INTRODUCTION
Variable patterns of body size in relation to latitude are known to occur in insects. Opposing intraspecific clines in body size and development time have been documented in ectothermic insects. First, the Bergmann's rule, states that body size increases with latitude (Blanckenhorn and Demont, Reference Blanckenhorn and Demont2004; Chown and Gaston, Reference Chown and Gaston2010). The mechanism behind the Bergmann's rule remains unclear, although it is commonly agreed that Bergmann's rule seems to be effected by temperature per se (Atkinson and Sibly, Reference Atkinson and Sibly1997). On the contrary, the converse Bergmann's rule, mediated by season length, predicts that developmental rate and body size decreases towards the poles (Masaki, Reference Masaki and Dingle1978; Nylin and Svärd, Reference Nylin and Svärd1991; Telfer and Hassall, Reference Telfer and Hassall1999). In addition, a third observed pattern, called counter-gradient seems to exist (Levinton and Monahan, Reference Levinton and Monahan1983; Conover and Present, Reference Conover and Present1990). This pattern has the same underlying mechanism than converse Bergmann's rule, but specifically refers to the genetic response involved in compensation to seasonal limitations at higher latitudes that results in faster growth compared to their low-latitude conspecifics. Observed field patterns can be also due to complex interactions, e.g. between Bergmann and converse Bergmann trends (Blanckenhorn and Demont, Reference Blanckenhorn and Demont2004). However, geographical patterns related to phenotypic characteristics may be essential for invasion capacity and dispersion success of various species, including parasitic insects. The present work provides new information about developmental and emergence characteristics in relation to latitude and body size in an invasive ectoparasite.
Insects that have short generation times in relation to the length of the growing season (at least more than 2–3 generations per season), but which inhabit temporal habitats like rotting fruit or fungi, could be expected to increase their body size and decrease development time with latitude (Chown and Gaston, Reference Chown and Gaston1999). In contrast, insects with a generation length more similar to the length of the season can instead be expected to be constrained by season length and decrease their mass at high latitudes and altitudes (Blanckenhorn and Demont, Reference Blanckenhorn and Demont2004).
