Introduction
Niche differentiation is the process by which species and individuals develop different forms of use of the available resources (MacArthur, Reference MacArthur1972). Considering feeding resources, competition influences interspecific and intraspecific trophic diversity, and niche overlap tends to be smaller due to trophic partitioning, allowing the coexistence of organisms that have similar dietary demands (MacArthur & Levins, Reference MacArthur and Levins1967; Pianka, Reference Pianka1981). Trophic partitioning among morphologically similar consumers may also vary according to individual feeding preference, and prey availability, diversity and stability (Araújo et al., Reference Araújo, Bolnick and Layman2011; Duarte et al., Reference Duarte, Flores, Vinagre and Leal2017).
The analysis of stable isotopes of carbon (δ13C) and nitrogen (δ15N) in the consumer tissues complements the understanding of its trophic ecology from other approaches, such as analysis of digestive tract, observation of feeding behaviour in the environment, and morphological and functional analysis of the digestive tract (Newsome et al., Reference Newsome, Martinez del Rio, Bearhop and Phillips2007, Reference Newsome, Yeakel, Wheatley and Tinker2012; González-Ortegón et al., Reference González-Ortegón, Perez-Miguel, Navas, Drake and Cuesta2021). The δ13C values generally indicate the origin of feeding resources (oceanic or coastal; pelagic or benthic) and are used to recognize the trophic habitat of consumers, while δ15N values are a measure of trophic position, especially useful when trophic ecology is not evaluated using the methods mentioned above (Cherel et al., Reference Cherel, Hobson, Bailleul and Groscolas2005; Fry, Reference Fry2008; Ferreira et al., Reference Ferreira, Monteiro and Di Beneditto2020).
Layman et al. (Reference Layman, Arrington, Montaña and Post2007) introduced ecomorphological metrics to summarize quantitative information from isotopic data to describe the trophic structure of a population or community. Subsequently, Jackson et al. (Reference Jackson, Inger, Parnell and Bearhop2011) developed the Bayesian approach to compare these metrics, allowing robust inferences about the isotopic niche of consumers. The stable isotopes provide quantitative information about the consumer's isotopic niche, which is associated with their feeding resources and trophic niche. Thus, the isotopic niche approach allows inferences on how consumers use the feeding resources available in their habitat (Layman et al., Reference Layman, Arrington, Montaña and Post2007; Newsome et al., Reference Newsome, Martinez del Rio, Bearhop and Phillips2007; Abrantes et al., Reference Abrantes, Barnett and Bouillon2014).
Shrimps of the Penaeidae family play an important role in benthic communities because they are omnivorous secondary consumers that feed mainly on organic detritus and other invertebrates, providing energy to the upper trophic levels of both benthic and pelagic food webs (Abarca-Arenas et al., Reference Abarca-Arenas, Franco-López, Peterson, Brown-Peterson and Valero-Pacheco2007; Di Beneditto et al., Reference Di Beneditto, Bittar, Camargo, Rezende and Kehrig2012). However, few studies have addressed the trophic relationships in shrimps using isotopic metrics (Willems et al., Reference Willems, De Backer, Kerkhove, Dakriet, De Troch, Vincx and Hostens2016; Ji et al., Reference Ji, Yokoyama, Reid, Fu and Zhou2019; Ferreira et al., Reference Ferreira, Monteiro and Di Beneditto2020), and there is still a lack of information on how species and individuals interact with each other.
This study compares the isotopic niche of three species of penaeid shrimps that are targets of fishing in south-eastern Brazil to understand their trophic relationships both interspecific and intraspecific. The hypotheses raised are based on the niche theory, in which the coexistence between organisms that have similar dietary demands is possible due to trophic partitioning. The two hypotheses raised are as follows: (I) species that share the habitat (fishing ground) have segregated isotopic niches; and (II) stages of maturity (juvenile and adult) and genders (male and female) vary with the trophic habitat (δ13C) and/or trophic position (δ15N) to minimize intraspecific feeding overlap.
