Introduction
Transgenic pigs are used in several areas such as genetic research, biomedicine or pig husbandry. Sperm-mediated gene transfer (SMGT), based on the spermatozoon’s capacity to capture and internalize exogenous DNA (eDNA) (Lavitrano et al., Reference Lavitrano, Maione, Forte, Francolini, Sperandio, Testi and Spadafora1997), is a good alternative for the production of transgenic animals compared with other methodologies, and is a cheaper option without the need for costly equipment. Also favourable is its efficiency with over an 80% success rate (Lavitrano et al., Reference Lavitrano, Forni, Bacci, Di Stefano, Varzi, Wang and Seren2003), its effectiveness and repeatability are controversial. An ideal transfection method must produce high transfection rates with low cell damage (Breunig et al., Reference Breunig, Lungwitz, Liebl and Goepferich2007), therefore physical or chemical methods (i.e. EP or PLF, respectively) that optimize this process, could be used to assist the spermatozoon to internalize the eDNA.
EP, also known as electropermeabilization, is a widely used method of cellular transfection. in which cells and eDNA of interest are placed in medium and a high voltage electric current is applied for very short time, causing cell membrane pores to open and allowing eDNA to enter the cell, with minimal compromise in cell viability (Pramod et al., Reference Pramod, Kumar and Mitra2016).
For spermatozoa, an efficient or successful EP method increases the transfection rate and simultaneously keeps the spermatozoon alive and retains its fertilization capability. Sperm motility could be affected by both electrical parameters and eDNA amount during EP, leading to decrease in fertilization, birth and gene transfer rates, as well as possible embryonic abnormalities (Tsai, Reference Tsai2000). Therefore, a medium is required that only allows passage of the electrical current to the cells and, at the same time, maintains sperm viability. In addition, the specific electric current (voltage) and the number and duration of the appropriate pulses need to be ascertained.
Many studies using EP have been reported that show diverse success rates using different protocols for bovine sperm (Gagne et al., Reference Gagne, Pothier and Sirard1991; Rieth et al., Reference Rieth, Pothier and Sirard2000; Simões et al., Reference Simões, Feitosa, Milazzotto, Nicacio, Barros, Gonçalves, Marques, Visintin and Assumpção2015), fish (Misgurnus anguillicaudatus) and mollusc (Haliotis diversicolor) (Tsai, Reference Tsai2000). However, there has been a lack of information for pig spermatozoa transfection. The only study available to review used a high unspecified voltage applied for 2.4 s, increasing the transfection rate by 5–10% (approximately 70–75%) in comparison with the control (Horan et al., Reference Horan, Powell, Bird, Gannon and Houghton1992).
Another possible sperm transfection method is PLF, which is a non-viral cell transfection method that uses cationic polymers as eDNA carriers (Fischer et al., Reference Fischer, Bieber, Li, Elsässer and Kissel1999). PEI is a cationic polymer that, due to its positive charge, binds DNA by electrostatic interaction, and forms PEI−DNA complexes (Bieber et al., Reference Bieber, Meissner, Kostin, Niemann and Elsasser2002). These complexes bind to the cell surface, interact electrically with the cell membrane and are internalized by endocytosis; the DNA molecules are then protected inside the endosome (Boussif et al., Reference Boussif, Lezoualc’h, Zanta, Mergny, Scherman, Demeneix and Behr1995; Tros de Ilarduya et al., Reference Tros de Ilarduya, Sun and Duzgunes2010). In cells, they migrate to the nucleus, where the DNA is released (Godbey & Mikos, Reference Godbey and Mikos2001; Tros de Ilarduya et al., Reference Tros de Ilarduya, Sun and Duzgunes2010). Another advantage of PEI is its low cytotoxicity for most cells (Fischer et al., Reference Fischer, Bieber, Li, Elsässer and Kissel1999); PEI efficiency is affected by its structure and molecular weight and these factors are highly correlated with cell damage (Kafil & Omidi, Reference Kafil and Omidi2011). In addition, to obtain satisfactory levels of PEI transfection, establishment of concentration and incubation time for this polymer is required.
PLF plus PEI has been evaluated previously in some cell types (Dang et al., Reference Dang, Wang, Qin, Zhang, Gu, Wang, Yang, Li and Zhang2011; Hsu & Uludag, Reference Hsu and Uludag2012). For pig spermatozoa, PEI-coated magnetic nanoparticles (MNP-PEI) alone were used (Fang et al., Reference Fang, Chen, Wang, Wang, Li, Zhu, Wang and Zeng2017), however PEI polyplex itself has not been tested on sperm from animal species. Using EP and PLF as the transfection method, various success rates have been observed in different cell types including spermatozoa from many species but not from swine. This study, therefore, aimed to standardize and optimize EP and PLF (with PEI) methods giving the most efficient and least cytotoxic conditions for swine spermatozoa transfection.
