Ventricular septal defect is the most common congenital heart disease. Reference Penny and Vick1 The incidence of ventricular septal defect ranges from 5 to 50 per 1000 live births while in adults its 0.3 per 1000. Reference Hoffman and Kaplan2 In the Philippines, there is no national database available to provide the number of ventricular septal defect cases per population.
Surgical treatment remains the gold standard for treating ventricular septal defect. Open-heart surgical ventricular septal defect patch closure provides a low post-operative mortality and morbidity. Reference Perrault, Drblik and Montigny3–Reference Meijboom, Szatmari and Utens5 The reported mortality rate is 0.3–0.6%. Reference Jacobs, O’Brien and Pasquali6 Complications such as atrioventricular block previously had an incidence of 8% but in the modern era, it decreased to 3%. Other complications such as reoperation occur in 25% due to significant residual leak, or for indications other than residual leak in up to 2%. Reference Jacobs, O’Brien and Pasquali6–Reference Tucker, Pyles, Bass and Moller8 Moreover, other concerns include the discomfort after the surgery, the scar, and possibilities of post-pericardiotomy syndrome, infection, or arrhythmia complications. Reference Meijboom, Szatmari and Utens5 Furthermore, cardiopulmonary bypass may have a long-term negative effect on the developmental and neurocognitive functions of the patient therefore affecting behavioural and school performance. Reference Meijboom, Szatmari and Utens5,Reference Roos-Hesselink, Meijboom and Spitaels9 These deterrent effects may limit this management.
Transcatheter closure of ventricular septal defect closure is now an acceptable alternative to surgical closure with low mortality and morbidity rate. A recent randomly assigned study of ventricular septal defect cases treated with either surgical or trancatheter closure showed that neither group showed significant morbidity and no mortality. Transcatheter closure was associated with fewer complications, myocardial injuries, transfusions, shorter procedural time, no requirement for intensive care unit (ICU) stay, shorter hospital stays, lower medical expenses, and faster recovery times. Reference Yang, Yang and Yu10
Literature review
In the early experience with the Amplatzer ventricular septal defect device, it was shown that transcatheter closure of ventricular septal defects is associated with complete heart block in 6–25% of cases, and as such is not yet FDA approved in the United States of America and Canada. Reference Butera, Carminati, Chessa, Piazza, Micheletti and Negura11,Reference Walsh, Bialkowski, Szkutnik, Pawelec-Wojtalik, Bobkowski and Walsh12 There are other possible complications that can occur immediately such as embolisation of the device, rhythm disturbances, haemolysis, thromboembolism, residual shunt, and endocarditis. Late complication of complete heart block is usually seen when a perimembranous type of ventricular septal defect has been closed. Reference Carminati, Butera and Chessa13–Reference Zhou, Pan, Guan and Ge17 The first generation Amplatzer perimembranous ventricular septal defect device has since been removed from the market.
With lessons learned from the initial Amplatzer device, new devices and modifications have decreased the incidence of these complications. The Lifetech Multifunctional occluder was considered in this study due to its availability over the Lifetech Cera Ventricular Septal Defect occluder in our institution. The Lifetech Multifunctional occluder is a ventricular septal defect device designed to close defect sizes from 2 to 10 mm. The occluder is a self-expandable, double disc device made from double nitinol wire mesh layers with 144 threads of nitinol wires. Both discs are linked together by a cone-shaped waist and a small waist, with a total unscretched length of 4 mm. The elongated waist is designed for a variety of ventricular septal defects and the soft woven mesh provides high conformability to reduce the risk of atrioventricular block by decreasing the stretch and clamp forces on the septum and conduction system. One screw hub is made on each disc (Fig 1). This double-sided screw provides procedural flexibility to facilitate antegrade or retrograde approach in delivering the device, while using a relatively low profile delivery sheath to minimiae unwanted vascular injury. The 1st four sizes from 5–3 mm to 8–6 mm are designed without any fabric membrane, which allows for a 4 F delivery system. Meanwhile the other sizes from 9–7 mm to 14–12 mm are sewn with polytetrafluoroehtylene membranes by nylon threads (Table 1). There is an unpublished study on the use of this device by Dr. Nageswara Rao Koneti in 2017 but there are no written reports regarding its outcomes and complications. Reference Koneti18