Materials and methods
Sampling
The shrimps were sampled in six fishing ports in south-eastern Brazil, located at states of Espírito Santo and Rio de Janeiro (Figure 1). The choice of fishing ports was defined by their representativeness in regional landings. Xiphopenaeus kroyeri (Heller, 1862), known as Atlantic seabob shrimp, was sampled in the six fishing ports. The Argentine stiletto shrimp (Artemesia longinaris Bate, 1888) and the southern white shrimp (Litopenaeus schmitti (Burkenroad, 1936)) were sampled in two fishing ports. The choice of the species sampled in each fishing port was based on their presence in the landings, verified in situ. Juvenile and adult individuals of both genders are captured in local fisheries (Fernandes et al., Reference Fernandes, Jardim, Di Beneditto, Silva and Keunecke2011, Reference Fernandes, Keunecke and Di Beneditto2014; Eutrópio et al., Reference Eutrópio, Mariante, Junior and Krohling2013) and were sampled for this study.

Fig. 1. Location of the six fishing ports in the states of Espírito Santo (ES) and Rio de Janeiro (RJ), south-eastern Brazil, where the shrimps were sampled.
The fisheries were done with bottom trawl net in coastal waters around the fishing ports, between 1–3 nautical miles from the coastline and 5–30 m deep (Ferreira & Di Beneditto, Reference Ferreira and Di Beneditto2021). Shrimp species are caught simultaneously during bottom trawl. Samplings were performed between June and August 2018, immediately after landing. In the ports of Conceição da Barra, Vitória and Atafona (Figure 1), fishing is focused on X. kroyeri. In Anchieta, Farol de São Tomé and Macaé (Figure 1), fishing is multispecific, with more than one target species in the landings.
For the analysis of δ13C and δ15N, 120 individuals of each species were sampled to represent the local stocks (30 adult males, 30 juvenile males, 30 adult females and 30 juvenile females). After sampling, each shrimp was identified at the species level and categorized according to the stage of maturity and gender, considering the morphology of the primary and secondary sexual traits. In males, the presence of gonopores in the fifth pair of pereiopods and the copulatory organ (petasma, which is fused only in adult individuals) were observed (Costa et al., Reference Costa, Fransozo, Melo and Freire2003). The females were recognized by the gonopores in the third pair of pereopods and by the presence of telic situated ventrally between the fourth and fifth pair of pereopods (Costa et al., Reference Costa, Fransozo, Melo and Freire2003). Gonads chromatic feature is the visual method to determine the maturation stage of the ovaries in penaeid shrimps (Brown & Patlan, Reference Brown and Patlan1974, Dumont & D'Incao, Reference Dumont and D'Incao2004; Peixoto et al., Reference Peixoto, Calazans, Silva, Nole, Soares and Frédou2018). The developing or developed adult females have voluminous and olive-coloured ovaries, and the spawned females exhibit flaccid ovaries with a white and translucent colour.
Isotopic analyses of carbon (δ13C) and nitrogen (δ15N)
In the laboratory, the abdominal muscle of each shrimp was removed, stored in a dry and sterile plastic tube, frozen (−20°C), lyophilized and homogenized in fine powder in a mortar and pestle. The exoskeleton and internal organs, such as gills, gonads, hepatopancreas and intestine, were removed before storage to prevent the analysis of tissues with different metabolic rates, compromising the isotopic interpretation.
Samples of 0.4 mg of muscle tissue (dry weight) were analysed to determine the isotopic composition in a Delta V Advantage interface Conflo IV, mass spectrometer (Thermo Scientific, Germany) coupled to the elemental analyser Flash 2000 at the Laboratório de Ciências Ambientais of Universidade Estadual do Norte Fluminense Darcy Ribeiro. The reference values for carbon and nitrogen isotopic analyses were Vienna Pee Dee Belemnite (VPDB) and atmospheric N2, respectively. The samples were analysed together with analytical blanks and urea analytical standards (VAT Analysentechnik -330802174; CH4N2O Mw = 60, C = 20%, N = 46%) with certified isotopic compositions (δ13C = −39.89‰ and δ15N = −0.73‰). Analytical control was performed every 10 samples using a certified isotopic standard (Elemental Microanalysis Protein Standard OEA): δ13C = −26.98‰ and δ15N = +5.94‰. The analytical reproducibility was based on triplicates for every 10 samples: ± 0.3‰ for δ15N and ± 0.2‰ for δ13C. The results were expressed in parts per thousand (‰).