Materials and methods
Animals
For each experiment commercial semen from 10 boars (Sus scrofa domesticus) strains AGPIC 337 (Agroceres Pic), NK 75 (Choice Genetics), LI 7600 SUPREMO (DB Genética Suína) and MS 115 (Embrapa) were kindly gifted by the Association of Pig Breeders of Santa Catarina (ACCS).
Sperm motility and concentration
Sperm (spzt) motility and concentration were evaluated soon after semen receipt. Sperm were then diluted in Beltsville Thawing Solution (BTS®) to give 200 μl solution containing 2×106 spzt/ml. Only samples with motility greater than 80% were used and were kept at 16–18°C until use. Both analyses were performed according to Brazilian School of Animal Reproduction preconditions (CBRA, 1998).
Flow cytometry
Experiments were carried out using a flow cytometer BD Accuri™C6 (Becton & Dickson, Santiago, Chile) coupled to an excitation source (488 nm) and three light filters (FL-1: 3533±30 nm; FL-2: 585±40 nm; FL-3: 675±25 nm). Plasma membrane damage (PMD) was determined using propidium iodide staining (PI; Sigma, 10 µg/ml). Acrosome damage rate (AD) was evaluated using FITC−PSA (FITC-conjugated lectin from Pisum sativum, Sigma 1.25 µg/ml). The mitochondrial membrane potential was measured using JC-1 as a probe (JC-1; Sigma-Aldrich 10 µg/ml) and the results were expressed as rate of sperm with low mitochondrial membrane potential (LMMP). Sperm chromatin integrity assay was carried out using acridine orange (Sigma) (Boe-Hansen et al., Reference Boe-Hansen, Morris, Ersboll, Greve and Christensen2005). DNA damage to sperm results were presented as rate of fragmented DNA (FDNA).
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was performed according to Bou’s protocol (Bou et al., Reference Bou, Sun, Lv, Zhu, Li, Wang, Li, Liu, Zheng, He, Kong and Liu2014), with adaptations. The tag for the plasmidial eGPF gene, a cyanine 5 (Cy5)-labelled probe was designed based on its genomic sequence (pmhyGenie5 maker). The chromosome SRY gene was designated as control and a cyanine 3 (Cy3)-labelled probe was designed based on GenBank database sequence (https://www.ncbi.nlm.nih.gov/nuccore/U49860.3?from=4546&to=5256&sat=4&sat_key=78588295&report=fasta). Both probes were obtained from Síntese Biotecnologia LTDA (Belo Horizonte, Brazil). For slide mounting, an anti-fade stain (Fluoroshield™ with DAPI, Sigma) was used for counterstaining, marking the sperm nuclei. Cells were examined on slides (100 cells/slide) using an epifluorescence microscope (Axio Observer A1, Carl Zeiss AG, Oberkochen, Germany).
Plasmid vector
The plasmid used in the transfection experiments was the self-inactivating hyperactive piggyBac transposase-based plasmid (pmhyGENIE-5) that encodes the eGFP gene. The plasmid was kindly donated by Dr Stefan Moisyadi from the University of Hawaii (Honolulu T, Hawaii, EUA).
Standardization and optimization of electroporation
Electroporation (EP) experiments were performed using semen samples diluted in 200 μl BTS (2×106 sptz/ml) and an EP device Multiporator® (Eppendorf AG. Hamburg, Germany). For EP, BTS was set at room temperature (22°C). For recovery plasma membrane integrity after EP, BTS was used at 17°C according to the protocol described by da Silva et al. (Reference da Silva, de Souza, Superti, Lima-Rosa and Marques2017).
Determination of voltage, pulse number and pulse duration for the electroporation
Different voltages (500 or 1000 volts), pulses (single or double pulse) and pulse duration (250 or 500 μs) were tested. Samples were then analyzed by flow cytometry to measure PI incorporation rate. Groups with major PI uptake were considered for use to test the effect of electroporation on pig sperm viability. The experimental steps are described in Fig. 1.
