INTRODUCTION
Babesia bovis remains a significant health and economic problem for the cattle industry, causing infections with significant mortality and morbidity rates in semi-tropical and tropical regions worldwide (Bock et al. Reference Bock, Jackson, de Vos and Jorgensen2004). Despite important research efforts in recent years focused on the development of improved methods of control, vaccination with live attenuated parasites is still the most effective method of prevention (Brown and Palmer, Reference Brown and Palmer1999; Bock et al. Reference Bock, Jackson, de Vos and Jorgensen2004; deWaal and Combrink, Reference de Waal and Combrink2006). Nevertheless, numerous B. bovis antigens with potential for development of recombinant subunit vaccines have been identified and some tested in vaccine trials, including the rhoptry associated protein-1 (RAP-1) (Norimine et al. Reference Norimine, Mosqueda, Suarez, Palmer, McElwain, Mbassa and Brown2003). Although RAP-1 is a component in a partially protective multi-antigen vaccine (Wright et al. Reference Wright, Casu, Commins, Dalrymple, Gale, Goodger, Riddles, Waltisbuhl, Abetz, Berrie, Bowles, Dimmock, Hayes, Kalnins, Leatch, McGrae, Montague, Nisbet, Parrodi, Peters, Scheiwe, Smith, Rode-Bramanis and White1992), neither full size nor truncated recombinant RAP-1 induced protection against challenge with virulent B. bovis, despite eliciting strong T-cell and B-cell immune responses in cattle vaccination trials (Norimine et al. Reference Norimine, Mosqueda, Suarez, Palmer, McElwain, Mbassa and Brown2003).
The rap-1 gene family of Babesia occurs in all babesial species examined to date (Goff et al. Reference Goff, Davis, Palmer, McElwain, Johnson, Bailey and McGuire1988; Suarez et al. Reference Suarez, Palmer, Jasmer, Hines, Perryman and McElwain1991a; Dalrymple et al. Reference Dalrymple, Casu, Peters, Dimmock, Gale, Boese and Wright1993; Ikadai et al. Reference Ikadai, Xuan, Igarashi, Tanaka, Kanemaru, Nagasawa, Fujisaki, Suzuki and Mikami1999; Skuce et al. Reference Skuce, Mallon and Taylor1996; Kappmeyer et al. Reference Kappmeyer, Perryman, Hines, Baszler, Katz, Hennager and Knowles1999; Zhou et al. Reference Zhou, Jia, Nishikawa, Fujisaki and Xuan2007). All members of this family have well-defined molecular features such as the presence of a signal peptide, strict conservation of 4 cysteine residues, a 14 amino-acid motif and several other short motifs present in the first 300 amino acids of the molecules (Suarez et al. Reference Suarez, Palmer, Jasmer, Hines, Perryman and McElwain1991a,Reference Suarez, McElwain, Stephens, Mishra and Palmerb; Reference Suarez, Thompson, McElwain, Hines and Palmer1994; Dalrymple et al. Reference Dalrymple, Peters, Böse and Wright1996). In B. bovis, only 2 identical rap-1 genes were initially identified (Dalrymple et al. Reference Dalrymple, Casu, Peters, Dimmock, Gale, Boese and Wright1993; Suarez et al. Reference Suarez, Palmer, Hötzel and McElwain1998). However, in B. bigemina the rap-1 gene family is present as a complex tandem array of 3 different types of rap-1a, rap-1b and rap-1c genes (Suarez et al. Reference Suarez, Palmer, Florin-Christensen, Hines, Hötzel and McElwain2003). In this parasite, the 3 types of rap-1 genes are transcribed in merozoites, but only the expression of RAP-1a proteins was demonstrated, suggesting the existence of mechanisms that tightly regulate the expression of rap-1 genes. Interestingly, genome sequencing revealed that genes encoding the characteristic RAP-1 motifs are also present in the genome of the related apicomplexan parasites Theileria parva and T. annulata (Pain et al. Reference Pain, Renauld, Berriman, Murphy, Yeats, Weir, Kerhornou, Aslett, Bishop, Bouchier, Cochet, Coulson, Cronin, de Villiers, Fraser, Fosker, Gardner, Goble, Griffiths-Jones, Harris, Katzer, Larke, Lord, Maser, McKellar, Mooney, Morton, Nene, O'Neil, Price, Quail, Rabbinowitsch, Rawlings, Rutter, Saunders, Seeger, Shah, Squares, Squares, Tivey, Walker, Woodward, Dobbelaere, Langsley, Rajandream, McKeever, Shiels, Tait, Barrell and Hall2005; Gardner et al. Reference Gardner, Bishop, Shah, de Villiers, Carlton, Hall, Ren, Paulsen, Pain, Berriman, Wilson, Sato, Ralph, Mann, Xiong, Shallom, Weidman, Jiang, Lynn, Weaver, Shoaibi, Domingo, Wasawo, Crabtree, Wortman, Haas, Angiuoli, Creasy, Lu, Suh, Silva, Utterback, Feldblyum, Pertea, Allen, Nierman, Taracha, Salzberg, White, Fitzhugh, Morzaria, Venter, Fraser and Nene2005). However, except for the highly related Babesia and Theileria intra-erythrocytic parasites, comprehensive or Blast-assisted database searches have failed to demonstrate conservation of the typical RAP-1 motifs in other available microbial or eukaryotic genomes to date.