Figure 1. The Lifetech MFO VSD Occluder.
Table 1. Specification of Lifetech Multifunctional occluder (MFO)

F = French
DELIVERY SYSTEM, STEEREASE DELIVERY SHEATH
Steerease delivery sheath 4 F, 5 F, 6 F and 7 F that consist of (a) Coil reinforce delivery sheath 800 mm, outer diameter 2.2 mm, inner diameter 1.8 mm; (b) Dilator 800 mm; (c) Haemostatic valve; (d) Loader; (e) Cable 1150 mm
Significance of the study
Our experience in using this new device started in February 2017. We report our procedural and short-term outcomes of patients who underwent transcatheter closure of ventricular septal defect using Lifetech Multifunctional occluder with an emphasis in evaluating the effectiveness and the safety of the intervention.
Methodology
This is a retrospective, descriptive, pilot study wherein 25 patients who underwent transcatheter closure of ventricular septal defect using the Lifetech Multifunctional occluder from February 2017 to January 2018 were included.
The medical records of each patient were reviewed. Relevant demographic characteristics such as weight (kg), height (cm), age, sex, comorbidities, electrocardiogram (ECG) rhythm disturbances, and heart failure New York Heart Association (NYHA) classification (I–IV) were extracted.
The initial transthoracic two dimensional echocardiogram reports and clips were reviewed, and the following measurements were extracted: ventricular septal defect type, ventricular septal defect right ventricular defect size (mm), distance of the defect to the aortic valve (mm), presence of tricuspid or aortic regurgitation (trivial/mild/moderate/severe), left atrial and left ventricular end diastolic diameter (mm & z score).
Cardiac catheterisation reports were reviewed, and procedural characteristics were recorded such as angiographic ventricular septal defect right ventricular defect size (mm), number of defects, mean pulmonary artery pressure (mmHg), fluoroscopy time, procedure time, antegrade/retrograde approach, ventricular septal defect device implanted, procedural success rate, need for changing the device size, periprocedural complication, and type of anesthesia used.
The length of hospital stay and the need for IUC stay were extracted. The patient’s transthoracic echocardiographic results on the 1st post-op day, 1 month, and after 6 months post-transcatheter closure were examined to document the closure rate of the defect, size of the left atrial/ventricular end diastolic diameter (mm & z score), presence of new onset tricuspid or aortic regurgitation, and the resolution of pre-procedure tricuspid or aortic regurgitation. Other clinical conditions recorded include incidence of rhythm disturbances, atrioventricular block, haemolysis, migration, embolisation, shedding of the occluder, thromboembolism, infective endocarditis, and device/procedural related serious adverse events.
Categorical data were described as frequencies and proportion. The collected data collected were encoded in Microsoft excel. Checking for inconsistencies and completeness of the data were done prior to analysis using Stata 12 statistical software.
The University of Philippines–Philippine General Hospital was the site for the study wherein patients were admitted, equipped with facilities to do the procedure, and address the risks of the study and medical records were retrieved. The University of Philippines Manila Research Ethics Board approved this study. All identifiable personal data were anonimised and kept confidential.
Results
Twenty-five patients who underwent transcatheter closure of ventricular septal defect using Lifetech Multifunctional occluder were included in the study (Table 2). The average age of patients was 9.32 years (±7.20) with a range from 1 to 32 years. The majority of the subjects were female (60%). Median weight and height were 22.25 kg and 121.45 cm.
Table 2. Baseline and procedural characteristics of patients with VSD who underwent transcatheter closure using Lifetech MFO