The deer ked, Lipoptena cervi (Diptera; Hippoboscidae), is a haematophagous ectoparasite which exploits several host species from the cervids (Hackman et al. Reference Hackman, Rantanen and Vuojolahti1983; Kadulski, Reference Kadulski1996). The deer ked occasionally attacks on humans and consequently the deer ked has drawn strong public attention during recent years in Finland because of relatively rapid increase in abundance and dispersion into new areas towards the north (Fig. 1a). During the last 5 decades the deer ked expanded its range almost 1000 km northward (Kaitala et al. Reference Kaitala, Kortet, Härkönen, Laaksonen, Härkönen, Kaunisto and Ylönen2009; Välimäki et al. Reference Välimäki, Härkönen, Härkönen, Kaitala, Kortet, Madslien, Malmsten, Redford, Ylönen and Ytherus2010). Until today there is no detailed knowledge on mechanisms that have enabled the rapid invasion of the deer ked into the northern conditions and almost nothing is known about possible plastic life-history traits of the deer ked (see Härkönen et al. Reference Härkönen, Härkönen, Kaitala, Kaunisto, Kortet, Laaksonen and Ylönen2010). Pupae of the deer ked drop passively from the host onto the ground or into the snow during the late autumn and winter. After overwintering in the pupal stage, development takes several months and adults emerge in the following late summer and autumn (review by Haarløv, Reference Haarløv1964). Thus, the pupal stage is one of the key developmental phases of the life cycle of the deer ked and likely the most sensitive to extrinsic mortality factors (Bequaert, Reference Bequaert1954; Haarløv, Reference Haarløv1964; Lehane, Reference Lehane2005).
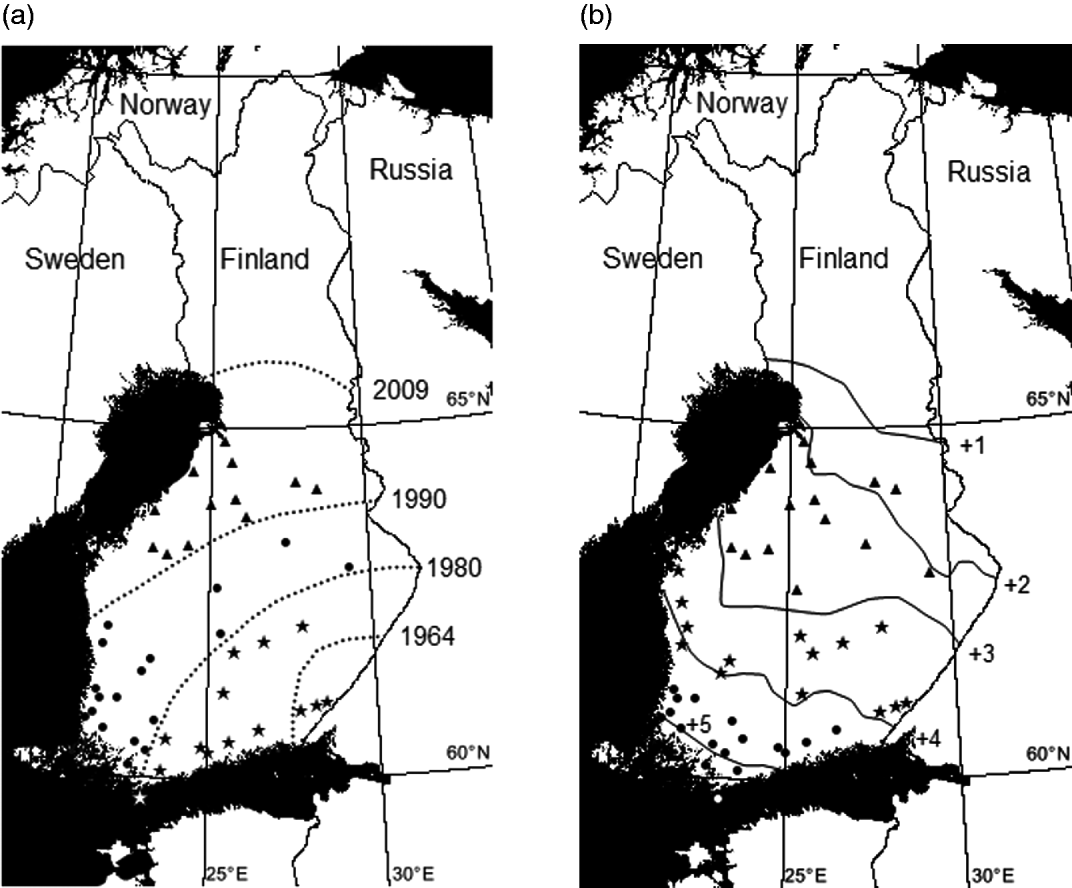
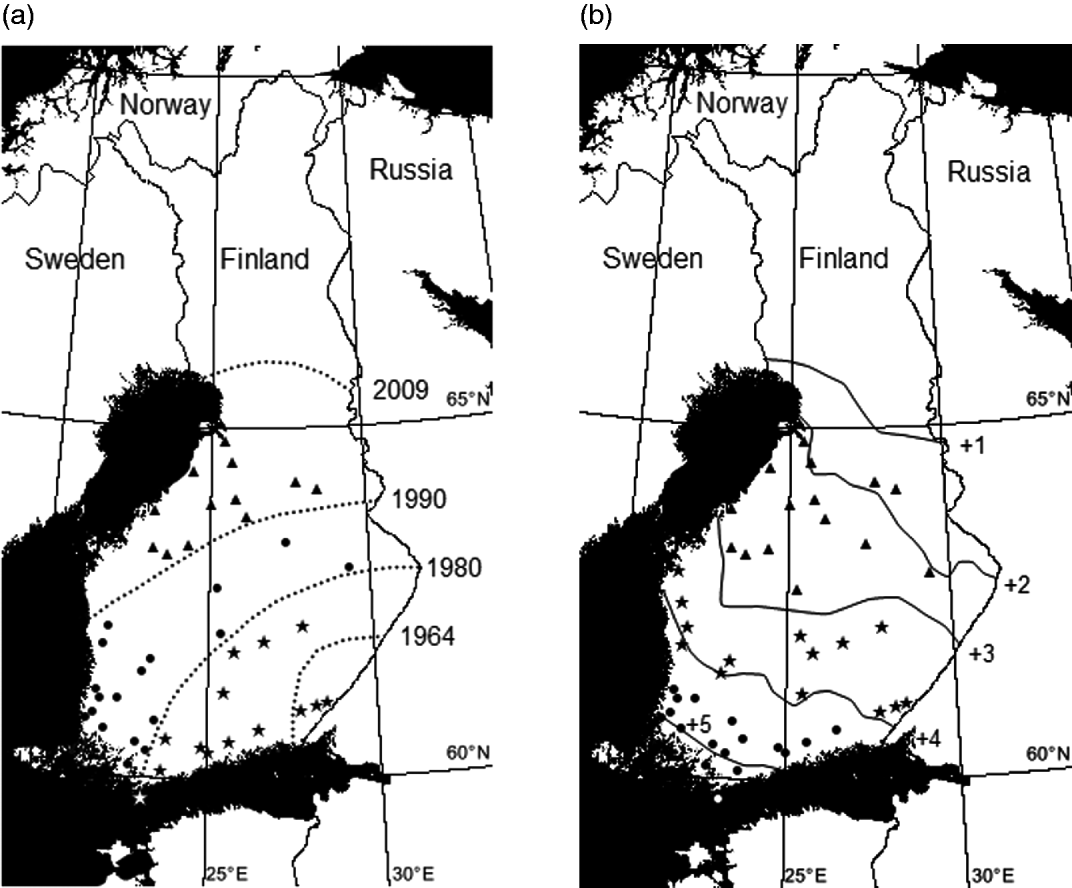
Fig. 1. (a) Invasion zones (=our study zones): (1) the south-eastern invasion zone (invasion years 1964–1975), (2) the middle invasion zone (years 1980–1990) and (3) the northernmost invasion zone (years 1990–2007), collection localities and the rough estimation of the northernmost limit of the current distribution area of the deer ked (year 2009) (according to Kaitala et al. Reference Kaitala, Kortet, Härkönen, Laaksonen, Härkönen, Kaunisto and Ylönen2009 and Välimäki et al. Reference Välimäki, Härkönen, Härkönen, Kaitala, Kortet, Madslien, Malmsten, Redford, Ylönen and Ytherus2010). Collection localities are marked by symbols: triangles (northernmost localities, n=14), circles (middle localities, n=18) and stars (south-eastern localities, n=14). (b) Temperature zones: (1) southern (hemiboreal), (2) central (southern boreal) and (3) northern (middle boreal) and the rough estimation of the northernmost limit (dashed line) of the current distribution area of the deer ked (Kaitala et al. Reference Kaitala, Kortet, Härkönen, Laaksonen, Härkönen, Kaunisto and Ylönen2009). Collection localities are marked using the symbols: triangles (northern localities, n=15), circles (middle localities, n=14) and stars (south-eastern localities, n=17). Values are annual average temperatures of 30-year average. (Figure has been modified according to the Finnish Meteorological Institute.)