Data analysis
All data analyses were performed in the R program (R Core Team, 2021), assuming an a priori error of 5% (α = 0.05). The assumptions of normality and homoscedasticity were tested by the Shapiro–Wilk and Levene tests, respectively.
To verify the first hypothesis that shrimp species that share the habitat have segregated niches, the fishing ports considered were those with multispecific fishing: Anchieta (X. kroyeri and L. schmitti), Farol de São Tomé (X. kroyeri and A. longinaris), and Macaé (X. kroyeri, A. longinaris and L. schmitti). The isotopic niche breadth is based on the position of individuals in the δ13C–δ15N space (Layman et al., Reference Layman, Arrington, Montaña and Post2007; Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011), calculated from the function of stable isotope Bayesian ellipses in the R program (Stable Isotope Bayesian in R – SIBER) (Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011; Parnell et al., Reference Parnell, Phillips, Bearhop, Semmens, Ward, Moore, Jackson, Grey, Kelly and Inger2013; R Core Team, 2021). The area of the standard ellipse, which represents the isotopic niche width, is based on the centroid of the group and sized with the chance of including 40% of subsequently sampled data. The area of each standard ellipse was compared probabilistically with the posterior Bayesian distributions, calculating the proportion of ellipses for group 1 which is greater than that for group 2 (Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011). The percentage of overlap of the area of the standard ellipses is the measure of isotopic niche overlap between species. To analyse how the values of δ13C and δ15N varied among the shrimp species in the multispecific fishing ports, one-way ANOVA and a posteriori Tukey's test were used.
To verify the second hypothesis that stages of maturity and genders vary in relation to the trophic habitat and/or trophic position to minimize intraspecific feeding overlap, two-way ANOVA was used. This test isolates the effects of each factor (stage of maturity and gender) separately, and measures the effect of the interaction between them.