There was 1 patient with a patent ductus arteriosus while the remaining 24 patients had isolated ventricular septal defects. Ninety-three percent of the subjects had no rhythm ECG disturbances while two patients with perimembranous ventricular septal defect had a baseline pre-ventricular septal defect device closure RSR patterns. One patient had RSR patterns seen in leads III, AVF, and AVL only. The other patient had RSR patterns seen in leads V1, V4R and V5R. The ECG tracings of these two patients remained unchanged from baseline up to 6 months post-transcatheter closure.
Twenty-one patients (84%) presented in NYHA class 1, while the remaining four patients had NYHA class 3 (16%). According to ventricular septal defect type, 21 (84%) were perimembranous, 2 (8%) had subaortic, 1 (4%) had doubly committed, and 1 (4%) with subaortic with right coronary cusp prolapse. All procedures were performed under intravenous anesthesia and no case required conversion to general anesthesia.
The Lifetech Multifunctional occluder ventricular septal defect device sizes used was chosen to be exactly the same size to up to 2 mm larger than the right ventricular side defect as measured on transthoracic echocardiogram and/or angiogram. The final size decision was on the discretion of the attending physician. The most commonly used devices are the 2 smaller sizes 5–3 mm (36%) and 6–4 mm (28%), accounting for 64%. The remaining incidences with regard to sizes used were 7–5 mm (20%), 9–7 mm (8%), 8–6 mm (4%), and 10–8 mm (4%). As measured by transthoracic echocardiography, the mean echo right ventricle side defect, distance from aortic valve, left atrium, and left ventricular end diastolic diameter were 3.84 cm (±0.97 cm), 4.85 mm (±2.16 mm), 25.79 mm (Z + 1.27), and 39.98 (Z + 0.41) respectively.
On angiography, the average number of right ventricular openings in the septal defects was 1.08 (±0.28). The average angiographic right ventricle defect size was 3.99 mm (±1.55 mm). The mean pulmonary artery pressure was 17.2 mmHg (±9.04 mmHg) and there were no cases with pulmonary arterial hypertension greater than 41 mmHg. Antegrade approach (Fig 2) was performed in 92% (23) of the cases, and 8% (2) had retrograde approach (Fig 3). The average fluoroscopy time was 17.25 minutes (±13.04 minutes) and procedure time was 78.96 minutes (±33.90 minutes). The average length of stay was 3.12 days (with a range of 3–6 days). No patient required ICU care post-transcatheter closure.

Figure 2. Steps in Antegrade approach. ( a ) Left ventricular angiogram showing the VSD. ( b ) The VSD is crossed retrogradely from the left ventricle using a modified cut pigtail or judkins right catheter and a 260 cm glide wire, which is advanced into the main pulmonary artery or superior vena cava. The wire is snared from a femoral vein approach and externalized creating an arteriovenous wire loop. ( c ) The delivery sheath is advanced from the femoral vein through the VSD and into the descending aorta. The wire is removed. ( d ) The defect is measured using angiographic measurements. The device was chosen to be exactly the same size to up to 2 mm larger than the right ventricular side defect as measured on transthoracic echocardiogram and/or angiogram. The device was advanced through the sheath and deployed across the VSD in a standard fashion. ( e ) Repeat left ventricular angiogram confirms the placement of the device within the defect. Aortogram is done to assess aortic regurgitation and transthoracic echocardiography to assess tricuspid regurgitation. ( f ) The device is released from the delivery system.

Figure 3. Steps in Retrograde approach. ( a ) Left ventricular angiogram showing the VSD. The defect is measured using angiographic measurements and the device was chosen to be exactly the same size to up to 2 mm larger than the right ventricular side defect as measured on transthoracic echocardiogram and/or angiogram. ( b ) The VSD is crossed retrogradely from the left ventricle using a modified cut pigtail or judkins right catheter and a 260 cm glide wire, which is advanced into the right ventricle. The pigtail or judkins right catheter is removed and the delivery sheath is advanced retrogradely from the femoral artery through the VSD and into the right ventricle. The wire is removed. ( c ) The device is advanced through the sheath and deployed across the VSD in a retrograde fashion. Transthoracic echocardiography is done to assess tricuspid regurgitation. The device is released from the delivery system.
All of the 25 patients had a successful transcatheter closure of their ventricular septal defect without the need for a change in the initially selected device size during the procedure.
Based on the available patient records, 42% (10/24) of the patients had complete closure of the ventricular septal defect on the 1st post-transcatheter closure day. The complete closure improved to 52% (12/23) at 1 month, and 81% (17/21) at 6 months (Table 3) (Figs 4 and 5). All four patients recorded with extended closure of the defect after 6 months post-transcatheter closure had minimal residual shunting. The echocardiographic measurements of the left atrium and left ventricular end diastolic diameter were observed to decrease by 1 month and continued to improve after 6 months, as is expected after the closure of the ventricular septal defect shunt lesion in congenital heart disease.
Table 3. Complete closure rate of the defect at 1 day, 1 month, and 6 months post-transcatheter closure