In this study, we explore possible differences in pupal characteristics along (1) geographical temperature zones and (2) historical invasion zones of the deer ked. We wanted to study whether the northward spreading deer ked already shows plastic and/or rapid evolutionary changes in pupal characteristics between populations after facing the new environment. The principal question is whether the size of the pupa (measured as pupal weight), varies between different temperature or invasion zones towards north in Finland. To study pupal development characteristics and variables associated with the development success (e.g. pupal size, completion of metamorphosis, proportion of eclosed pupae and development duration) in relation to the temperature gradient and invasion zones we measured wild-collected deer ked pupae divided in respect to their origin and reared them in identical temperature and light conditions. We measured also the size of the emerged adults. Throughout this article, we use also the comparable term, eclosion, which refers to adult emergence from the pupal case (see e.g. Qiu and Hardin, Reference Qiu and Hardin1996; Watari, Reference Watari2002).
We hypothesized that the most northern pupae are smaller than the southern pupae regardless of a relatively small age difference between populations. We based this assumption, following the converse Bergmann's rule, on the relative long generation time of the deer ked which could be constrained by season length (see Blanckenhorn and Demont, Reference Blanckenhorn and Demont2004). The other main question considered the pupal development duration and whether it varies between individuals originating from 3 different latitudinal zones, or whether pupae express synchronized emergence characteristics, when reared in similar temperature and light conditions regardless of the different origins of the pupae. We hypothesized that the pupal development duration would be shorter among individuals originating from the northern latitudes. Since invasion history is relatively short, we did not have specific predictions for possible variation between invasion zones.
MATERIALS AND METHODS
Natural history of the deer ked
The original distribution of the deer ked covers Central and Eastern Europe, some parts of Northern Europe and Siberia, northern China, northern Africa (Algeria) but it has also been introduced to North America (e.g. Maa, Reference Maa1969; Dehio et al. Reference Dehio, Sauder and Hiestand2004). In Finland, the deer ked has rapidly spread towards the north during the last decades (Fig. 1a). The deer ked population was established in South Eastern Finland in the early 1960s. The Finnish population has expanded its range at an average rate of 11 km/year toward the north during the period 1960–2009 and 11 km toward the west during the period 1960–1990, when the west coast was colonized and no further westward expansion was possible (Välimäki et al. Reference Välimäki, Härkönen, Härkönen, Kaitala, Kortet, Madslien, Malmsten, Redford, Ylönen and Ytherus2010). At present, the limit of the distribution area of the deer ked in Finland follows and even crosses the southernmost areas of the reindeer husbandry (Kaitala et al. Reference Kaitala, Kortet, Härkönen, Laaksonen, Härkönen, Kaunisto and Ylönen2009; Välimäki et al. Reference Välimäki, Härkönen, Härkönen, Kaitala, Kortet, Madslien, Malmsten, Redford, Ylönen and Ytherus2010). Hence the Finnish south-eastern population is approximately 45 years old. The age difference between south-eastern and northernmost populations, used in this study, is roughly estimated as 15 years.
Original Central European breeding hosts (sensu Bequaert, Reference Bequaert1953) supporting reproduction of the deer ked are the red deer (Cervus elaphus), the roe deer (Capreolus capreolus), and, to a lesser extent, the fallow deer (Dama dama) (Haarløv, Reference Haarløv1964). The main host of the deer ked in Fennoscandia and in Finland is the moose (Alces alces) (Paakkonen et al. Reference Paakkonen, Mustonen, Roininen, Niemelä, Ruusila and Nieminen2010). The deer ked is also able to use the wild forest reindeer (Rangifer tarandus fennicus) (Kaunisto et al. Reference Kaunisto, Kortet, Härkönen, Härkönen, Ylönen and Laaksonen2009) with lower prevalence, and occasionally the semi-domestic reindeer as breeding hosts (Kynkäänniemi et al. Reference Kynkäänniemi, Kortet, Härkönen, Kaitala, Paakkonen, Mustonen, Nieminen, Härkönen, Ylönen and Laaksonen2010). The white-tailed deer (Odocoileus virginianus) is also among the breeding hosts of the deer ked in North America, but the prevalence of infection seems relatively low (Matsumoto et al. Reference Matsumoto, Berrada, Klinger, Goethert and Telford2008). Evidence of the white-tailed deer as a breeding host in Finland is inadequate so far (Välimäki et al. Reference Välimäki, Härkönen, Härkönen, Kaitala, Kortet, Madslien, Malmsten, Redford, Ylönen and Ytherus2010). Some individuals of this invasive ectoparasite can make a mistake and attack also on humans when seeking breeding host (Kortet et al. Reference Kortet, Kaunisto, Härkönen, Hokkanen, Härkönen, Kaitala and Ylönen2010).