Results
Interspecific comparison
The isotopic niche overlap between shrimp species was absent or low (Figure 2). The percentage of overlap of the area of the standard ellipses in Anchieta (X. kroyeri vs L. schmitti) was 34% (Figure 2A); in Farol de São Tomé (X. kroyeri vs A. longinaris) was absent; and in Macaé, it was 9% between A. longinaris and L. schmitti and absent in the comparisons involving X. kroyeri. Niche segregation was mainly supported by X. kroyeri, which showed more enriched values of δ13C compared with the other species (Figure 2, Table 1).
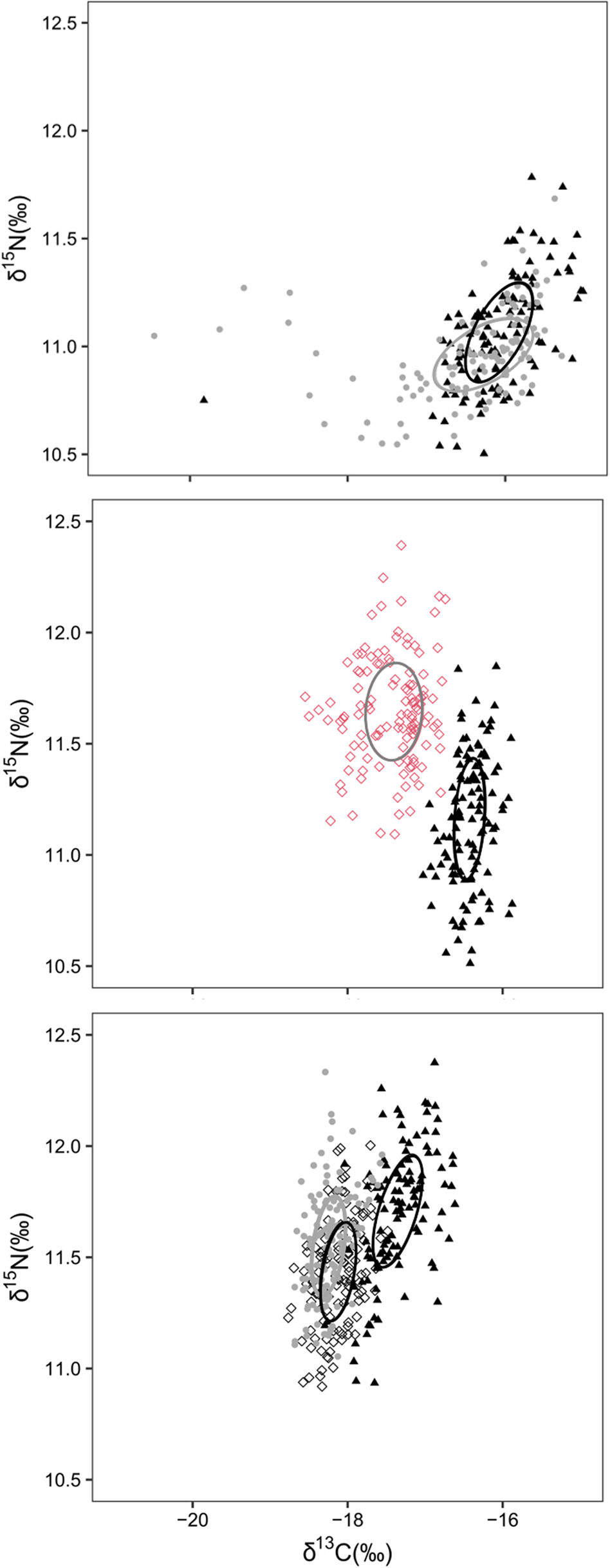
Fig. 2. δ13C and δ15N values of shrimp species from multispecific fishing ports in south-eastern Brazil (A: Anchieta; B: Farol de São Tomé; C: Macaé). The Triangle, lozenge and circle represent Xiphopenaeus kroyeri, Artemesia longinaris and Litopenaeus schmitti, respectively. The rows represent the data ellipses (40% confidence interval) for the isotopic niches.
Table 1. Mean and standard deviation (SD) of δ13C and δ15N in shrimp species from six fishing ports in the states of Espírito Santo (ES) and Rio de Janeiro (RJ), south-eastern Brazil

Intraspecific comparison
The values of δ13C differed significantly between maturity juvenile and adult individuals in all species, with higher values in adults (two-way ANOVA, F = 103.49, df = 1, P = 2 × 10−16) (Figure 3A). The exception was A. longinaris in Farol de São Tomé, with juveniles more enriched in δ13C. Meanwhile, no significant difference was found in the three species comparing gender males and females (two-way ANOVA, F = 1.491, df = 1, P = 0.22) (Figure 3A). No interactions were detected between maturity and gender factors (two-way ANOVA, F = 0.38, df = 1, P = 0.68).
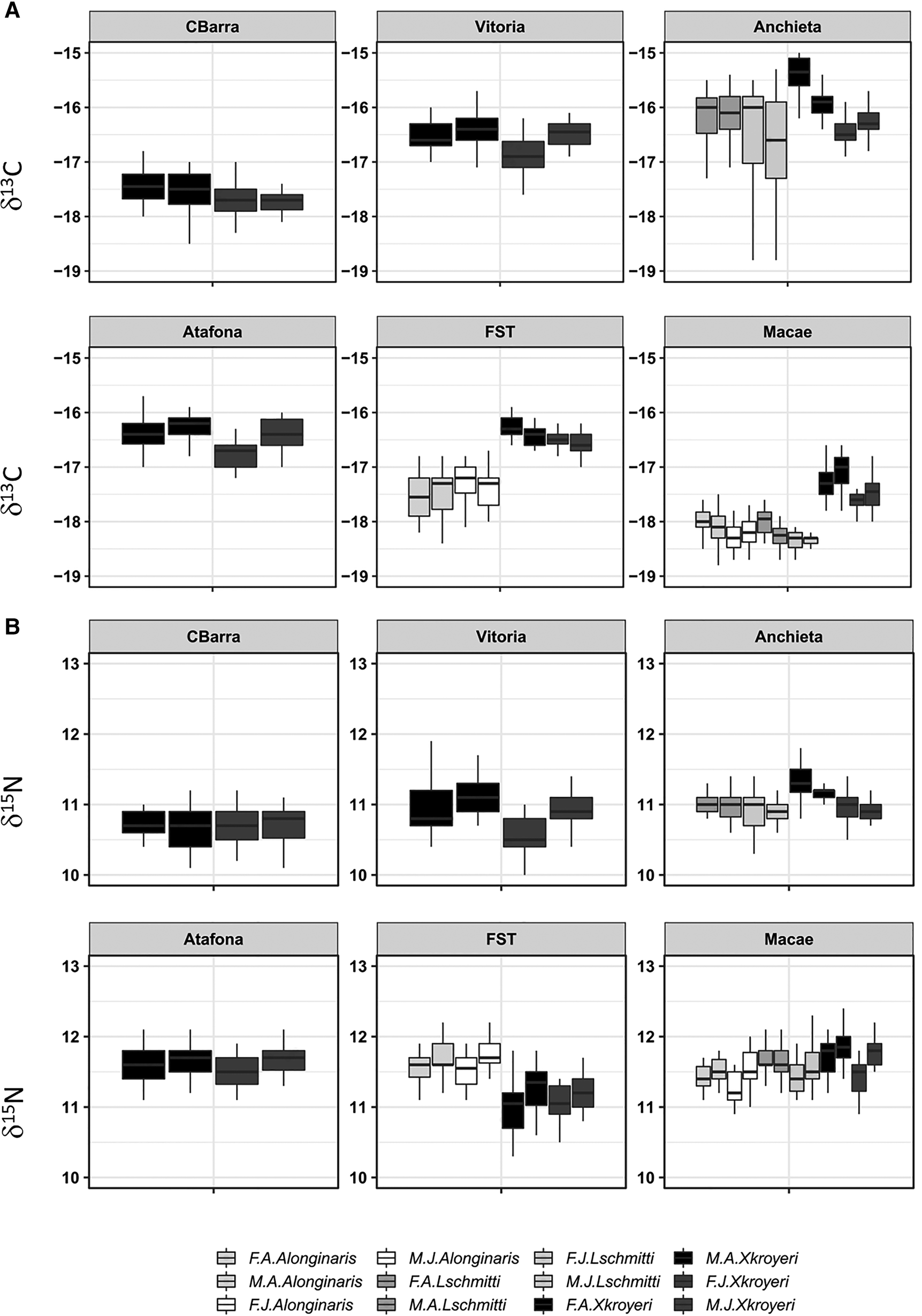