Typically, RAP-1 molecules are highly immunogenic and Babesia-infected animals normally mount high levels of anti-RAP-1 antibodies, a feature that was utilized for the development of several RAP-1-based methods for serological diagnosis (Boonchit et al. Reference Boonchit, Xua, Yokoyama, Goff, Wagner and Igarashi2002, Reference Boonchit, Xuan, Yokoyama, Goff, Waghela, Wagner and Igarashi2004; Goff et al. Reference Goff, Molloy, Johnson, Suarez, Pino, Rhalem, Sahibi, Ceci, Carelli, Adams, McGuire, Knowles and McElwain2006; Zhou et al. Reference Zhou, Jia, Nishikawa, Fujisaki and Xuan2007). Yet, despite extensive molecular and immunological characterization, the functional role of the members of the Babesia rap-1 gene family remains mostly undefined. It was shown that RAP-1 proteins are expressed at least in the sporozoite and merozoite stages of B. bovis (Mosqueda et al. Reference Mosqueda, McElwain, Stiller and Palmer2002). In addition, consistent with their rhoptry localization (Sam-Yellowe, Reference Sam-Yellowe1996), experimental evidence suggests that RAP-1 proteins are involved in erythrocyte invasion. This is supported by the observations that B. bovis RAP-1 is able to bind the surface of erythrocytes (Yokoyama et al. Reference Yokoyama, Suthisak, Hirata, Matsuo, Inoue, Sugimoto and Igarashi2002), and antibodies against RAP-1 are able to partially block invasion of bovine erythrocytes by merozoites and sporozoites (Yokoyama et al. Reference Yokoyama, Suthisak, Hirata, Matsuo, Inoue, Sugimoto and Igarashi2002; Mosqueda et al. Reference Mosqueda, McElwain, Stiller and Palmer2002).
Taking into account the limitations previously shown by recombinant versions of RAP-1 as vaccine candidates and partial neutralization data, together with its limited capacity for sequence variation (Suarez et al. Reference Suarez, Palmer, Hötzel and McElwain1998), suggests that expression of RAP-1-related molecules is required for B. bovis survival in the face of the strong anti-RAP-1immune responses known to occur in vaccinated or chronically infected cattle. Thus, we hypothesize that redundant and poorly immunogenic molecules containing the RAP-1 strictly conserved motifs could function as equivalents of RAP-1, thus providing alternative ways to contribute to erythrocyte invasion and/or egression by B. bovis, and overall to the survival of the parasite during infection.
In this report we describe the molecular and antigenic characterization of a novel RAP-1-related antigen (RRA) encoded in the genome of B. bovis (Brayton et al. Reference Brayton, Lau, Herndon, Hannick, Kappmeyer, Berens, Bidwell, Brown, Crabtree, Fadrosh, Feldblum, Forberger, Haas, Howell, Khouri, Koo, Mann, Norimine, Paulsen, Radune, Ren, Smith, Suarez, White, Wortman, Knowles, McElwain and Nene2007). RRA contains all the signature motifs found in all members of the rap-1 gene family of Babesia; it is encoded by a single copy gene, is highly conserved among geographical isolates of B. bovis, contains neutralization sensitive epitopes, and is expressed in B. bovis merozoites both in vitro and during infection in cattle.
MATERIALS AND METHODS
Parasites
The Mo7 biological clone of B. bovis was derived by limiting dilution of the Mexico strain as described elsewhere (Rodriguez et al. Reference Rodriguez, Buening, Gree and Carson1983; Hines et al. Reference Hines, McElwain, Buening and Palmer1989) and maintained as a cryopreserved stabilate in liquid nitrogen (Palmer et al. Reference Palmer, Buening and Carson1982). The Argentina strains R1A and S2P (Anziani et al. Reference Anziani, Guglielmone, Abdala, Aguirre and Mangold1993) (kindly provided by Ignacio Echaide, INTA-Rafaela) and the Texas B. bovis strain T2Bo have been previously described (Goff et al. Reference Goff, Davis, Palmer, McElwain, Johnson, Bailey and McGuire1988). The Mexican strain of B. bovis used for infecting steer C97 was described previously (Brown et al. Reference Brown, Zhao, Rice-Ficht, Logan and Woods1992). Parasites were grown in long-term microaerophilus stationary-phase culture by previously described techniques (Levy and Ristic, Reference Levy and Ristic1980; Hines et al. Reference Hines, McElwain, Buening and Palmer1989).