Figure 4. TTE 5 chamber view showing a left to right shunting through the perimembranous VSD.

Figure 5. TTE 5 chamber view showing a Lifetech MFO with complete occlusion of the defect.
Two (8%) of the 25 cases had periprocedural complications. One patient had an inadvertent urinary bladder puncture upon right femoral venous access. The left femoral venous access was used instead. The complication was addressed by inserting an indwelling foley catheter, and then the introducer sheath that punctured the urinary bladder was removed. She was treated with antibiotics and repeat cytogram was done after 5 days prior to the removal of the foley catheter. One patient had inadvertent device detachment and embolisation during device manipulation using an antegrade approach. The device was easily retrieved using a three loop snare in the mid right pulmonary artery. The same device was successfully deployed across the ventricular septal defect through a retrograde approach. The double-sided screw of the device is an advantage versus other device. It provided procedural flexibility to facilitate antegrade or retrograde approach in delivering the device.
The incidence of pre-procedure tricuspid regurgitation was 52% (13/25) (Table 4). One of the 25 patients 4% (1) had severe tricuspid regurgitation, 4% (1) had moderate, 32% (8) had mild tricuspid, and 12% (3) had trace tricuspid regurgitation. Forty-eight percent (12/25) of the patients had no baseline pre-procedure tricuspid regurgitation.
Table 4. Incidence of pre-procedure tricuspid regurgitation

Out of the 13 patients with pre-procedure tricuspid regurgitation, the regurgitation disappeared after 1 day, 1 month, and 6 months post-transcatheter closure by 8% (1), 23% (3), and 38% (5), respectively.
The pre-procedure tricuspid regurgitation decreased by 23% (3) from moderate and severe regurgitation to mild and mild to trace after 1 day post-transcatheter closure. The patients maintained the same trace or mild regurgitation after 1 month and 6 months thereafter at 75% (9) and 61% (8) respectively (Table 5).
Table 5. Course of pre-procedure tricuspid regurgitation

Of the 12 patients without pre-procedure tricuspid regurgitation, 66% (8/12) had new onset tricuspid regurgitation after 1 day post-transcatheter ventricular septal defect closure. Of which, three patients had mild tricuspid regurgitation while five had trace tricuspid regurgitation. At 6 months post-transcatheter ventricular septal defect closure, there was resolution of the tricuspid regurgitation in five cases. Only one patient (1/12) 8% maintained the mild tricuspid regurgitation while two (2/12) 17% continued to have a trace tricuspid regurgitation (Table 6).
Table 6. Incidence of new tricuspid regurgitation

The pre-procedure aortic regurgitation was present in two of the patients (8%). One had mild aortic regurgitation with a subaortic ventricular septal defect with right coronary cusp prolapse and another case had trace aortic regurgitation with a subaortic ventricular septal defect. Both maintained the same status after transcatheter closure and throughout the 6-month follow up.
New aortic regurgitation after the 1st day post-transcatheter closure of the ventricular septal defect was present among three patients (3/23), 13% of which had trace aortic regurgitation. The trace aortic regurgitation eventually disappeared after 1 month post-transcatheter closure. The two patients with a subaortic and doubly committed ventricular septal defect with no baseline aortic regurgitaiton did not develop any aortic regurgitation in the course of follow up.
One patient failed to undergo an ECG at 6-month follow up. Of the remainder, one of the 24 patients (4%) developed a widened QRS complex in all limb leads shortly after the Lifetech Multifunctional occluder device was released (Fig 6). This was a 4-year-old boy who weighed 16.5 kg, with a 7 mm perimembranous ventricular septal defect that was closed using a Multifunctional occluder 9–7 mm device via an antegrade approach. The ECG normalised with a narrow QRS complex at 1-month follow up and has remained normal up to the 6th month post-transcatheter follow up.