Fig. 3. δ13C and δ15N values of stages of maturity (juvenile and adult) and genders (male and female) of the shrimp species from six fishing ports in south-eastern Brazil. Horizontal lines in the box indicate median of boxplot. (F.A., adult female; F.J., juvenile female; M.A., adult male; M.J., juvenile male; CBarra, Conceição da Barra; FST, Farol de São Tomé).
In general, the values of δ15N varied significantly between stages of maturity (two-way ANOVA, F = 53.02, df = 1, P = 6.09 × 10−13) and genders (two-way ANOVA, F = 51.62, df = 1, P = 1.20 × 10−12) (Figure 3B). No interactions were detected between maturity and gender factors (two-way ANOVA, F = 0.48, df = 1, P = 0.61). Adult individuals were more enriched than juveniles, and males had more enriched values than females. The exceptions were X. kroyeri in Conceição da Barra and A. longinaris in Farol de São Tomé, where there was no significant difference between both stages of maturity (F = 0.486, df = 1, P = 0.48 for X. kroyeri, F = 0.087, df = 1, P = 0.76 for A. longinaris) and genders (F = 0.121, df = 1, P = 0.72 for X. kroyeri, F = 0.061, df = 1, P = 0.80 for A. longinaris).
Discussion
Interspecific comparison
The interspecific comparison of the isotopic niche of coexisting shrimp species corroborated the first hypothesis raised in the study. The results support the assumption of niche theory that species that share habitats and have similar feeding demands tend to have segregated niches with low overlap (MacArthur & Levins, Reference MacArthur and Levins1967; Pianka, Reference Pianka1981). Trophic partitioning among shrimps, inferred by stable isotopes, has already been recorded as a strategy to minimize the possible effects of interspecific competition in other decapod crustaceans (Cummings et al., Reference Cummings, Lee, Simpson, Booth, Pile and Holmes2011) and other animal taxa (Alfaro et al., Reference Alfaro, Thomas, Sergent and Duxbury2006; Hyodo, Reference Hyodo2015; Figgener et al., Reference Figgener, Bernardo and Plotkin2019).