DNA and RNA analysis, cloning and sequencing
Genomic DNA was extracted from cultured merozoites by the standard phenol-chloroform procedure. Sequencing was performed with a Prism Ready Reaction DyeDeoxy Terminator cycle sequencing kit and read with an ABI PRISM 373 genetic analyzer (Applied Biosystems). Protein secondary structure analysis and B cell epitope prediction were performed using the BCM Search Launcher: Protein Secondary Structure Prediction available at http://searchlauncher.bcm.tmc.edu/seq-search/struc-predict.html. Phylogenetic analysis was performed using the program Phylip available at the following website: http://www.genebee.msu.su/services/phtree_reduced.html. Total RNA was extracted with TRiZol (Invitrogen) following the manufacturer's instructions. RT-PCR was performed using total RNA as described previously (Suarez et al. Reference Suarez, Palmer, Florin-Christensen, Hines, Hötzel and McElwain2003), and RRA mRNA was amplified using primers RR-f and RR-r described below.
Synthetic peptides, expression of proteins, and production and detection of antibodies
The open reading frame (ORF) of rra was amplified from DNA extracted from the Mo7 strain by PCR using primers RR-f (5-atg aca aat tgt tat ttc atg-3′) and RR-r (5′-tat att gtt tat gtt tga tgc-3′). PCR-amplicons were cloned into the vector pBAD/Thio-Topo (Invitrogen) for expression of recombinant RRA (rRRA). Primers RR-f and RR-r were designed to allow in-frame cloning of the inserts into the vector to produce expressed thioredoxin fusion proteins. Inclusion bodies from bacteria induced with 0·2% arabinose were prepared by sonication and high-speed centrifugation and dissolved in 6 m urea, 0·15 m NaCl, 0·1 m Tris-HCl, pH 8·0. Solubilized protein was purified by affinity chromatography on Ni++columns. Relative antigen purity was confirmed in Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels. It must be noted that the predicted size of the recombinant RRA, as shown in Fig. 4A (∼56 kDa) is increased ∼15 kDa when compared to the predicted size of the native protein (∼41 kDa) due to the presence of a ∼15 kDa thioredoxin domain at the N-terminal end and a HIS-tag at the C-terminal end of the recombinant protein. The synthetic peptide sRRA-1: RHM EGK RFR SKS KSI TLR RQQ SES YGW DKM KGN KKA KE represents a combination of 3 predicted B-cell epitopes of RRA encompassed between amino acids 146–159; 300–312; and 322–336 of the putative rra orf. The synthetic peptide sRRA-1 was coupled to KLH via glutaraldehyde, and used for rabbit immunization, performed by Affinity BioReagents. Briefly, 1 rabbit was inoculated by the intramuscular route with 250 μg of conjugated KLH-peptide mixed in an equal volume of complete Freund's adjuvant. Two further immunizations were similarly performed every 2 weeks with KLH-peptide mixed in incomplete Freund's adjuvant. The anti-sRRA-1 rabbit serum from the last bleeding (collected 3 weeks after the last immunization) was used in the immunoprecipitation, immunofluorescence and neutralization assays. Metabolic labelling of B. bovis merozoites in in vitro cultures with [35S]-methionine followed by immunoprecipitations, Western-blot analysis, and fixed immunofluorescence was performed as described previously (Johnson et al. Reference Johnson, Perryman and Goff1997; Suarez et al. Reference Suarez, Palmer, Florin-Christensen, Hines, Hötzel and McElwain2003; Goff et al. Reference Goff, Davis, Palmer, McElwain, Johnson, Bailey and McGuire1988 respectively). To minimize non-specific reactivity with E. coli antigen present in the rRRA protein in Western blot analysis, serum samples were incubated for 2 h at 37°C with 50 μg/ml of a lysate of E. coli TOP10 cells transformed with the pBAD/TOPO® ThioFusion™ vector (previously ligated with the B. bovis non-related gene, Am780 from Anaplasma marginale (Brayton et al. Reference Brayton, Kappmeyer, Herndon, Dark, Tibbals, Palmer, McGuire and Knowles2005)). The purified rAm780 protein was used as an irrelevant recombinant protein control in the Western blot analysis shown in Fig. 4A.
Babesia bovis-infected cattle
Cow C97 (Brahman×Angus cross) was infected with the Mexico strain of B. bovis (Brown et al. Reference Brown, Logan, Wagner and Tetzlaff1991) and, upon the onset of clinical signs of acute babesiosis, was treated with 3·0 mg of berenil (diaminazine aceturate: Sigma)/kg of body weight as previously described (Brown et al. Reference Brown, Logan, Wagner and Tetzlaff1991). For immunoprecipitation, serum from a B. bovis T2Bo-hyperimmunized steer was used, as previously described (Johnson et al. Reference Johnson, Perryman and Goff1997). Serum samples from 3 field B. bovis-infected cattle in Argentina as diagnosed by immunonofluorescence, were kindly provided by Dr Ignacio Echaide.