Deer ked abundance on an individual host may be very high, even up to above 16 000 flies have been noted (Vikøren et al. Reference Vikøren, Ytrehus and Handeland2008; Paakkonen et al. Reference Paakkonen, Mustonen, Roininen, Niemelä, Ruusila and Nieminen2010). Taking the high infestation intensity into account, deer keds may have negative and detrimental effects on their hosts. As the controlled experimental deer ked infections demonstrated, deer keds can lower the physical condition of semi-domesticated reindeer and cause short-term histological, physiological, and behavioural changes (Kynkäänniemi et al. Reference Kynkäänniemi, Kortet, Härkönen, Kaitala, Paakkonen, Mustonen, Nieminen, Härkönen, Ylönen and Laaksonen2010). In Norway and Sweden, an epizootic of alopecia was associated with massive deer ked infestation in moose (Statens Veterinärmedicinska Anstalt, 2007, 2008; Vikøren et al. Reference Vikøren, Ytrehus and Handeland2008). In addition, deer ked is a nuisance and an obstacle to traditional human outdoor activity (see Kortet et al. Reference Kortet, Kaunisto, Härkönen, Hokkanen, Härkönen, Kaitala and Ylönen2010). The deer ked may cause serious health problems and symptoms, including chronic deer ked dermatitis (Rantanen et al. Reference Rantanen, Reunala, Vuojolahti and Hackman1982) and occupational allergic rhinoconjunctivitis in humans (Laukkanen et al. Reference Laukkanen, Ruoppi and Mäkinen-Kiljunen2005). The species is also a potential vector of various diseases (Ivanov, Reference Ivanov1974; Rantanen et al. Reference Rantanen, Reunala, Vuojolahti and Hackman1982; Dehio et al. Reference Dehio, Sauder and Hiestand2004; Matsumoto et al. Reference Matsumoto, Berrada, Klinger, Goethert and Telford2008).
The annual life cycle of the deer ked includes the 4 following phases; (1) emergence of a new adult generation in autumn, (2) short flying period and host colonization, (3) adult life on the host and pupaparous pupal production and (4) pupal stage of variable length on the ground before the synchronized emergence of new adults (Haarløv, Reference Haarløv1964; Hackman et al. Reference Hackman, Rantanen and Vuojolahti1983). Adult deer keds emerge and seek hosts in Finland from late summer to the end of autumn (Hackman, Reference Hackman1977). Almost immediately after finding a host, both males and females drop their wings (Bequaert, Reference Bequaert1953; Hackman et al. Reference Hackman, Rantanen and Vuojolahti1983) and start to suck blood and interstitial fluid from the suitable hosts (Ivanov, Reference Ivanov1974). The adults spend wintertime and the rest of their lives on the same host (Bequaert, Reference Bequaert1953; Haarløv, Reference Haarløv1964). It is not known for how long adults can live on the host, but deer ked females produce viviparously pupae one by one, at least from the autumn until following late winter.
Pupae collection and storage conditions
Volunteers collected deer ked pupae from all over its current distribution area in Finland. They collected pupae between 9 February and 19 March 2007. During winter, the deer ked pupae drop off the parasitized hosts onto the snow cover and pupae can most probably be seen on bedding sites of the moose (see Kaunisto et al. Reference Kaunisto, Kortet, Härkönen, Härkönen, Ylönen and Laaksonen2009). We asked the volunteers (mostly hunters) to fill out the data forms concerning exact information about collection dates and places, as well as host species. According to filled forms the pupae used in this research were collected from the bedding sites of moose.
After collection, we asked voluntary collectors to keep pupae at a low temperature (ca. +3°C, e.g. in a fridge) and in adequate moisture in a tube filled with a piece of cotton. We asked volunteers to send pupal samples via the post to the Konnevesi Research station as soon as possible after collection. After arrival at the research station, we stored the samples under identical conditions. We stored pupae in a cold room (+3°C) until the experiment began and we handled pupal samples with particular care to avoid warming.
Geographical and invasion historical zone division
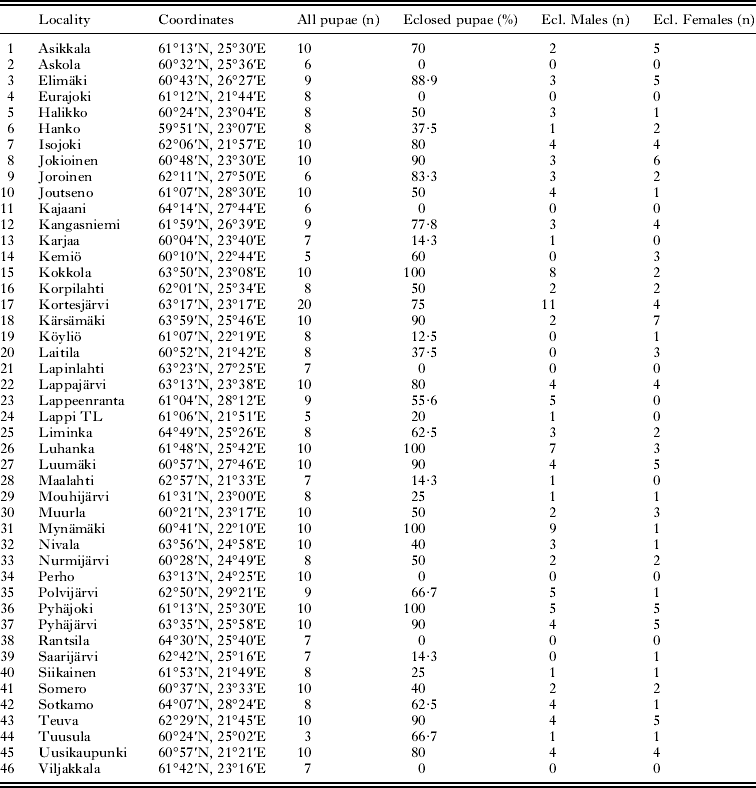
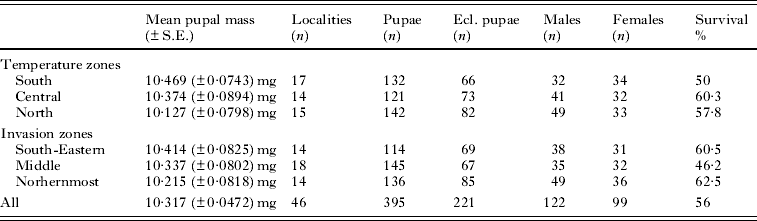