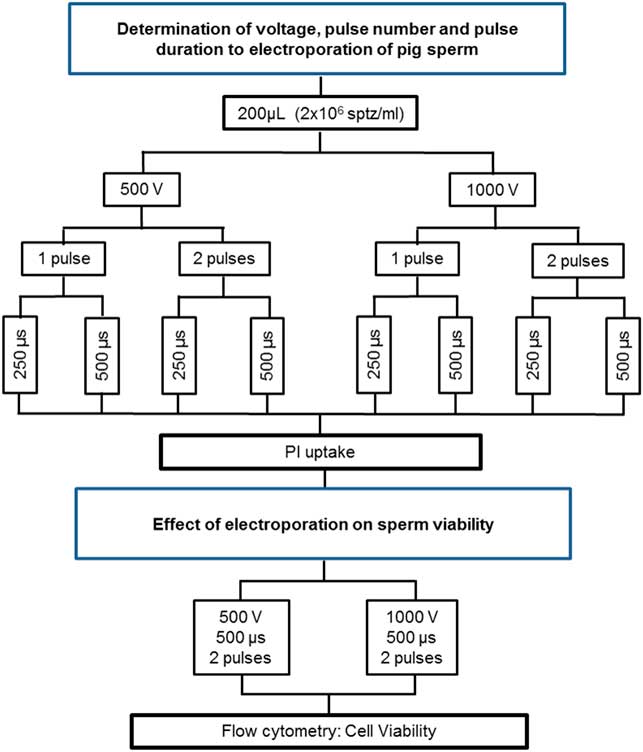
Figure 1 Flow chart of experiments performed to determine the best parameters for electroporation and to evaluate the effect of PEI on the sperm viability.
Effect of electroporation on viability of pig sperm
As described previously, samples were electroporated using the following conditions: EPα (500 volts, 500 μs and two pulses) or EPβ (1000 volts, 500 μs and two pulses). Sperm viability was assessed (PMD, AD, LMMP and FDNA). The experiment was performed as set out in Fig. 1.
Standardization and optimization of polyfection using PEI
Polyfection experiments were performed using semen samples diluted in 200 μl BTS (2×106 sptz/ml) using branched PEI with a molecular weight of 25 kDa and purchased from Sigma-Aldrich.
Determination of PEI concentration and incubation time using PEI/FITC
PEI was labelled with FITC according to Saito and Saitoh (Reference Saito and Saitoh2012) with minor modifications. To our knowledge there have been no previous studies reporting the use of PEI in sperm, therefore PEI concentration and incubation time used were based on a previous study on fibroblasts (Hsu and Uludag, Reference Hsu and Uludag2012). Different concentrations (0.5, 1, 2 and 4 mg/ml) of FITC-labelled PEI (PEI/FITC) at different time intervals (10 min, 2 h and 4 h) were tested. The PEI/FITC uptake was assessed using flow cytometry. The best PEI/FITC concentration and incubation time were chosen for subsequent experiments, as set out in Fig. 2.
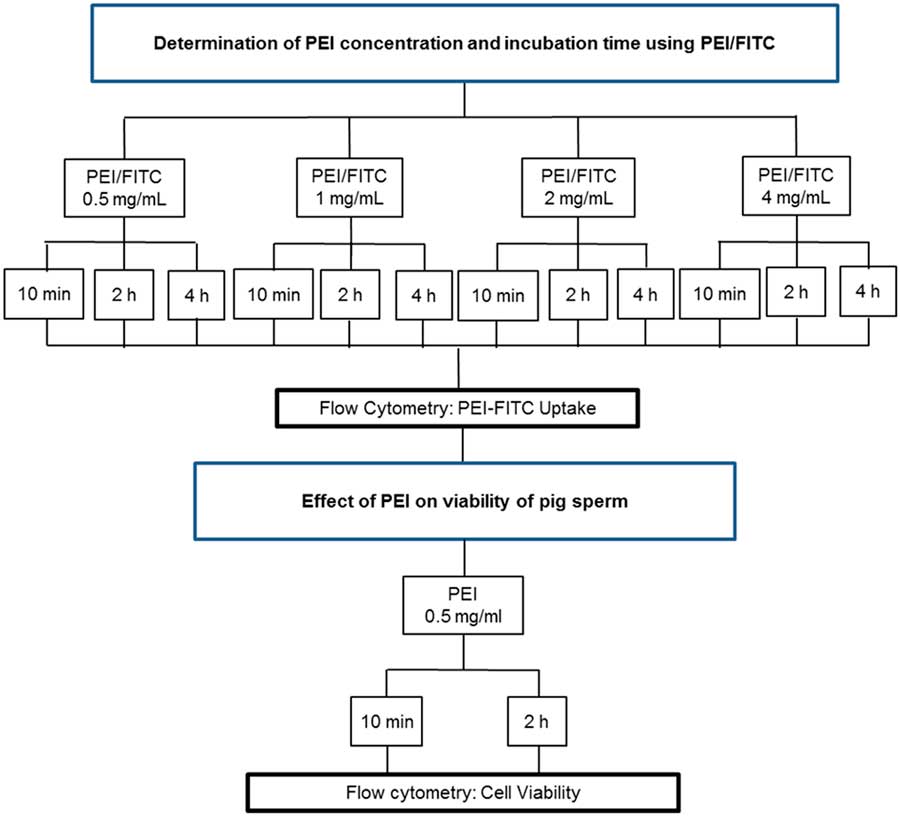
Figure 2 Flow chart of experiments performed to determine the best PEI concentration and evaluate the effect of PEI on the sperm viability.