T-cell proliferation assay
A short-term T-cell line (CL) was generated by stimulating peripheral blood mononuclear cells (PBMC) derived from a B. bovis-infected animal (C97) with B. bovis-membrane and organelle-enriched pellet (CM) for 1 week and resting for 1 week without antigen in the presence of irradiated autologous PBMC as described (Norimine et al. Reference Norimine, Suarez, McElwain, Florin-Christensen and Brown2002). For proliferation assays, 3×104 T-cells and 2×105 irradiated autologous PBMC were cultured with antigen in a total volume of 100 μl of complete RPMI 1640 medium in triplicate wells of round-bottom 96-well plates at 37°C in a 5% CO2-humidified atmosphere. Antigens used in the proliferation assays were CM, recombinant RAP-1, recombinant RRA, and control recombinant protein MSP-5 (A. marginale). All recombinant proteins were produced and purified with the same methodology described for RRA. To measure proliferation, cells were incubated for the final 18 h of culture with 0·25 μCi of [3H]thymidine (Dupont, New England Nuclear, Boston, MA, USA), after which, radio-isotope labelled nucleic acids were harvested onto glass filters and counted in a beta counter.
In vitro neutralization test
Inhibition of B. bovis merozoites was performed as described elsewhere (Suarez et al. Reference Suarez, Florin-Christensen, Hines, Palmer, Brown and McElwain2000). Briefly, 5×105 Mo7 B. bovis merozoites were incubated with pre-immune or anti-sRRA-1 rabbit immune sera, diluted 1:5 in culture medium, for 30 min at 4°C. An equal volume of 5% (v/v) of bovine erythrocytes in culture medium was added prior to incubation in triplicate wells of 96-well plates at 37°C in a 5% CO2 atmosphere. The percentage of parasitized erythrocytes (PPE) was determined after 24, 48 and 72 h by microscopical examination of 2000 erythrocytes in Giemsa-stained smears prepared from each well. Results from 3 replicates were analysed by one-tailed Student's t-test with a P value of <0·05 and a Bonferroni correction for multiple comparisons.
RESULTS
The Babesia bovis T2Bo genome sequence contains a previously uncharacterized RAP-1- related gene
Blast searches of the T2Bo B. bovis genome with the B. bovis RAP-1 predicted amino acid sequence resulted in highly statistically significant identity (Blast E value: 2e−20) with the predicted ORF of a previously unknown gene (NCBI Reference Sequence: XP_001610950.1). Virtual translation of the protein encoded by this gene, here termed RAP-1-related antigen (RRA), revealed an ORF of 349 amino acids, predictive of a 41·5 kDa protein containing a signal peptide of 22 amino acids. The RRA predicted amino acid sequence shows 30% identity and 55% overall homology when compared to the B. bovis RAP-1. Sequence alignment of both ORFs is shown in Fig. 1A. The regions of sequence identity amongst these 2 ORFs are restricted to the first 300 amino acids of the proteins and they include the strict conservation of 4 cysteine residues (Fig. 1A). Additionally, there is partial conservation of other RAP-1 signature motifs, such as the 14-mer sequence (Suarez et al. Reference Suarez, Palmer, Jasmer, Hines, Perryman and McElwain1991a, Reference Suarez, McElwain, Stephens, Mishra and Palmerb) (Fig. 1A), while RRA lacks the series of repeats present in the C-terminal end of the B. bovis RAP-1 (Suarez et al. Reference Suarez, Palmer, Jasmer, Hines, Perryman and McElwain1991a). Both rap-1 genes and the rra gene are located on chromosome 4. However, the rra gene is located approximately 90 kbp downstream from the rap-1 locus, thus not closely linked in the B. bovis genome (Brayton et al. Reference Brayton, Lau, Herndon, Hannick, Kappmeyer, Berens, Bidwell, Brown, Crabtree, Fadrosh, Feldblum, Forberger, Haas, Howell, Khouri, Koo, Mann, Norimine, Paulsen, Radune, Ren, Smith, Suarez, White, Wortman, Knowles, McElwain and Nene2007). The rra gene is not flanked by other rap-1 related or any predicted surface protein sequences. Phylogenetic analysis of all known RAP-1 sequences from Babesia, using MSA-1 as an outlier (Fig. 1B) shows 2 major clusters of RAP-1 sequences, with one of the branches containing RAP-1b of B. bigemina and RRA, suggesting that RRA is more closely related to the RAP-1b sequence from B. bigemina than to B. bovis RAP-1. Blast searching of the T2Bo genome using the RRA sequence indicates that this strain contains a single gene copy of the rra gene. To determine whether the RRA sequence is conserved among geographically distinct B. bovis strains, we compared the sequences of the rra gene in the T2Bo (Texas) and the Mo7 strains with rra-PCR amplicons obtained from the R1A and S2P strains from Argentina. The predicted amino acid sequence of RRA is strictly conserved (100% identity) among all strains analysed (data not shown), thus similar to RAP-1, RRA appears to be highly conserved among distinct geographical isolates of B. bovis.