To study the effect of the temperature zones on pupal characteristics we divided pupae into 3 blocks according to their origin in relation to the temperature zones (measured as annual average temperatures) and current distribution area of the deer ked in Finland: (1) southern (hemiboreal), (2) central (southern boreal) and (3) northern (middle boreal) (Fig. 1b). According to this division the age difference between populations is more unsubstantial, because the temperature and invasion zones are not cohesive, but they crisscross (Fig. 1a and b). The southern population and the northern population are clearly situated in different temperature zones. The annual average temperature varies between ca. +2·0°C and +3·0°C in the zone where the northern population is situated. In the central zone the average temperature varies between +3·0°C and +4·0°C and in the southern zone the annual average temperature varies between ca. +4·5°C and +5·5°C. We studied pupae from 46 localities with respect to the temperature zones and pooled the data within each temperature zone (Table 1).
Table 1. Details of the 46 research localities (all pupae studied, eclosed and uneclosed pupae and number of specific gender per locality)

When exploring potential differences between the invasion zones we categorized the localities above again into 3 blocks according to the invasion history and current distribution area of the deer ked in Finland: (1) the south-eastern invasion zone (invasion years 1964–1975), (2) the middle invasion zone (years 1980–1990) and (3) the northernmost invasion zone (years 1990–2007) (Fig. 1a). This approach emphasizes age differences between populations.
Pupal size measurements
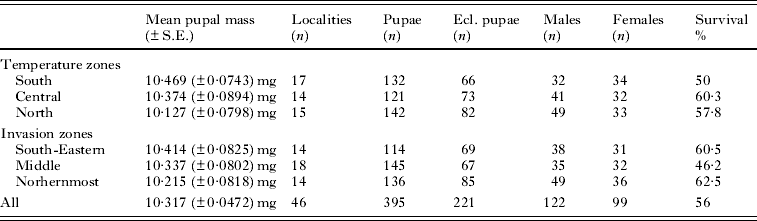
Before measuring the fresh mass of pupae, we kept them on a tray in a dry place for 2 h so that any possible additional external moisture evaporated. We measured the pupal mass in March when we supposed all pupae to be in the same overwintering stage. We weighed altogether 395 pupae by steelyard (Sauter AR 1014). In the temperature zone division we explored 142 northern origin pupae, 121 pupae from the central zone and 132 south origin pupae, while in the invasion zone analyses 136 pupae came from the northernmost invasion zone, 145 pupae from the middle zone and 114 pupae from the south-eastern invasion zone (Table 2).
Table 2. Details of the temperature and invasion zone division analyses
Rearing conditions of the pupae
We placed all the individually weighed pupae into coded rearing containers for later identification of their geographical origin. Rearing containers were translucent plastic tubes (3 cm in diameter and 5 cm long), which we covered with a small-gilled net, preventing adult deer keds escaping after their emergences. In addition, we placed a piece of Sphagnum moss into each rearing container with one pupa. The purpose of the moss was to prevent the pupae from becoming mouldy due to antiseptic effects and drying of the pupae by balancing moisture conditions.
Throughout the study we used similar temperature, light and moisture conditions in the rearing environment. We kept pupae for 2 months after collection in a low temperature (+3°C) at the laboratory, assuming pupae to continue their overwintering. After that low temperature period, we moved weighed pupal samples to the climate chamber (+17°C) to monitor their development at the end of May. Hackman (1979) reported that the first adult emergence took place after 2 months when brought to room temperature. Those pupae were collected in Finland in March from the snow and reared the whole time in room temperature. It seems probable that while staying in the soil the pupae spend the most of their time in diapause (Haarløv, Reference Haarløv1964). Because of asynchrony in dropping time of pupae (may vary several months between some individuals) from the fur of a host (see Keilbach, Reference Keilbach1966), we expected pupae to terminate diapause depending on temperature (see e.g. Tauber et al. Reference Tauber, Tauber and Masaki1986; Härkönen et al. unpublished observations).
We reared pupae in a long day rhythm (16 h of light per day) from the end of March. According to unpublished observations made by Härkönen and coworkers the development of deer ked pupa is not light dependent. In addition, we kept the relative humidity of the air between 70 and 90% by using an air conditioner, and we watered the pupae once per week depending on the appearance of the surrounding moss.
Observation of pupal development duration
When we expected the period of adult emergence to begin, we performed the observations of the pupae daily. After the first adult emerged, the pupae were observed a few times per day and the emergence dates of the adults were noted. After the end of the experiment, all the uneclosed pupae were opened under the microscope.