Effect of PEI on viability of pig sperm
Based on the results of PEI/FITC uptake, a new flow cytometry experiment was carried out to evaluate sperm viability (PMD, AD, LMMP and FDNA) using the preferential PEI conditions (0.5 mg/ml, 10 min and 2 h). A control group (without PEI) was also evaluated and the experiment was performed as described in Fig. 2.
Evaluation of PLF and EP on sperm viability and efficiency after transfection with plasmid
For PLF, the plasmid was first complexed with PEI (PEI−plasmid). After dilution, each sample was incubated with 0.5 mg of PEI/plasmid (corresponding to 400 ng of plasmid per sample) for 10 min at 17°C. For EP, the complex was mixed with the samples and these were electroporated (500 volts, 500 μs and two pulses). The procedure described by Lavitrano et al. (Reference Lavitrano, Forni, Bacci, Di Stefano, Varzi, Wang and Seren2003) with modifications was used as the standard direct transfection method and included a control group (without transfection). Finally, plasmid uptake was quantified using FISH, as described previously. A new flow cytometry experiment was carried out to evaluate sperm viability (PMD, AD, LMMP and FDNA).
Statistical analysis
All data were submitted to statistical analysis using PROC MIXED from SAS 9.2® software (Statistical Analysis System, SAS Institute Incorporation, Cary, NC, USA) for Windows. LSMEANS (mean of the minimum squares) test was used to obtain the adjusted means for treatments, with comparisons using Tukey’s Test. The significance level used to reject H0 was 5%, that is a significance level less than 0.05 (P<0.05), was considered to indicate significant difference between analyzed variables or interactions between these variables.
Results
EP standardization and optimization
For electroporation (Fig. 3), all analysed parameters (voltage, pulse number and duration) showed no interaction. When analysed singly, no significant difference between pulse number and pulse duration was seen, however significant difference occurred between voltages (P=0.0276). When 500 volts were applied the PI incorporation rate was 33.55±3.89%, while for 1000 volts the PI incorporation rate was 45.97±3.89%. Therefore, to evaluate sperm viability, it was decided to use two groups: (1) 500 Volts, 500 μs and two pulses; and (2) 1000 Volts, 500 μs and two pulses, prior to PI incorporation.

Figure 3 Rates of PI uptake by electroporated sperm cells. The largest value indicates the most efficient electroporation parameters for sperm cell transfection. Data represent least squares means (LSM)±standard error of the mean (SEM). a,bDifferent letters indicate significant differences among groups (P<0.05).
For sperm viability (Fig. 4), there was no significant difference between groups when evaluating plasma membrane integrity. For acrosome integrity, the AD of the control group was 41.06±2.54%, with no significant difference for EPα (36.71±2.54%) (P=0.4957), but a significant difference for EPβ (55.77±2.54%) (P=0.0011). There was no significant difference between the EPα and EPβ groups (P<0.0001). The FDNA rate of the control group was 1.19±0.34%, with no significant difference for EPα (1.64±0.34%) (P=0.6185), but with significant difference for EPβ (2.53±0.34%) (P=0.0241). There was no difference between the EPα and EPβ groups (P=0.1684). Only EP, when subjected to a high electric currency, disrupted chromatin integrity when compared with the control group, however when a low electric currency was applied there was no increase in DNA damage rates. The LMMP rate of the control group was 15.47±2.32%, differing from EPα (−8.13±2.32%) (P<0.0001) and EPβ (−7.57±2.32%) (P<0.0001), however there was no significant difference between EPα and EPβ (P=0.9840). These results showed that EP increased mitochondrial membrane potential.

Figure 4 Cell viability of electroporated sperm cells. Rates of (A) acrosome damage, (B) plasmatic membrane damage, (C) fragmented DNA and (D) low mitochondrial membrane potential. Data represent least squares means (LSM)±standard error of the mean (SEM). a,bDifferent letters indicate significant differences among groups (P<0.05).
All these results provide evidence for efficient EP in pig sperm that allows high transfection rates coupled with low sperm loss, using a regime of 500 volts, 500 μs and two pulses. After EP, cell membrane integrity should be measured by incubation of electroporated sperms in BTS at 17°C.