Fig. 1. (A) Sequence comparison between RAP-1 and RRA from Babesia bovis. Conserved cysteine residues are indicated with red arrows. The 14 amino acid RAP-1 conserved region among RAP-1 and RRA (14mer) is underlined. (B) Phylogram of the amino acid sequences from all known ORFs derived from rap-1 gene family of B. bovis, B. divergens, B. canis, B. ovis and B. bigemina, produced using the program Phylip with bootstrap values indicated at each branch point. The B. bovis MSA-1 amino acid sequence was used as an outlier. The most parsimonious tree is shown. GenBank Accession numbers are: B. bovis rra: XP_001610950.1; B. bovis rap-1 (bovis): AAB84267; B. caballi rap-1 (caballi): BAA83725; B. divergens rap-1 (divergens): Z49818; B. canis rap-1 (canis): CAA01285; B. ovis rap-1 (ovis): AAA27805; B. bigemina rap-1a-p58 (Bg-1a): 1906304A; B. bigemina rap-1b (Bg-1b): AAB72094; B. bigemina rap-1c (Bg-1c): AAN84523; and B. bovis msa-1 (msa-1-bov): AAK07773.
Expression in Babesia bovis merozoites and immunogenicity of RRA
We initially analysed the presence of rra transcripts in cultured merozoites by Northern blot analysis on total RNA from B. bovis Mo7 merozoites, using rap-1 and rra specific probes. Intense hybridization bands were evident in the Northern blot using the rap-1 specific probe whereas a weaker signal was detected using the rra probe (red box, Fig. 2A). In control dot blots, the 2 dig-labelled probes generated comparable signals (data not shown).
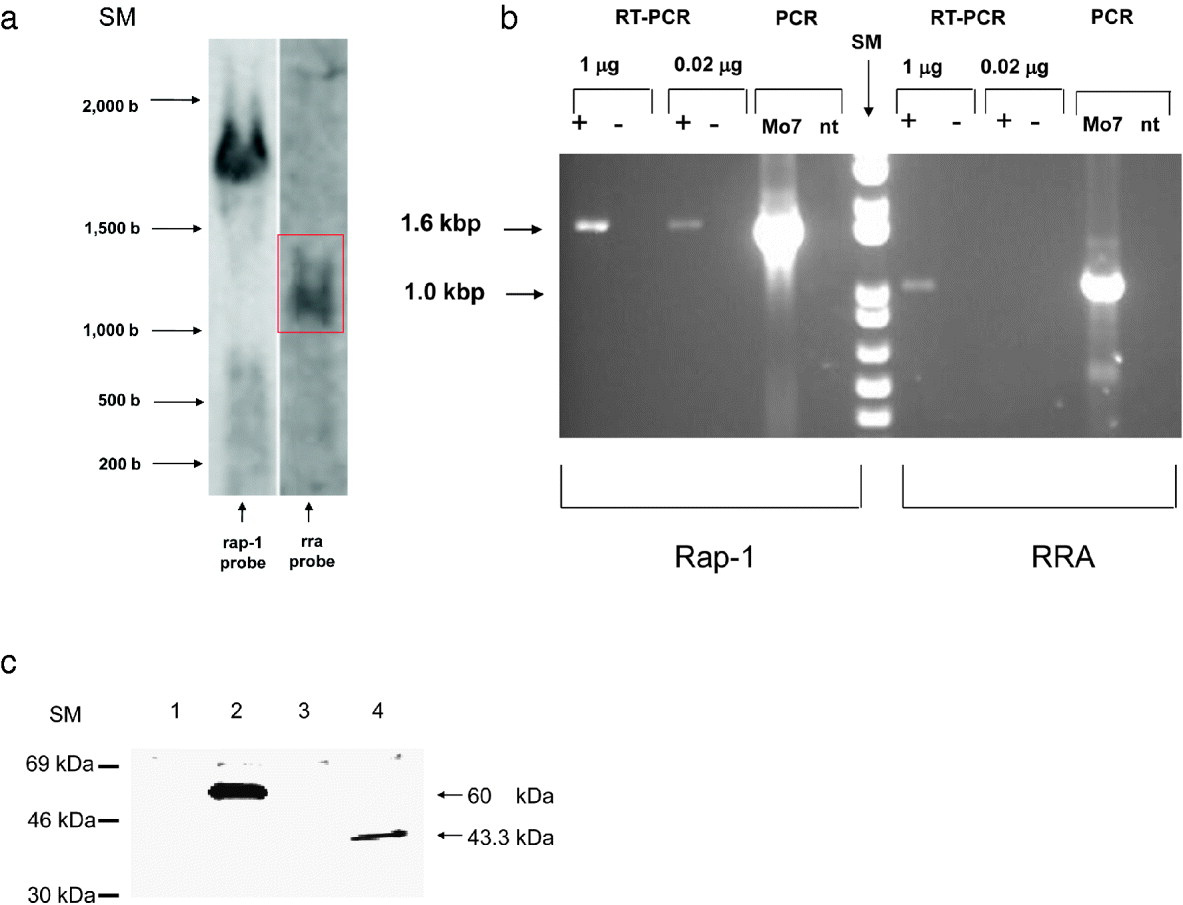
Fig. 2. Expression of RRA. (A). Northern blot analysis. Total RNA extracted from Babesia bovis Mo7 strain merozoites was separated in a 2% denaturing agarose gel, transferred to a Nylon membrane and hybridized with dig-labelled rap-1 (left panel) and rra (right panel) probes. Size markers in base numbers are marked on the left. (B) RT-PCR amplification of the rap-1 and rra ORFs from total RNA extracted from B. bovis Mo7 strain merozoites and control PCR amplifications of genomic DNA from B. bovis Mo7 strain merozoites, and no template (nt). +: reverse transcriptase reactions;-: control reactions without reverse transcriptase. The set of primers used for each PCR reaction is indicated at the bottom. Size markers (SM) in base pairs are indicated on the left. (C) Immunoprecipitation of [35S]-methionine metabolically labelled B. bovis proteins with negative control mouse monoclonal antibody Tryp (lane 1), monoclonal antibody BABB75 against RAP-1 (lane 2), control pre-immune rabbit serum (lane 3), and serum from a rabbit immunized with sRRA-1, a synthetic peptide representing putative B-cell epitopes of RRA (lane 4). Position of size markers (expressed in kDa) are indicated on the left. The size of the immunoprecipitated proteins is shown on the right.