The isotopic niche segregation was mainly supported by the more enriched values of δ13C in X. kroyeri. The distribution pattern of this species justifies the difference. Castilho et al. (Reference Castilho, Pie, Fransozo, Pinheiro and Costa2008) analysed the abundance of eight species of shrimp in south-eastern Brazil in relation to environmental variables, including X. kroyeri, A. longinaris and L. schmitti. The authors found that the occurrence of X. kroyeri was limited in waters with temperatures below 28°C and beyond 15 m depth, suggesting that the species is more abundant in and dependent on warmer and shallower waters than other species. Values of δ13C are more enriched in shallow coastal waters of the benthic environment (Fry, Reference Fry2008), which is the preferred habitat of X. kroyeri. Typical values for benthic microalgae reported in the literature range between −12 and −20‰ (Bouillon et al., Reference Bouillon, Connolly and Gillikin2011). The benthic environment is consistently more enriched in δ13C than its pelagic counterpart (~7‰) due to isotopic fractionation (France, Reference France1995). This bentho-pelagic difference can be reflected in shrimp δ13C values, and thus the microalgae and phytoplankton can be one of the main primary sources of resources for these species (Fry, Reference Fry2008). Isotopic analysis proves to be a powerful tool to distinguish between benthic and pelagic food sources for coastal animals.
The greatest dispersion of shrimps along the δ15N axis, illustrated in Figure 2, suggests that the individuals have high food plasticity. The isotopic results showed the feeding plasticity of penaeid shrimps, which was already verified in previous studies with digestive tract analysis (Boschi, Reference Boschi1969; Branco & Moritz Junior, Reference Branco and Moritz Júnior2001; Albertoni et al., Reference Albertoni, Palma-Silva and Esteves2003; Spanjersberg et al., Reference Spanjersberg, Roux and Caille2006; Willems et al., Reference Willems, De Backer, Kerkhove, Dakriet, De Troch, Vincx and Hostens2016).
The density of individuals of any exploited population by commercial fishing must be high in each fishing ground to maintain the viability of the economic activity (King, Reference King2007). Food availability is only one of the features for the maintenance of shrimp populations. Other environmental features, such as type of sediment, salinity, depth and water temperature are also determinant or limiting to the establishment of the species (Gulland & Rotschild, Reference Gulland and Rotschild1981).
Xiphopenaeus kroyeri was the only species sampled in the six fishing ports, indicating that both availability of feeding resources and other environmental features are adequate for maintaining their fishing stocks. Conversely, A. longinaris prefers areas with colder water (Costa et al., Reference Costa, Fransozo, Castilho and Freire2005), and above 22°S (i.e. north of the port of Farol de São Tomé) there is no record of this species in landings. Litopenaeus schmitti preferentially inhabits sandy bottom (Boos et al., Reference Boos, Costa, Santos, Dias-Neto, Severino-Rodrigues, Rodrigues, D'Incao, Ivo, Coelho, Pinheiro and Boos2016), with larger particle size than fine sand, silt and clay, characteristic of coastal areas with strong fluvial influence. This would explain, for example, the absence of the species in the port of Atafona, which is influenced by the Paraíba do Sul River, the largest river input in south-eastern Brazil (Souza et al., Reference Souza, Godoy, Godoy, Moreira, Carvalho, Salomão and Rezende2010).
Intraspecific comparison
The second hypothesis that there is variation in the trophic habitat and/or trophic position of juvenile and adult individuals, and males and females to minimize feeding overlap, was confirmed. In general, the same trends were noted for the three shrimp species. Differences between stages of maturity were observed for δ13C and δ15N. The most enriched (less negative) values of δ13C in adults and less enriched (more negative) in juveniles may indicate feeding activity mainly associated with benthic environment and water column, respectively (Fry, Reference Fry2008).
The less enriched values of δ15N in juveniles may be related to the size (and trophic position) of the prey consumed. The feeding habits of the three shrimp species were never analysed at the six fishing ports considered by this study. However, previous studies done in other areas and based on digestive tract analysis showed that for X. kroyeri (Cortés & Críales, Reference Cortés and Críales1990; Branco & Moritz-Júnior, Reference Branco and Moritz Júnior2001; Willems et al., Reference Willems, De Backer, Kerkhove, Dakriet, De Troch, Vincx and Hostens2016) and A. longinaris (Boschi, Reference Boschi1969; Spanjersberget al., Reference Spanjersberg, Roux and Caille2006), there is a difference in the size of prey consumed by juveniles (smaller prey) and adults (larger prey). As crustaceans develop and undergo morphological and physiological changes, their prey capture skills and nutritional needs are altered (Dall et al., Reference Dall, Hill, Rothlisberg and Sharples1990). Additionally, there are studies with A. longinaris (Gimenez et al., Reference Gimenez, Garcıa-Carreno, Del Toro and Fenucci2002) and L. schmitti (Lemos et al., Reference Lemos, Garcia-Carreno, Hernández and Del Toro2002) that showed variations in digestive enzymes between juveniles and adults. This represents variations in their food metabolism, which most likely interferes with the selection of consumed resources.