PLF standardization and optimization
There was no correlation between concentration (0.5, 1.0, 2.0 and 4.0 mg/ml) and time (10 min, 2 and 4 h) (P=0.7317), therefore the effect of these variables was evaluated separately. As shown in Fig. 5A , use of different concentrations of PEI had no effect on the outcome. Only changes in incubation time showed significant effects in terms of PEI/FITC uptake. Figure 5B shows that a 10 min or 2 h incubation resulted in higher uptake (97.82±0.32% and 97.06±0.32%, respectively) but these rates did not differ significantly. Conversely, a significant reduction in uptake was observed after 4 h (86.89±0.32%, P<0.0001) compared with a 2 h incubation.
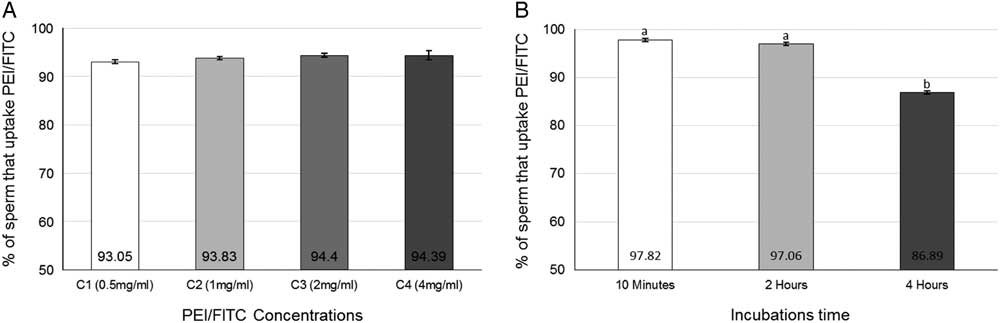
Figure 5 (A) Uptake of different concentrations of PEI-FITC. (B) Uptake of PEI-FITC in different incubation times. Data represent least squares means (LSM)±standard error of the mean (SEM). a,bDifferent letters indicate significant differences among groups (P<0.05).
For sperm viability (Fig. 6), no significant difference was observed among the groups for acrosome damage (AD), LMMP or fragmented DNA rates. The extent of PMD did not change after a 10 min incubation (18.27±2.70%) compared with the control group (19.48±2.70%), but an incubation time of 2 h increased the PMD (66.46±2.70%, P<0.0001).
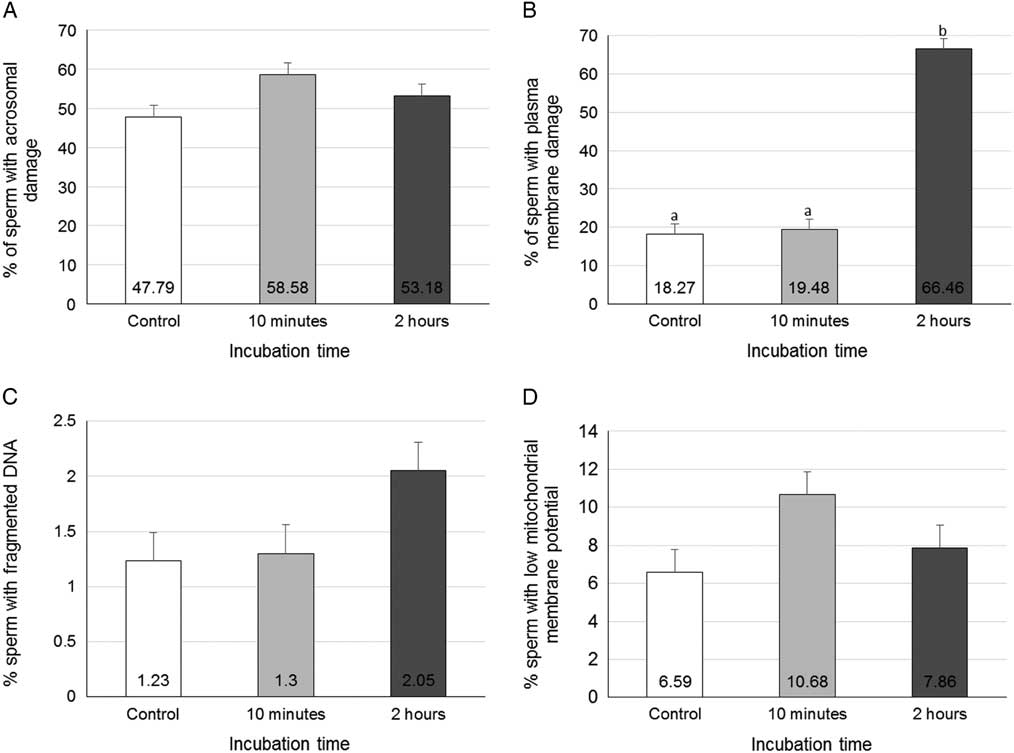
Figure 6 Cell viability of sperm cells treated with PEI. Rates of (A) acrosome damage, (B) plasmatic membrane damage, (C) fragmented DNA and (D) low mitochondrial membrane potential. Data represent least squares means (LSM)±standard error of the mean (SEM).a,bDifferent letters indicate significant differences among groups (P<0.05).