To confirm transcription of the rra gene in B. bovis merozoites and to estimate relative amounts of rap-1 and rra transcripts, RT-PCR was performed using either 1 μg or 0·02 μg of total B. bovis merozoite RNA using rap-1 and rra specific primers (Fig. 2B). A stronger ∼1·7 kbp band was obtained when using control rap-1 specific primers in RT-PCR amplification experiments on 1 μg of total RNA compared to the ∼1 kbp rra amplicon (Fig. 2B). However, when using 50-fold diluted total RNA (0·02 μg), no amplification products were obtained for the rra transcript, whereas the ∼1·7 kbp rap-1 transcript was still amplified. The ∼1·0 kbp RT-PCR product obtained for rra was identical in size to the product of PCR amplification of genomic DNA using identical sets of primers (Fig. 2B). No amplification product was obtained when the RT was omitted in the RNA PCR amplifications, or when the reaction was performed in the absence of genomic DNA (Fig. 2B). Sequencing of the rra-RT-PCR amplification product showed 100% identity with the rra gene. Thus, taken together, these results confirmed that cultured B. bovis merozoites contain rra transcripts, but the data suggest that rra transcripts are relatively less abundant than rap-1 transcripts.
To confirm expression of the RRA protein in B. bovis merozoites, we performed immunoprecipitation of 35S metabolically labelled parasite proteins using rabbit antibodies prepared against sRRA-1, a synthetic peptide representing putative antigenic regions of RRA. The rabbit anti- sRRA-1 peptide antibodies precipitated a metabolically labelled B. bovis antigen of ∼43 kDa (Fig. 2C, lane 4), a size that is consistent with the predicted molecular weight of the RRA ORF. Immunoprecipitation of RAP-1 with anti-RAP-1 monoclonal antibody BABB75A4 is shown as a control on lane 2. No labelled products were precipitated by monoclonal control antibody or by rabbit pre-immune sera (lanes 1 and 3 respectively).
Fixed IFA using the rabbit antibodies directed against the synthetic sRRA-1 peptide and with the monoclonal antibody BABB75A4 reactive with RAP-1 are shown in Fig. 3. IFA revealed reactivity of the anti-sRRA-1 antibodies with B. bovis-infected erythrocytes only when incubated at a low antiserum dilution (1:10). Higher dilutions of the antiserum (1:100) failed to show significant fluorescence (not shown). Taken together, the transcription analysis, immunoblot and IFA data show that the RRA is expressed in B. bovis merozoites. Additionally, the results suggest that RRA is present at lower levels when compared to RAP-1 in cultured merozoites.

Fig. 3. Immunofluorescence analysis of Babesia bovis Mo7 merozoites in bovine erythrocyte culture using anti sRRA-1 rabbit antibodies (top left panel), or control pre-immune rabbit serum (top right panel) diluted 1:10, and anti-RAP-1 monoclonal antibody BABB75A4 (bottom left panel) or control monoclonal antibody Tryp (bottom right panel) used at 20 μg/ml.
We further tested the ability of sera from a B. bovis hyperimmune cow to recognize recombinant RRA in Western blots, which showed a faint band at the correct molecular weight (Fig. 4A, lane 4). This result also confirms RRA expression by B. bovis parasite stages that occur in infected cattle. However, additional immunoblots performed with 3 sera from naturally infected cattle that recognized RAP-1, failed to recognize recombinant RRA (data not shown).