Adult sex identification and size measurements
We identified genders of the adults by studying the sex organs under the stereomicroscope. In addition, we measured the adult size parameters by using the stereomicroscope with a scale. We measured the head at the widest point and the body length from the tip of the abdomen to the base of the haustellum.
Statistical analyses
For data analyses we used SPSS for Windows (version 13.0). We used ANOVA for exploring the possible influence of the zone on the pupal size by selecting the zone (temperature or invasion zone) as a factor and pupal size as a dependent variable. We conducted this for all pupae (uneclosed and eclosed pupae). We did not include gender for this model, because we knew the gender only for eclosed pupae. For eclosed pupae, we used the ANOVA model to study the effect of the zone (temperature or invasion zone) and the gender on pupal size by selecting zone and gender as fixed factors and pupal size of eclosed pupae as a dependent factor. For the temperature zone division analysis, we used ANOVA to detect possible differences in pupal size between eclosed and uneclosed pupae in the north and the south by selecting pupal status (value: 1=uneclosed, 2=eclosed) as a definitive factor and pupal size as a dependent factor. We conducted this test separately among northern and southern pupae.
Relating also to the pupal success, we explored potential differences between the zones (temperature or invasion zone) in the proportion of pupae that eclosed (i.e. survival rate) separately for each zone pair by using the Chi-square test. In addition, we studied potential differences between the zones (temperature or invasion zone) in the proportion of pupae completing metamorphosis by using the Chi-square test.
We used one single ANCOVA model to test influence of pupal size, zone (temperature or invasion zone) and gender on the pupal development duration by selecting pupal size as a covariate, zone and gender as fixed factors and developmental rate as a dependent variable for the model.
We conducted the ANCOVA model also to detect possible associations behind the adult size parameters, selecting pupal size as a covariate, zone (temperature or invasion zone) and gender as fixed factors and adult size parameter as a dependent variable. We performed separate ANCOVA models for different adult size parameters (width of the head and adult body length as dependent variables).
We used the Bonferroni correction for the two independent comparisons (statistical analyses for the temperature and the invasion zone divisions) and lowered the significance level α from 0·05 to 0·025.
RESULTS
The geographical pattern in size and possible influence of the gender on the pupal size
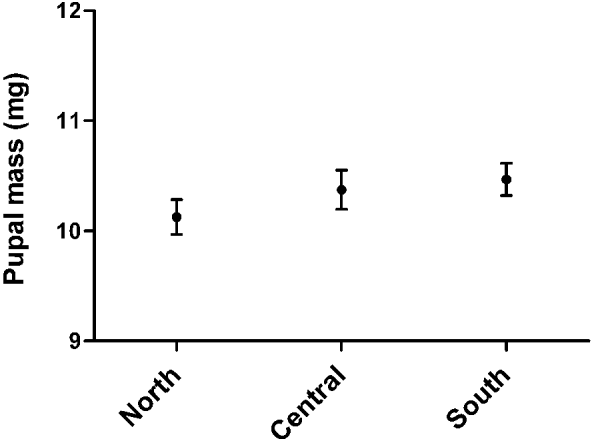
In the temperature zone division, pupae from the 3 temperature zones differed significantly from each other in terms of pupal size (ANOVA; F2,392=4·292, P=0·007). The difference in pupal size between the northern and the southern temperature zones was statistically significant when all pupae (eclosed and uneclosed) were included (Tukey HSD post hoc test; P=0·007). Southern origin pupae were statistically heavier than pupae from the northernmost temperature zone (Fig. 2, Table 2). In this model, zone was a fixed factor and pupal size a dependent factor. However, when only pupae that eclosed were included, there was no difference between the temperature zones in terms of pupal size when the temperature zone and the gender were fixed factors and the pupal size was a dependent factor for the model (ANOVA; F2,215=1·138, P=0·32). The gender did not affect the pupal size (ANOVA; F1,215= 1·406, P=0·24). There was no interaction between the zone and the gender (ANOVA; F2,215=0·233, P=0·79). The difference in relation to the pupal size disappeared among eclosed pupae between the temperature zones, likely because eclosed pupae were heavier than uneclosed ones in the northernmost temperature zone used in this study when analysed separately the northern and the southern populations (ANOVA; F1,140=10·573, P=0·001).
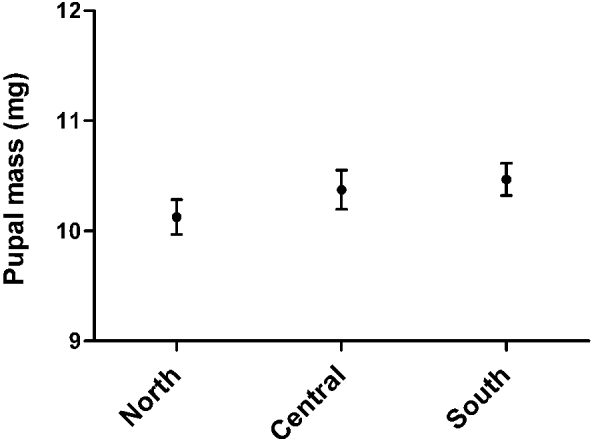
Fig. 2. Mean pupal mass of the deer ked including all weighed pupae (uneclosed and eclosed pupae) in 3 temperature zones with a 95% confidence interval.