Polyfection versus electroporation comparison
Figure 7A shows transfection rates measured using FISH (Fig. 7B ). All treatment outcomes differed significantly (P<0.0001). Incubation, which is the standard method of SMGT and is used as a transfection control, showed a transfection rate of 17.80±1.07%, the EP transfection rate was 36.70±2.78% and PLF reached 76.8±3.09%. Therefore the developed protocols were very efficient for transfection of swine spermatozoa.
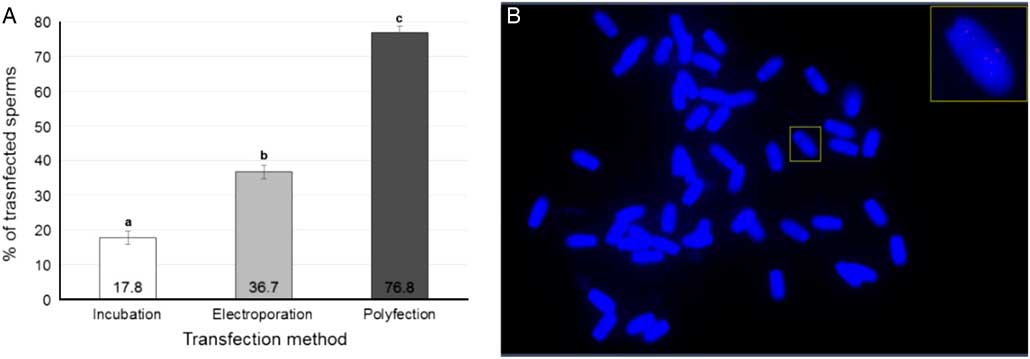
Figure 7 Transfected pig sperm. (A) Rates of transfected sperm cells in each transfection method. (B) FISH of transfected swine spermatozoa. Visualization of the plasmid vector pmhyGenie5, in Cy5 labelling, internalized to the sperma nucleus. Data represent least squares means (LSM)±standard error of the mean (SEM). a,b,cDifferent letters indicate significant statistical differences among groups (P<0.05).
Sperm viability (Fig. 8), assessed by flow cytometry, showed that each method altered each parameter differently. For acrosome integrity analysis (Fig. 8A ) the control (31.68±3.61%) showed no significant difference for direct incubation (19.49±3.61%) (P=0.247), but there were significant differences for PLF (70.79±3.68%) (P<0.001) and for the EP groups (71.82±3.68%) (P<0.001). Incubation time showed significant difference for PLF (P <0.001) and for the EP groups (P<0.001). PLF and EP groups did not differ between themselves (P=0.8306). For the plasma membrane (Fig. 8B ), there was no significant difference between the control (21.91±3.55%), incubation (20.6±3.81%) and EP (27.2±3.55%). These groups differed from the PLF group (66.97±3.55%) (P<0.001). Therefore, only PLF treatment presented a deleterious effect on the plasma membrane. In terms of DNA fragmentation rates (Fig. 8C ), only transfection by incubation (3.67±0.03%) showed a significant difference in relation to the other groups (P<0.001). Results for the control (0.58±0.08%), PE (1.27±0.29%) and PLF (0.81±0.16%) groups did not differ from each other. LMMP rates (Fig. 8D ) showed that the PLF group (37.46±2.02%) differed significantly from the other groups (P<0.0001). Incubation (23.07±1.88%) did not differ from the control (16.52±1.88%) (P=0.0211) nor from the EP (29.58±1.88%) (P=0.0942), however the results in the control and EP groups differed significantly from each other (P=0.0084).