Fig. 4. Immunogenicity of RRA. (A) Antibodies from hyperimmune Babesia bovis specific serum weakly recognize recombinant RRA in a Western blot. Lane 1, uninfected erythrocytes. Lane 2, B. bovis-infected erythrocytes. Lane 3, irrelevant recombinant control protein rAm780. Lane 4, recombinant RRA. The locations of the size markers (SM) are indicated on the left, and the calculated size of the product recognized by antibodies is indicated on the right with an arrow. (B). T-cell stimulation. A short-term T-cell line (CL) was generated by stimulating peripheral blood mononuclear cells derived from a B. bovis-immune animal C97 with B. bovis membrane- and organelle-enriched pellet (CM) for 1 week and rested for 1 week. The proliferation assay was carried out using 3 concentrations of CM, recombinant RAP-1, recombinant RRA, and negative control recombinant MSP-5 from A. marginale. Results are expressed as mean cpm (counts per minute) incorporated into 3 parallel cultures +/−S.D.
To further analyse CD4 T-cell responses to the RRA, we tested the ability of the recombinant RRA to stimulate B. bovis-immune T-cells ex vivo. As shown in Fig. 4B, no significant stimulation was obtained using recombinant RRA as antigen whereas both B. bovis RAP-1 and crude parasite membrane antigens strongly stimulated T-cell proliferation in the same assay. Collectively, these data suggest that in contrast to RAP-1, RRA acts as a subdominant antigen during in vivo infection of cattle. However, T-cells from only 1 B. bovis immune animal were available for testing in this study, and further analysis of a larger population of B. bovis immune cattle is required.
The RRA contains neutralization-sensitive epitopes
To determine the possible functional relevance of RRA, we tested whether rabbit antibodies against sRRA-1 containing predicted B-cell epitopes that were shown to immunoprecipitate a native ∼43 kDa antigen, could inhibit B. bovis infection of erythrocytes using in vitro neutralization assays. Incubation of free merozoites with rabbit antiserum specific for sRRA-1 resulted in a statistically significant decrease of PPE of in vitro cultures when compared with pre-immune rabbit serum, with P values of 0·00236 and 0·0113 at 3 and 4 days of culture, respectively (Fig. 5). When comparing medium with antiserum specific for sRRP-1, there was a significant difference at day 4, with a P value of 0·00056, but not at day 3 (P=0·03). These results suggest that the sequence in the synthetic peptide contains B-cell epitopes that are neutralization-sensitive and support that, similar to RAP-1, RRA might play an important role in the process of invasion of red blood cells.
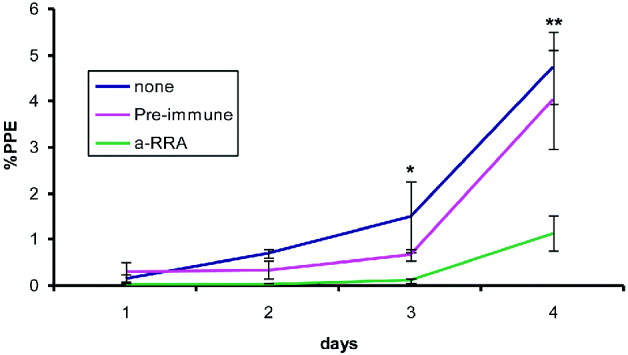
Fig. 5. In vitro growth curves. Babesia bovis strain Mo7 merozoites were exposed either to pre-immune rabbit serum, anti-sRRA-1 peptide rabbit serum, or no sera, and incubated with bovine erythrocytes and cultured for 4 days, as described in the Materials and Methods section. PPE in each culture well were calculated every 24 h. The data show the mean±1S.D. of the percentage of infected erythrocytes in 3 independent assays. *P<0·025 for control vs anti-rRRP-1 sera; **P<0·025 for all comparisons.
DISCUSSION
A gene that codes for a RAP-1-like protein was identified in the B. bovis genome and termed RAP-1 related antigen (RRA). Interestingly there are parallels between the RRA of B. bovis and its putative orthologue in B. bigemina, RAP-1b (Suarez et al. Reference Suarez, Palmer, Florin-Christensen, Hines, Hötzel and McElwain2003). However, in contrast to what was previously found in B. bigemina where multiple copies of tandemly arranged rap-1b genes are present (Suarez et al. Reference Suarez, Palmer, Florin-Christensen, Hines, Hötzel and McElwain2003), the rra gene of B. bovis is present as a single copy and it is not closely linked to the rap-1 locus.
Similar to rra, B. bigemina rap-1b transcripts are relatively less abundant than rap-1a, and rap-1b transcripts are either not translated or RAP-1b protein is present in undetectable amounts in B. bigemina merozoites. In addition, both recombinant versions of B. bovis RRA and B. bigemina RAP-1b failed to stimulate proliferation of T-cells either from B. bovis or B. bigemina immune cattle (data presented in this study and W. Brown, personal communication, and Brown and Palmer, Reference Brown and Palmer1999). It is possible that the inability of RRA to stimulate T-cells in our proliferation experiment is as a result of the low level of expression of RRA in merozoites during bovine infection, which may be insufficient to induce a detectable level of T-cell response. Alternatively, the amount of RRA in the merozoite membrane extract used for establishing the T-cell line might be too low to stimulate the growth of RRA-specific T cells in culture. Additionally, sequence comparison demonstrates that all T-cell epitopes mapped in B. bovis RAP-1 using CD4+ T cells from animal C97, which was also used in the current study, are poorly conserved or absent in RRA (Norimine et al. Reference Norimine, Suarez, McElwain, Florin-Christensen and Brown2002). Lack of conservation of these epitopes could also explain the lack of response of animal C97 T cells to RRA.