In the invasion zone division, pupae from the 3 invasion zones did not significantly differ between the zones in terms of pupal size when all pupae (eclosed and uneclosed) were compared (ANOVA; F2,392=1·450, P=0·236) (Table 2). In this model, zone was a fixed factor and pupal size a dependent factor. Among only the eclosed pupae, there was no interaction between invasion zone and gender when zone and gender were fixed factors and the pupal size was a dependent factor (ANOVA; F2,215=0·377, P=0·69). The invasion zones did not differ from each other in relation to pupal size of eclosed pupae (ANOVA; F2,215=0·124, P=0·88). The gender did not also affect the pupal size (ANOVA; F1,215=1·623, P=0·20).
Adult emergence and success
The adult emergence period began at the end of July. The first adult emerged on 30 July 2007 and the emergence period lasted for 31 days, until 30 August. Only 34 out of 172 uneclosed pupae (19·8%) had seemingly completed the metamorphosis from larvae to adult inside the puparium but the adult emergence did not take place for some reason and adults had died inside the puparium.
Between the temperature zones there were no differences in the proportion of pupae completing metamorphosis inside the puparium nor in the survival rate (i.e. proportion of pupae that eclosed).
Between the invasion zones, there were also no differences in the proportion of pupae completing metamorphosis inside the puparium. The survival rate was lowest in the middle zone compared to the south-eastern (X 2=5·248, d.f.=1, P=0·022) and northernmost (X 2=7·502, d.f.=1, P=0·006) invasion zone. Between the northernmost and the south-eastern invasion zone there was no difference in terms of survival rate (X 2=0·102, d.f.=1, P=0·749).
Influence of the geographical origin and the gender on the pupal development duration
Temperature zone had no significant influence on pupal development duration when testing pupal size as covariate, temperature zone and gender as fixed factors and pupal development duration as a dependent factor for the model (ANCOVA; F2,209=0·255, P=0·775). Hence, the pupal development duration did not differ between adults from the 3 temperature zones when reared in similar conditions. The gender also had no effect on the pupal development duration (ANCOVA; F1,209=0·140, P=0·709). There were no interactions of any kind in this ANCOVA model.
In the invasion zone division, there were no interactions of any kind in the ANCOVA model, when pupal size was a covariate, invasion zone and gender were fixed factors and pupal development duration was a dependent factor for the model. Invasion zone had no significant influence on the pupal development duration (ANCOVA; F2,209=1·412, P=0·246). Hence, the pupal development duration did not differ between adults from 3 invasion zones with different invasion times when reared in similar conditions. The gender also had no effect on the pupal development duration (ANCOVA; F1,209=0·331, P=0·566).
Influence of the pupal size on the pupal development duration and adult morphology
In the temperature zone division, pupal size had a significant influence on pupal development duration when testing pupal size as covariate, other factors such as temperature zone and gender as fixed factors, and pupal developmental rate as a dependent factor for the model (ANCOVA; F1,209=9·052, P=0·003). The positive relationship between pupal size and development duration was equal, hence there were no interactions, in all 3 geographical zones (ANCOVA; F2,209=0·120, P=0·887) and in both genders (ANCOVA; F1,209=0·001, P=0·971).
There were no interactions of any kind in the ANCOVA model, when testing pupal size as covariate, temperature zone and gender as fixed factors and adult size parameter (width of the head or body length) as a dependent factor for the model. We found a positive association between pupal size and adult size parameters like width of the head (ANCOVA; F1,128=28·03, P<0·001) and body length (ANCOVA; F1,102=23·10, P<0·001).
In the invasion zone division, when testing pupal size as covariate, invasion zone and gender as fixed factors and pupal developmental rate as a dependent factor for the ANCOVA model, pupal size had also a significant influence on pupal development duration, indicating that smaller pupae eclose earlier (ANCOVA; F1,209=10·829, p=0·001). The relationship between pupal size and development duration was equal, hence there were no interactions, in all 3 invasion zones (ANCOVA; F2,209=0·899, P=0·409) nor in both genders (ANCOVA; F1,209=0·043, P=0·836). In addition, when pupal size was used as covariate, invasion zone and gender as fixed factors and adult size parameter (width of the head or body length) as dependent factor for the model, there was a positive association between pupal size and adult size parameters like width of the head (ANCOVA; F1,128=24·92, P<0·001) and body length (ANCOVA; F1,102=21·71, P<0·001). There were no interactions of any kind in this ANCOVA model.
DISCUSSION
Our data indicate that the expansion of the deer ked's distribution area towards the north seems to have caused plastic changes in the pupal characteristics along the temperature gradient. The invasion history, or the time of establishment of the parasite to the environment and its host specimen, did not seem to affect life-history characteristics of the deer ked. The deer ked pupae from the southernmost temperature zones were heavier than pupae from the northernmost temperature zones (used in this study) when compared between temperature regions. However, the pupal size did not vary between invasion zones in Finland. Pupal development duration did not vary between individuals having a different temperature origin or invasion history, contrasting our expectations. However, the adult emergence period was relatively synchronized regardless of different origins (temperature and invasion history) when the individuals were reared under the same temperature and light conditions. Among the tested variables, only pupal size was associated positively with pupal development duration, indicating that smaller pupae eclosed earlier in both zone divisions.
