripening. Indeed, as demonstrated by Jeanson et al. (Reference Jeanson, Hilgert, Coquillard, Seukpanya, Faiveley, Neveu, Abraham, Georgescu, Fourcassié and Beuvier2009), the redox potential of milk treated with different gasses was constant over time in absence of bacterial growth.
In a second trial, lower quantities of KIO3 (0·05 and 0·1%) were added to the cheeses in order to have an effect on the redox potential without influencing microbial growth. Cheeses made with the addition of 0·1 and 0·05% KIO3 had a significantly higher redox potential compared to the control cheese and positive values of +316 and +179 mV, respectively, were maintained for the first 2 weeks of ripening (Fig. 3). At 1 month of ripening, E h of cheeses made with 0·1 and 0·05% KIO3 decreased slightly and after 2 months the E h reached values closed to that of the control cheese (Fig. 3). In the past, studies on the addition of oxidising salts to the milk designed for Edam cheese manufacture have been conducted in order to prevent the development of anaerobic butyric acid bacteria (Vos, Reference Vos1948; Peltola & Antila, Reference Peltola and Antila1953; Galesloot, Reference Galesloot1960, Reference Galesloot1961a, Reference Galeslootb) and a trend in redox potential similar to our results was found. Among the salts studied by Galesloot (Reference Galesloot1961a), KNO3 was able to keep the E h at values about 100 mV higher than the control cheese for 10 d and after that period the E h decreased to values close to those of the control cheese. The inability to keep the E h at constant positive values throughout ripening when oxidising agents were added to the cheese could be due to the bacterial growth in the cheese. The oxidising agents added might be reduced by bacterial redox systems present in the cheese (Jacob, Reference Jacob, Norris and Ribbons1979; Martin et al. Reference Martin, Cayot, Vergoignan, Journaux, Gervais and Cachon2010) or by chemical reaction with indigenous reducing compounds. In our study, the decrease in E h of the experimental cheeses containing oxidising agents (KIO3 at 0·05 and 0·01%) occurred in conjunction with the growth of non-starter LAB.
Volatile analysis
Study of the volatile compounds in the cheeses analysed at 2 and 6 months of ripening by SPME GC-MS identified 40 compounds. The compounds identified are typical of Cheddar cheese (Singh et al. Reference Singh, Drake and Cadwallader2003; Hannon et al. Reference Hannon, Kilcawley, Wilkinson, Delahunty and Beresford2007) and their concentrations are similar to other studies (Hou et al. Reference Hou, Hannon, McSweeney, Beresford and Guinee2014).
Principal component analysis (PCA) was performed to assess the relationship within and among the cheeses and the volatile compounds identified. PCA of the volatile data was performed at 2 (Fig. 4a) and 6 (Fig. 4b) months on control cheeses and cheeses made with the addition of 0·1 and 0·05% KIO3, 0·1% Na2S2O4 and 2% Cys on the averaged peak areas of separate trials. Cheeses made with 1% KIO3 and 0·05% Na2S2O4 were excluded from the PCA analysis since they were produced only in Trial 1.

Fig. 4. Principal component analysis of data of volatile compounds identified in control Cheddar cheeses (Control), cheeses made with the addition of potassium iodate at 0·1 (KIO3 0·1%) and 0·05% (KIO3 0·05%), cheeses made with the addition of sodium hydrosulfite at 0·1% (Na2S2O4 0·1%) and cheeses made with the addition of 2% cysteine (Cys 2%) at two (A) and six (B) months of ripening. Values are mean of the different trials. At 2 months of ripening, principal components (PC) 1 and 2, accounted for 37 and 31% of the variation, respectively, and at 6 months, PC 1 and 2 accounted for 35 and 34%, respectively. The volatile compounds identified as written in red and the cheeses are shown in blue. Cheeses made with the addition of 1% KIO3 and 0·05% Na2S2O4 were excluded from the analysis as they were made only in one trial.