Interestingly, previous experiments demonstrated that (1) immunization with RAP-1 or an N-terminal truncated version of RAP-1 (RAP-1-NT) conserved region is not sufficient to elicit protective immune responses despite strong immunological stimulation (Norimine et al. Reference Norimine, Mosqueda, Suarez, Palmer, McElwain, Mbassa and Brown2003); (2) RAP-1 is an immunodominant, monomorphic antigen, and cattle remain persistently infected despite high antibody titres against RAP-1 (Brown et al. Reference Brown, McElwain, Ruef, Suarez, Shkap, Chitko-Mckown, Tuo, Riece-Ficht and Palmer1996; Suarez et al. Reference Suarez, Palmer, Hötzel and McElwain1998; Boonchit et al. Reference Boonchit, Xua, Yokoyama, Goff, Wagner and Igarashi2002), and (3) anti-RAP-1 antibodies can only partially neutralize erythrocyte invasion by both B. bovis merozoites and sporozoites (Yokoyama et al. Reference Yokoyama, Suthisak, Hirata, Matsuo, Inoue, Sugimoto and Igarashi2002; Mosqueda et al. Reference Mosqueda, McElwain, Stiller and Palmer2002). Consistently, in this study we also observed strong but incomplete neutralization effects of B. bovis merozoites using anti-sRRA-1 antibody in in vitro neutralization assays. Although the exact functional roles of RAP-1 and RRA remain unknown, the partial antibody-mediated neutralization effects of anti-RAP-1 antibodies supports that an additional RAP-1-like molecule may be used for B. bovis survival in the phase of strong anti-RAP-1 immune response in infected cattle. Conservation of the RAP-1 signature motifs in RRA supports functional roles common between these two molecules. However, the T-cell proliferation assay and the weak antibody response suggest that, in contrast to RAP-1, RRA is poorly antigenic during infection. Thus collectively, the data suggest that RRA is expressed in relatively small amounts and is therefore unable to elicit significant immunity in infected cattle, but yet it may play a role in parasite invasion and survival. Therefore, because it is a weak immunogen during infection yet potentially important for erythrocyte invasion (as demonstrated in in vitro neutralization assays), we hypothesize that RRA may provide alternative means for the parasite to escape from anti-RAP-1 antibody-mediated neutralization. This could explain the lack of total inhibition of invasion observed in the in vitro neutralization studies using either anti RAP-1 or RRA antibodies. Furthermore, as immunodominant antigens such as RAP-1 (Norimine et al. Reference Norimine, Mosqueda, Suarez, Palmer, McElwain, Mbassa and Brown2003) and MSA-1 (Hines et al. Reference Hines, Palmer, Jasmer, Goff and McElwain1995) fail to induce protective immunity, subdominant antigens like RRA may be better candidates for developing vaccines which could potentially elicit a neutralizing antibody response in vivo (Brown et al. Reference Brown, Norimine, Goff, Suarez and McElwain2006). In this scenario, it is also possible to speculate that the immunodominant RAP-1 molecule acts as a decoy, diverting potential immune responses against other functionally relevant molecules required for invasion by the parasite such as RRA, thus assuring persistent infection of cattle by B. bovis.
In summary, a previously unreported and highly conserved RAP-1-related protein encoded by B. bovis is expressed during infection of the bovine host. Based on the conservation of the RAP-1 signature motifs in RRA, we hypothesize that RRA is a subdominant functional equivalent of RAP-1 that is expressed at a low level in B. bovis merozoites. Thus it is possible that expression of RRA is strongly regulated in order to minimize host immunity during infection and contribute to persistent infection. This feature could provide a useful mechanism for the parasite to escape the immune response directed against highly immunogenic antigens such as RAP-1 as well as be a new target for immunization strategies. The apparent low level of expression and the subdominant characteristics of the B. bovis RRA raise interesting questions. The possible functional relationships of RRA and RAP-1, whether the expression of RRA is differentially regulated during the B. bovis life cycle, and its possible role in erythrocyte invasion and in the induction of protective immune responses need to be addressed in future studies.
ACKNOWLEDGMENTS
Technical support provided by Paul Lacy and Lupita Leiva is greatly appreciated. We thank Dr Kevin Lahmers for his help with statistical analysis, David Herndon for his help with the B. bovis cultures, Dr Ignacio Echaide for providing B. bovis strains from Argentina and sera from B. bovis infected cattle and Dr Don Knowles for his support.