The first result concerning the relationship of pupal size and temperature zone was in accordance with our first hypothesis which predicted decreasing size towards the north (see Masaki, Reference Masaki and Dingle1978; Mousseau, Reference Mousseau1997; Blanckenhorn and Demont, Reference Blanckenhorn and Demont2004). Thus, one would expect a latitudinal pattern of decreasing size, because of the seasonal constraints (i.e. shorter and colder growing season in the north) as the converse Bergmann's rule states (e.g. Masaki, Reference Masaki and Dingle1978). That rule has often been observed in insects and other arthropods (Mousseau, Reference Mousseau1997). In many insects, (e.g. crickets and grasshoppers), body size appears to be largely genetically determined so that northern populations are smaller (e.g. Masaki, Reference Masaki and Dingle1967). On the other hand, deer ked adults live and reproduce their whole life in constant temperature inside the fur and, consequently, are relatively unaffected by outdoor temperature. Why then should they follow the converse Bergmann's rule? Alternatively, the smaller body size in the north can also be a result from phenotypic plasticity when an organism's life cycle is linked to season length (e.g. Leimar, 1996). Environmentally induced phenotypic plasticity might explain the observed geographical variation in the deer ked pupal size between temperature zones (cf. West-Eberhard, Reference West-Eberhard2003). The mechanism behind this could be that females in the northern latitudinal zones show plasticity in their investment to smaller pupae. In addition, the age difference between individuals from northernmost and southernmost temperature zones may not be so high for adaptation due to the overlapping invasion and temperature zones. One possible explanation for the lack of the clear pattern between the invasion zones is that the real differences in season length and associated temperatures are small or missing.
After taking into account only the pupae that eclosed, there was no significant difference between temperature zones in terms of pupal size. This means that eclosed pupae were heavier than uneclosed ones in the northern zone. It may suggest that our selected constant rearing temperature potentially interfered with the developing process of the smallest pupae and may have hindered us to observe the adaptation to local environmental conditions more closely.
In both division analyses (the temperature zone and invasion zone division) we found a positive correlation between the pupal size and pupal development duration. Adults that emerged from smaller pupae seemed to emerge earlier than adults from larger pupae under identical conditions. This supports a general expectation that smaller individuals contain less metabolic reserves, which indicates a decreased ability to sustain a long non-feeding pupal stage (e.g. Feder et al. Reference Feder, Powell, Filchak and Leung2010). There was no difference between the male and the female pupae in terms of pupal size. In the northern temperature zone, smaller pupae with earlier eclosion could have an advantage because lower summer temperatures prolong the developmental period and shorten the suitable host search time by several weeks. Pupal size also correlated positively with adult size parameters (width of the head and body length) indicating that larger pupae produced larger adults in all zones and in both sexes when reared under identical temperature conditions. However, it is not known how the short growing season with lower temperatures in the north could affect this association. Studies report that pupal development temperature affects adult phenotype and size in some species (e.g. Stevens, Reference Stevens2004). This could be an important topic in future research also with the deer ked. According to these associative results pupal size and, for example, adult body length and width of the head could be used as a tool for estimating the morphological differences between deer ked populations in future investigations.
Geographical origin and sex, instead, did not affect pupal development duration. Despite the different temperature origin and different invasion history of the pupae, the adult emergence period was relatively synchronized when reared under identical temperature and light conditions, which was also contrary to our expectations. This synchronization of eclosion, despite the different origin, could suggest that the northernmost pupae have not yet genetically adapted to their new environment or, perhaps the deer ked had not yet faced the total limit of its distribution, which would force differences to outcome through adaptations.
Among various insects, males usually emerge earlier than females. This phenomenon is known as a protandry, which can be understood in terms of sexual selection acting on males to maximize the number of matings, or on females to increase reproductive success by minimizing the pre-reproductive period (e.g. Wiklund et al. Reference Wiklund, Lindfors and Forsberg1996; Taylor et al. Reference Taylor, Anderson and Peckarsky1998). This may not be so in the case of the deer ked, perhaps because the host searching periods of adults is relative short or this type of method was not enough to raise possible differences in adult emergence between genders.
To conclude, we know currently that adult deer keds face constant and relatively safe conditions within the host's fur, but pupae are surrounded by the very variable extrinsic mortality factors (Välimäki et al. Reference Välimäki, Härkönen, Härkönen, Kaitala, Kortet, Madslien, Malmsten, Redford, Ylönen and Ytherus2010). Our results about pupal size between temperature zones may verify the great plasticity of this species. Thus, the deer ked likely has a notable capability to continue its dispersion towards the north into new areas. This is indeed predictable, since a colder and shorter growing season in northern Finland may not totally constrain deer ked invasion according to transplant experiments conducted beyond the current range of the deer ked (see Härkönen et al. Reference Härkönen, Härkönen, Kaitala, Kaunisto, Kortet, Laaksonen and Ylönen2010). In general, documented and forecast changes in the local species richness due to climate change likely signify that geographical ranges of species will continue to shift substantially polewards (e.g. Beaumont and Hughes, Reference Beaumont and Hughes2002; Parmesan, Reference Parmesan2006; Vanhanen et al. Reference Vanhanen, Veteli, Päivinen, Kellomäki and Niemelä2007).
ACKNOWLEDGEMENTS
We want to thank all the hundreds of volunteer pupa collectors for their help in the field and staff of Konnevesi Research station during the laboratory period of this study. We also thank Raine Kortet and two very careful anonymous referees for providing useful comments to this manuscript.
FINANCIAL SUPPORT
This study was partly funded by the Vanamo, Finnish Biologists’ Association (to S.K), Ella and Georg Ehrnrooth Foundation (to S.K), Pro Societas Flora et Fauna Fennica (to S.K.) and the Finnish Ministry of Agriculture and Forestry (to S.K.).