Figure 6. Post transcatheter closure tracing showing widened QRS complexes in all limb leads.
There were no atrioventricular block, haemolysis, migration, shedding of the occluder, thromboembolism, infective endocarditis, and device/procedure related serious adverse events.
There were two patients with ventricular septal defects that were associated with a right ventricular outflow tract obstruction. The first case is a 1-year-old male who weighed 9 kg with a 7 mm ventricular septal defect perimembranous defect. On pre-closure, there was a mild infundibular pulmonary stenosis with a gradient of 38 mmHg (Figs 7–9). The gradient decreased to 19 mmHg immediately post-closure and resolved at 1 month post-closure. The second case is an 8-year-old female weighing 22.5 kg who had a 6 mm perimembranous ventricular septal defect with a mild infundibular pulmonary stenosis of 26 mmHg that remained unchanged 9 months post-closure (Figs 10–11).

Figure 7. TTE short axis view showing a perimembranous VSD with mild infundibular pulmonary stenosis with a gradient of 38 mmHg.
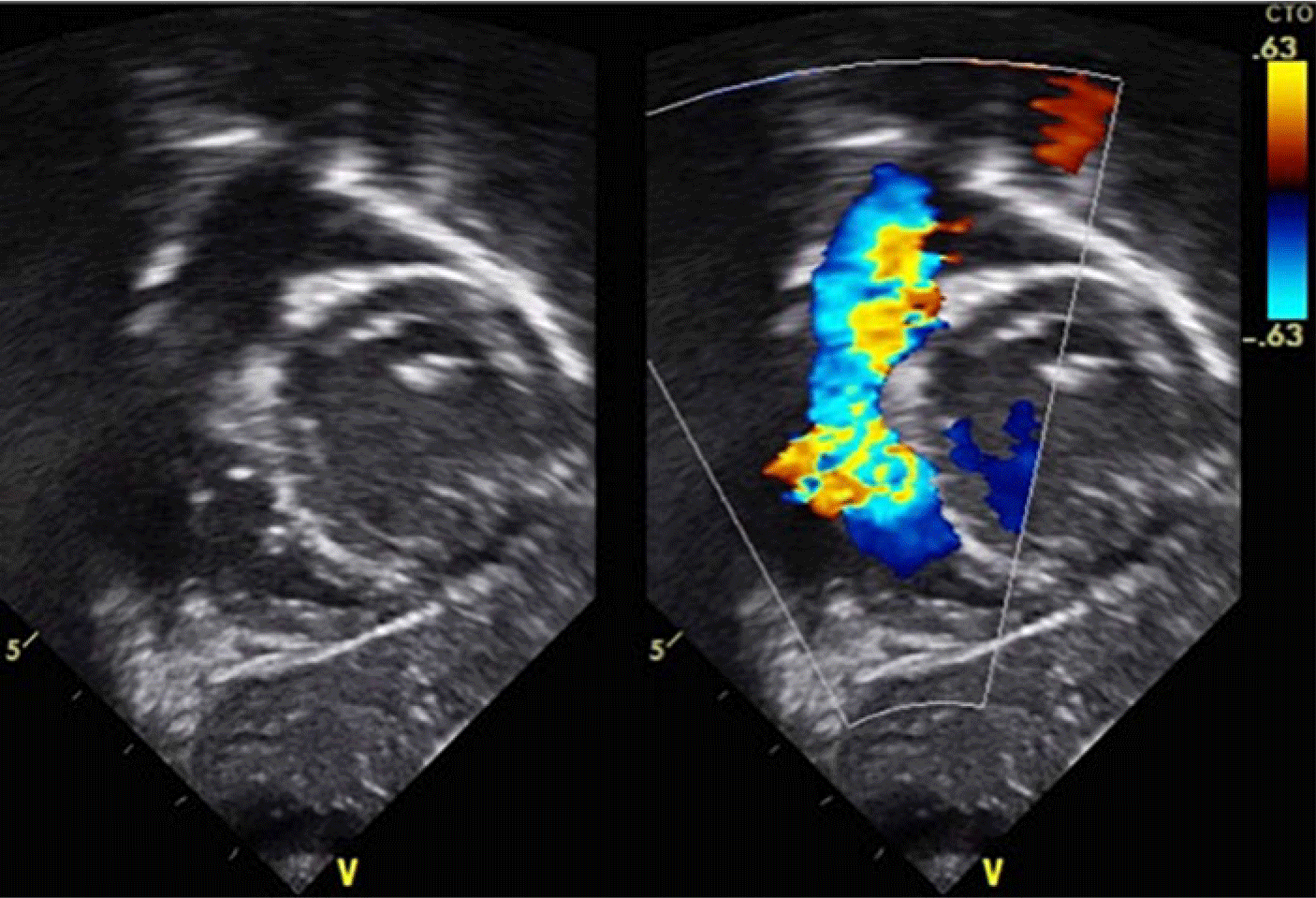
Figure 8. TTE subcostal view showing a perimembranous VSD with mild infundibular pulmonary stenosis with a gradient of 38 mmHg.
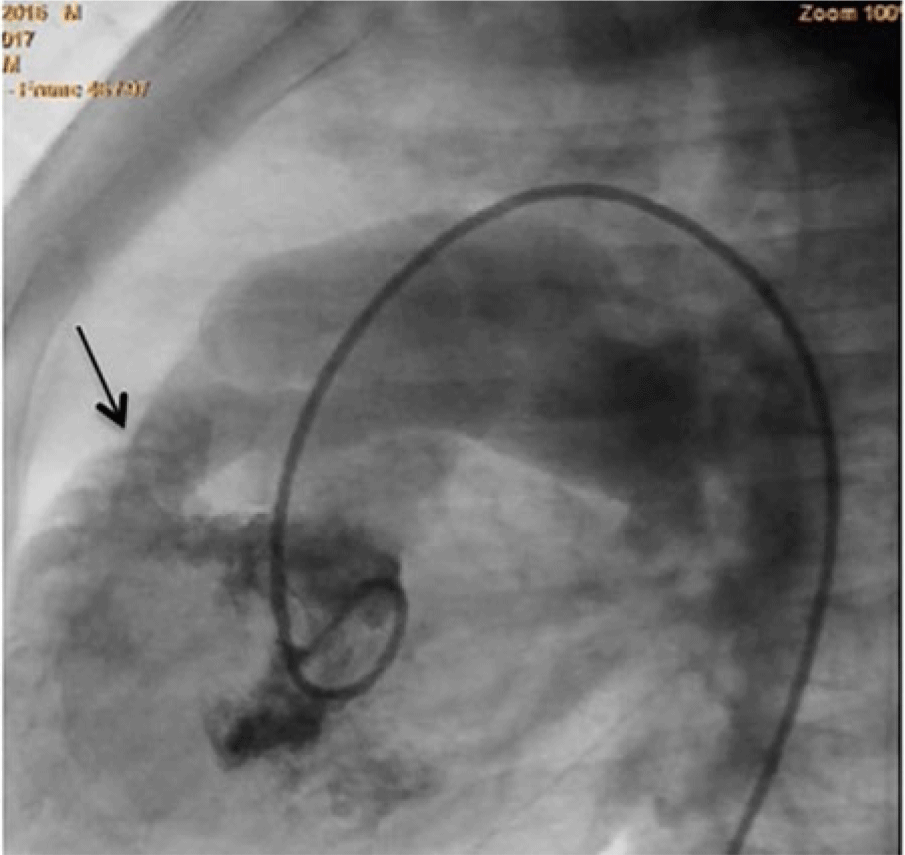
Figure 9. Left ventriculogram in LAO Cranial view showing an egress of dye through the perimembranous VSD to the right ventricle with mild infundibular pulmonary stenosis.

Figure 10. TTE short axis view showing a perimembranous VSD with mild infundibular pulmonary stenosis with a gradient of 26 mmHg.