The results showed that the trophic habitat (δ13C) was shared between genders, but the values of δ15N varied (males > females). This finding partially diverges from previous studies based on the digestive tract analysis (Boschi, Reference Boschi1969; Cortés & Críales, Reference Cortés and Críales1990; Branco & Moritz-Júnior, Reference Branco and Moritz Júnior2001; Spanjersberg et al., Reference Spanjersberg, Roux and Caille2006; Willems et al., Reference Willems, De Backer, Kerkhove, Dakriet, De Troch, Vincx and Hostens2016), which found males and females sharing the feeding resources. In this case, as well as for juveniles and adults, the range of δ15N values was not large enough to define a trophic level (trophic enrichment of 1.4‰ or higher, according to McCutchan et al., Reference McCutchan, Lewis, Kendall and McGrath2003). The difference in δ15N between males and females can be explained by the size of prey consumed, as assumed for juveniles and adults.
Besides differences in the size of prey consumed, other possible explanations for the intraspecific difference between genders regarding the δ15N values should be considered. The efficiency of δ15N as trophic marker is sensitive to the nutritional status of the consumer and quantitative and qualitative differences in food intake and assimilation (Hobson et al., Reference Hobson, Schell, Renouf and Noseworthy1996; Jennings et al., Reference Jennings, Pinnegar, Polunin and Warr2002; Das et al., Reference Das, Lepoint, Leroy and Bouquegneau2003). In experimental tanks with Litopenaeus vannamei, Moss & Moss (Reference Moss and Moss2006) tested the effects of gender and body size on food acquisition time. Bardera et al. (Reference Bardera, Owen, Façanha, Sloman and Alexander2020), in a study with the same species, compared the pattern of feeding activity between males and females. The results of both studies indicated that even with a smaller body size compared with females, which is a morphological feature of all penaeid shrimps (Dall et al., Reference Dall, Hill, Rothlisberg and Sharples1990), males showed a competitive advantage in the time of food acquisition, ingesting greater quantities in the same time interval. These differences in feeding behaviour could explain the variations in the δ15N values among genders, as recorded in the study.
Conclusion
The isotopic niche of the penaeid shrimps targeted by fisheries in south-eastern Brazil and their isotopic composition (δ13C and δ15N) showed interspecific and intraspecific trophic partitioning, respectively. Therefore, the results are in accordance with the assumptions of niche theory. Meanwhile, the isotopic niche model (δ13C × δ15N) is a simplified 2D geometry representing only a part of the species’ real niche. As discussed in Shipley & Matich (Reference Shipley and Matich2020), to represent a more realistic and multidimensional niche space, such as described by Hutchinson (Reference Hutchinson1957), additional niche axes will need to be considered, including habitat, time, ratio size and inter-individual differences.
The utilization of stable isotopes as an indirect measure of the trophic ecology of consumers, such as the penaeid shrimps, was supported by the results of this study. The partitioning of trophic resources, along with other environmental factors, contributes to the maintenance of these shrimp stocks for exploitation by extractive fishing.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315422000558.
Acknowledgements
We are indebted to fishers from the six fishing ports for providing us with the shrimps for this study. This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (grant 302.598/2021-9), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ (grant E-26/200.797/2021), and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Author contributions
Keltony de Aquino Ferreira: Conceptualization, Sampling, Methodology, Investigation, Formal analysis, Writing. Ana Paula Madeira Di Beneditto: Conceptualization, Funding acquisition, Writing, Adriane Cristina Araújo Braga: Writing – review.
Conflict of interest
The authors declare that they have no known conflict financial interests or personal relationships that could have appeared to influence the work reported in this paper.