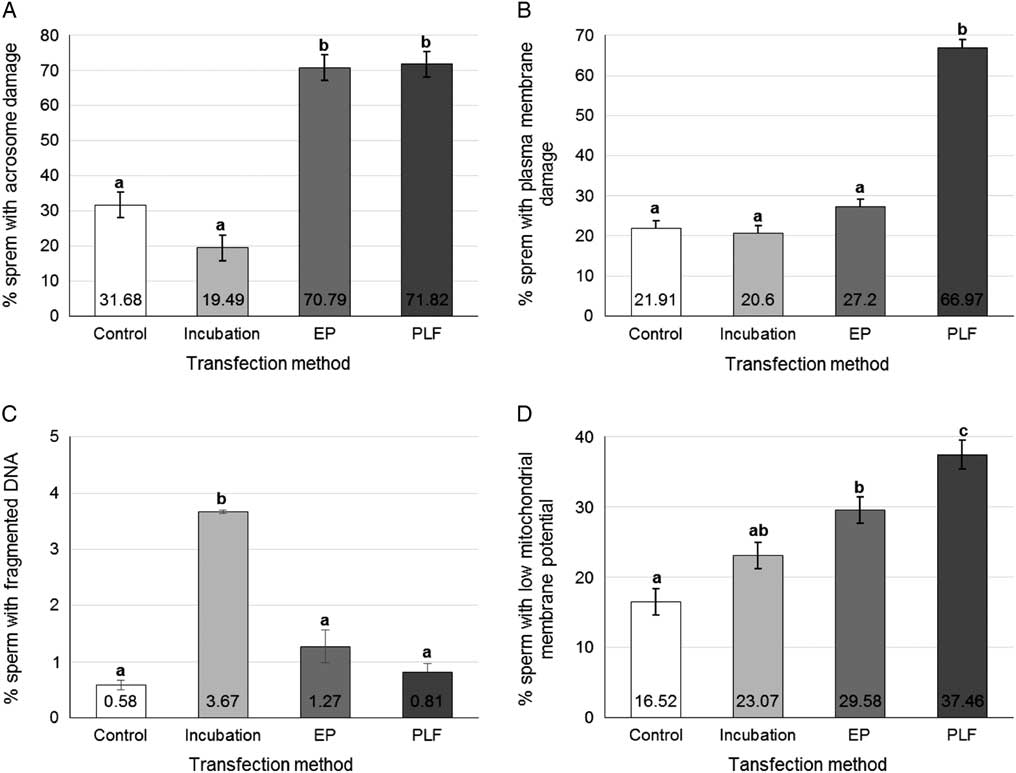
Figure 8 Sperm viability of transfected sperms. Rates of (A) acrosome damage, (B) plasmatic membrane damage, (C) fragmented DNA and (D) low mitochondrial membrane potential, in pig sperm transfected by different methods. Data represent least squares means (LSM)±standard error of the mean (SEM). a,b,c.Different letters indicate significant statistical differences among groups (P<0.05).
Discussion
The results presented for PEI-mediated polyfection were significantly higher than those obtained previously and those cited in the literature for other cell types. Hsu & Uludag (Reference Hsu and Uludag2012) utilized 10-fold higher PEI concentration for 2 to 6 h to maintain satisfactory levels of normal human foreskin fibroblast (NHFF; CRL2522) transfection in culture. These differences are due to several factors such as the type of cell culture (culture in suspension), which allows greater PEI−cell interaction. The spermatozoon internalizes DNA more easily than most cells. A recent study (Fang et al., Reference Fang, Chen, Wang, Wang, Li, Zhu, Wang and Zeng2017) used approximately 500 μl of 800 mg/ml plasmid solution and an equal volume (500 μl) of polyMAG-1000 for transfection of porcine spermatozoa, however transfection rates and sperm viability were not measured.
Incubation conditions used here did not cause damage to the cytoplasmic membrane, due to the use of BTS medium at 17°C, a condition for swine semen preservation. However, internalization of the plasmid occurred during this procedure (conventional SMGT method) via the MHC II and CD4 mechanism (Lavitrano et al., Reference Lavitrano, Maione, Forte, Francolini, Sperandio, Testi and Spadafora1997), which favours plasma membrane integrity.
PLF was the only method that negatively affected the plasma membrane. These data corroborate that of Hunter & Moghimi (Reference Hunter and Moghimi2010), who found that incubation of the cell with PEI for 1 h increased membrane damage by increasing phosphatidylserine translocation.
In the incubation method, internalization of the plasmid by the cell occurs preferentially in the post-acrosomal region where MHC II is expressed (Francolini et al., Reference Francolini, Lavitrano, Lamia, French, Frati, Cotelli and Spadafora1993). As a result, the acrosome remains intact. In addition, the medium and temperature conditions established in this study led to maintenance of acrosomal integrity, as the same conditions as those for preservation of cooled sperm semen were used.