Figure 11. Angiogram in the right ventricular outflow tract in lateral view showing a mild infundibular stenosis.
Discussion
We had a procedural success of 100% in the transcatheter closure of ventricular septal defect, with none needing a change of the initially chosen device size. The device size was chosen to be exactly the size up to 2 mm larger than the right ventricular side defect as measured on transthoracic echocardiogram and/or angiogram. In our series, the closure rate was at 42% (10/24), 52% (12/23), and 80% 16/20 (80%) at 1 day, 1 month, and 6 months follow up. These closure rates are similar to Amplatzer muscular ventricular septal defect occluder closure rates at 47.2% (34/72), 69.6% (32/46), and 92.3% (24/26) at 1 day, 6 months, and 12 month follow up. Reference Holzer, Balzer, Cao, Lock and Hijazi19 To establish the long-term effectiveness of this device, a larger sample size and a longer duration of monitoring are warranted.
Other echocardiographic measurements include regurgitation of the tricuspid and aortic valves. After transcatheter closure, in the 13 cases with pre-procedure tricuspid regurgitation, 5 (38%) resolved at 6-month follow up. One patient with severe tricuspid regurgitation secondary to a Gerbode type B defect (Figs 12–14) had near total resolution of the tricuspid regurgitation after transcatheter closure (Figs 15 and 16). The type B Gerbode, or Indirect type, has blood flow from the left ventricle shunting through the ventricular septal defect into the right ventricle and then through the septal leaflet defect of the tricuspid valve into the right atrium. Reference Riemenschneider and Moss20 There was also no progression in the remaining eight cases.

Figure 12. Gerbode Defects. The type A or Direct type, the blood shunts from the left ventricle through the membranous septum above the tricuspid valve into the right atrium. The type B Gerbode, or Indirect type, the blood flow from the left ventricle shunting through the VSD into the right ventricle and then through the septal leaflet defect of the tricuspid valve into the right atrium. Image taken from Vijayalakshmi IB. Device Closure of Small Ventricular Septal Defects: When and Why? BMH Medical Journal (ISSN 2348-392X), 1(3): 56-63 (2014).

Figure 13. TTE 4 chamber view showing a perimembranous VSD with shunting of the flow through the septal tricuspid leaflet causing tricuspid regurgitation.

Figure 14. TTE 4 chamber view showing a severe tricuspid regurgitation from a type B or Indirect Gerbode defect.

Figure 15. TTE 5 chamber view showing occlusion of the Geborde type B defect with no residual shunt.