At 2 months of ripening, principal components (PC) 1 and 2, which accounted for 37 and 31% of the variation, respectively, clearly separated the cheeses made with the reducing agent from cheeses made with the oxidising agents (Fig. 4a). Cheeses made with the addition of reducing agents were characterized by the presence of sulphur and ketone compounds. Sulphur compounds like dimethylsulfide (DMS) and dimethyltrisulfide (DMTS) are considered important aroma compounds that characterize mature Cheddar cheese (Singh et al. Reference Singh, Drake and Cadwallader2003) and the addition of reducing agents favoured the production of sulphur compounds already at 2 months of ripening. Similarly, a study by Green & Manning (Reference Green and Manning1982) reported that at 6 weeks of ripening concentrations of hydrogen sulphide and methanethiol were higher in the cheeses made with the addition of a reducing compound (Cys). Moreover, Martin et al. (Reference Martin, Cachon, Pernin, De Coninck, Gervais, Guichard and Cayot2011) found an increase in DMS in yogurt made under reducing conditions throughout 28 d of storage. In contrast to our results, Kieronczyk et al. (Reference Kieronczyk, Cachon, Feron and Yvon2006), in a study on the flavour compounds produced by amino acid catabolism in vitro of two strains of Lactococcus under reducing and oxidising conditions, found that methanethiol and dimethyldisulfide were mainly produced under oxidising conditions. However, among the volatile sulphur compounds identified by the authors, DMTS was present in higher level in reducing system than in oxidising system.
Moreover, the PCA of volatiles data at 2 month of ripening suggests that cheeses made with oxidising agents were characterized mainly by aldehydes, in particular benzaldehyde and acetaldehyde (Fig. 4a). These results are in agreement with previous studies. Kieronczyk et al. (Reference Kieronczyk, Cachon, Feron and Yvon2006) reported a higher quantity of benzaldehyde when LAB were grown in vitro at positive redox potential. A study by Martin et al. (Reference Martin, Ebel, Rojas, Gervais, Cayot, Cachon and Kongo2013) analysed the volatile compounds produced by yogurts under reducing or oxidising conditions, made by the usage of gasses. The authors found that yogurts made under positive redox potential had higher quantities of acetaldehyde and diacetyl and lower level of DMS.
Furthermore, the PCA at 2 month of ripening showed that other volatile compounds like esters and hydrocarbons were present at higher level in the control cheese and cheese made with 0·05% KIO3 than in other cheeses (Fig. 4a).
At 6 month of ripening, PC 1 and 2 accounted for 35 and 34% of variation in the data, respectively, and separation between the cheeses was less pronounced (Fig. 4b). Control cheese and cheese made with the addition of Cys were separated by PC2 from the cheeses made with the addition of oxidising agents and Na2S2O4.
Conclusion
In this study addition of oxidising and reducing agents was done at the salting stage of Cheddar cheese manufacture in order to understand the influence of oxidation-reduction potential on Cheddar cheese ripening.
Our findings support the hypothesis that redox potential has an influence on the development of cheese flavour during ripening. This study confirms that a negative redox potential is essential for the development of sulphur compounds (Green & Manning, Reference Green and Manning1982; Kristoffersen, Reference Kristoffersen1985) and those compounds were present already at 2 months of ripening when reducing agents were added to the cheese at the salting stage.
Moreover, it seems that the cheese microflora has an important effect on the redox potential of Cheddar cheese. In absence of LAB, caused by the addition of a high concentration of KIO3, the redox potential of our experimental cheese was positive over 6 months of ripening. When KIO3 was added at 0·1 and 0·05% to the cheese, the E h was positive for about 2 months of ripening and it decreased to negative values when the number of non-starter LAB increased. This suggests that LAB might be able to use the oxidising agents added, produce reducing metabolites and drive the environmental redox potential to values close to the E h of the control cheese. Another hypothesis could be the reaction of the oxidising agents with naturally occurring reducing compounds and a consequence reduction on redox potential.
Redox potential can modify microbial activity and metabolic bacteria paths and as consequence act on the flavour development (Ledon & Ibarra, Reference Ledon and Ibarra2006).
In conclusion, understanding and controlling redox potential can be useful to guide aroma formation in dairy products.
The authors would like to thank the financial support provided by the Food Institutional Research Measure administered by the Department of Agriculture, Food and the Marine, Ireland.