Electroporation caused injury to the acrosome when the voltage was too high (1000 V) or in presence of the plasmid. Acrosome damage in spermatozoa occurs due to depolarization of the cell membrane (Schackmann et al., Reference Schackmann, Christen and Shapiro1981), and EP promotes this depolarization, which occurs at higher intensity at higher voltages. In addition, EP also opens pores on the acrosomal membrane; the plasmid (14.5 kb), because of its size, may have contributed to the acrosome lesion as it passed through the open pores in the membrane,
As occurred for the plasma membrane, PLF negatively affects acrosome integrity, however this effect was evidenced only in the presence of the plasmid. The biochemical pathway triggered by PEI passage across the acrosomal membrane as it enters the cell is the same pathway activated in the cytoplasmic membrane. The acrosome also contains phosphatidylserine on the outside of the membrane (Kurz et al., Reference Kurz, Viertel, Herrmann and Muller2005). Therefore, the phosphatidylserine pathway may affect both the acrosome membrane and the cytoplasmic membrane.
The occurrence of DNA fragmentation was verified by two conditions: electroporation at high voltage (1000 V) or transfection by incubation. After entering the cell nucleus, PEI can damage DNA by interacting with nucleic acids (Kafil & Omidi, Reference Kafil and Omidi2011). These authors evaluated chromatin integrity using the comet assay on PEI-treated A431 cells and found a high rate of DNA fragmentation (28.7±7.9% mV zeta potential). DNA damage associated with use of PEI was not evidenced in our study. This finding may be because, in spermatozoa, DNA is folded with protamine and condensed more densely compared with DNA packaged with histones, which gives greater protection to sperm DNA. Incubation negatively affected chromatin integrity, possibly due to sperm’s intrinsic ability to internalize eDNA into the nucleus and integrate it into its genome (Zoraqi & Spadafora, Reference Zoraqi and Spadafora1997).
Plasmid internalization occurs in the SMGT by an active process, which requires energy expenditure by spermatozoa. Mitochondria provide the energy, and are responsible for glucose metabolism. Uptake of larger size plasmids, like pmhyGenie-5, increases the amount of required energy. This requirement increases mitochondria activity, leading to a loss in mitochondrial membrane potential due to the greater effort made by the organelle to supply energy demand. Evidence of this is seen when using smaller plasmids; for a 4 kb plasmid there was no decrease in LMMP (Feitosa et al., Reference Feitosa, Milazzotto, Simoes, Rovegno, Nicacio, Nascimento, Goncalves, Visintin and Assumpcao2009) compared with the 14.5 kb plasmid used here. Possibly, for this reason, in the presence of the vector the transfection groups had increased spermatozoa with low mitochondria potential.
Transfection rate following incubation reached around 17.4%, which was similar to that found using the standard protocol (Lavitrano et al., Reference Lavitrano, Forni, Bacci, Di Stefano, Varzi, Wang and Seren2003), which produced 20% swine spermatozoa when the plasmid had been internalized in the nucleus. These authors, however, mentioned that the SMGT rate could reach 80%. The use of electroporation allowed a transfection rate significantly higher than that obtained by Horan et al. (Reference Horan, Powell, Bird, Gannon and Houghton1992), who achieved an increase of 5−10% in DNA uptake in electroporated swine spermatozoa. In the present study, we optimized previously used EP parameters to achieve a better outcome, and found an increase of almost 20% in eDNA uptake compared with the incubation procedure.
The percentage of spermatozoa transfected by PLF plus PEI was higher than that described by Hsu & Uludag (Reference Hsu and Uludag2012), who obtained success rates of 30−35%. The success obtained here was possibly due to optimization of parameters, therefore demonstrating the need to standardize protocols before use.
Our results showed that transfection using sperm incubation and plasmid pmhyGenie-5, despite presenting similar results to those found in the literature, gave the lowest transfection rates, whereas electroporation gave intermediate transfection rates and polyfection gave the highest transfection indices. Despite this, both methods, EP and PLF, caused acrosome, cytoplasmic or both, membrane lesions. Because of these lesions, we suggest that these methods may be best used in association with reproductive biotechniques, such as intracytoplasmic sperm injection or in vitro fertilization. In addition, the need for more studies aimed at alternatives to promote the recovery of spermatozoa after transfection, especially in relation to polyfection, is highlighted, as this is the first study to report its use in spermatozoa.
Conclusion
These results suggest that EP and PEI could be an efficient and low cost transfection method for swine sperm. Treated cells showed higher PMD and/or AD indices, therefore it would be interesting to combine this procedure with biotechniques that facilitate fecundation (i.e. in vitro fertilization or intracytoplasmic sperm injection) or even include antioxidants or anti-apoptotic drugs to improve spermatozoa viability.
Acknowledgements
The authors thank Dr Stephan Moysiad, from University of Hawaii, for donation of the pmhyGenie5 plasmid.
Financial support
Z.S. was supported by a masters scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. This project was supported by Empresa Brasileira de Pesquisa Agropecuária (Embrapa).
Conflicts of interest
There were no conflicts of interest.