Figure 16. TTE 4 chamber view showing resolution of the tricuspid regurgitation after occlusion of the Gerbode defect.
The new onset tricuspid regurgitation at 6-month follow up was mild (8%) and trace (17%) in 1 or 2 cases out of 12 cases, respectively. This new onset tricuspid regurgitation may be due to distortion of the tricuspid subvalvar apparatus from the right-side wing of the Lifetech Multifunctional occluder device, or from the catheter manipulations during device deployment. The right-side wing of the Lifetech Multifunctional occluder device is 1–2 mm larger than the right-side wing of the Lifetech Cera Ventricular Septal Defect occluder device which could be a contributory factor. 21 This larger wing of the Multifunctional Occuder device on the right side may pose a disadvantage due to development of tricuspid regurgitation. Although the development of trace and mild tricuspid regurgitation maybe clinically not significant and can usually be considered as normal findings in normal hearts, further monitoring for the progression of tricuspid regurgitation is warranted to elucidate the right venticular wing interaction with the septal leaflet chordae of the tricuspid valve. If there is progression to a severe tricuspid regurgitation, transcatheter retrieval of the device and change to a smaller right-wing disk may be done or surgical retrieval of the device, ventricular defect patch closure and repair of the tricuspid valve via open heart surgery can be a backup strategy.
New onset aortic regurgitation was noted on the 1st day post-transcatheter closure in 3 of 23 cases (13%). However, these findings were transient and disappeared at 1-month follow up. There were two cases with baseline mild and trace aortic regurgitation associated with a subaortic ventricular septal defect. After the transcatheter ventricular septal defect closure, the degree of aortic regurgitation remained the same throughout the 6-month follow up. Closure of the defect did not improve or worsen the aortic regurgitation. In two other cases, one with a subaortic and another with a doubly committed ventricular septal defect, both did not develop any aortic regurgitation in the 6-month of follow up. The elongated waist of the device is well adapted for a variety of ventricular septal defects. The soft nitinol and the flexible design allow the Lifetech Multifunctional Occluder to conform not only to the perimembranous type of ventricular septal defect but also to subaortic and doubly committed ventricular septal defects. As long as there is a 2 mm distance from the aortic valve to the ventricular septal defect, transcatheter closure of the ventricular septal defect using Lifetech Multifunctional occluder is safe with regards to the aortic valve function. Long-term follow up as to the effect of the device to the subarterial defects is still required. If there is any progression to a moderate aortic regurgitation, surgical retrieval of the device, ventricular septal defect patch closure, and repair or replacement of the aortic valve via open heart surgery is a backup strategy.
Only one (4%) case with a 7 mm perimembranous ventricular septal defect, which was closed using a Lifetech Multifunctional occluder 9–7 mm device, developed a widened QRS complex of 0.12 seconds post-closure from a baseline of 0.08 seconds. This widened QRS eventually resolved at 1-month follow up with no intervention done. It is surmised that the frictional rubbing of the catheter or the device may cause an inflammatory response within the conduction tissue. Reference Carminati, Butera and Chessa22 The soft woven mesh of nitinol wires of the Multifunctional occluder device along with its increased flexibility and pivot of the right wing, probably reduces the risk of atrioventricular block, by reducing the clamp and stenting forces compared to the other ventricular septal defect devices. Long-term follow up is still warranted to ascertain the possibility of developing atrioventricular block.
Our study was consistent with the findings of Yang et al Reference Yang, Yang and Yu10 it also showed no significant morbidity and no mortality. There were no myocardial injuries, need for transfusions, and no case requiring ICU stay. There was shorter procedural time, shorter hospital stay, lower hospital expenses, and faster recovery times.
In a perimembranous ventricular septal defect, the most common cause of right ventricular outflow tract obstruction is a right ventricular infundibular hypertrophy or prominent muscle bundles in the right ventricle (double-chambered right ventricle). Reference Mahesh, Kumar, Satheesh and Jayaraman23 In two cases, we had a perimembranous ventricular septal defect with a mild infundibular hypertrophy and there was no membranous ventricular septal aneurysm obstruction seen. Post-transcatheter closure, the 1-year-old patient had resolution of the infundibular hypertrophy but the 9-year-old patient had persistence of the mild infundibular hypertrophy even after 6 months. If the ventricular septal defect is closed at an early age, the chances of hypertrophy resolution may be higher, as long as the infundibular hypertrophy is mild. However, if the infundibular hypertrophy is moderate or severe, surgical resection of the prominent right ventricle muscle or hypertrophy is needed with patch closure of the ventricular septal defect.
Conclusion
Our retrospective review of procedural and short-term outcomes of transcatheter closure of ventricular septal defect sizes 2–10 mm using Lifetech Mutlifunctional Occuder appears to be safe and effective. However, a larger sample size and long-term monitoring is warranted.
Limitations of the study and recommendation
The retrospective nature of the study and the absence of a protocol account for some lacking data in the review of the case records.
Based on this review, a 5–10-year prospective study that monitors the course of the treatment, possible long-term complications, morbidity, and quality of life of these patients is recommended.
Acknowledgements
I would like to thank our mentors at the section of Pediatric and Congenital Cardiac Catheterization and Interventional Cardiology at the Philippine General Hospital for the guidance. To my co fellows, my wife Tina and kids Nick and Joachim, thank you for the support.
Financial support
The author funded this study. There was no financial support or grants from the industry.
Conflict of interest
There was no conflict of interest from financial, proprietary considerations of the author or the study site.
Ethical standards
The University of the Philippines Manila Research Ethics Board approved the study. Strict confidentiality was imposed and the anonymity was protected.

























