INTRODUCTION
The worldwide occurrence of biological invasions still represents a challenge for the ecologists. The introduction of non-indigenous species (NIS) can affect the integrity and functioning of ecosystems, being one of the most important causes of native biodiversity loss. In addition, NIS can introduce diseases and parasites to an ecosystem and they can potentially impact on the dynamics of endemic parasites (Poulin et al. Reference Poulin, Paterson, Townsend, Tompkins and Kelly2011). As pointed out by Kelly et al. (Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009) and by Poulin et al. (Reference Poulin, Paterson, Townsend, Tompkins and Kelly2011) introduced species can influence, directly or indirectly, the transmission of native parasites among native species in different ways: (1) they can act as competent hosts for endemic parasites, amplifying the infection which can ‘spill back’ to native fauna, (2) they can act as sinks for parasites thus diluting disease risk for native hosts, (3) they can alter the abundance of the parasite's hosts, enhancing or reducing disease transmission to other native hosts in the parasite's life cycle and (4) they can alter disease incidence and severity in native hosts through trait-mediated effects (e.g. by altering the behaviour or immunocompetence of native hosts, introduced species could change their exposure or susceptibility to infections.)
The reef-forming polychaete Ficopomatus enigmaticus (Fauvel, 1923) (Serpulidae) is a successful invasive species in several lagoons and estuaries of the world (Bruschetti et al. Reference Bruschetti, Luppi, Fanjul, Rosenthal and Iribarne2008). In the Mar Chiquita coastal lagoon, a World Reserve of Biosphere since 1996, located in the Buenos Aires Province, Argentina (37°32′S, 57°19′W), the presence of F. enigmaticus was first recorded in the early 1970s (Orensanz and Estivariz, Reference Orensanz and Estivariz1972). During the last decades the large circular reefs built by this species, which can reach up to 7 m in diameter (Obenat and Pezzani, Reference Obenat and Pezzani1994; Schwindt et al. Reference Schwindt, Iribarne and Isla2004a) have increased in density and size, covering up to 86% of the brackish portion of the lagoon (Schwindt et al. Reference Schwindt, De Francesco and Iribarne2004b). Besides increasing the topographical complexity and benthic diversity in the lagoon (Schwindt et al. Reference Schwindt, Bortolus and Iribarne2001), F. enigmaticus has important ecosystem engineering effects. The biological activity of this polychaete affects overall estuarine primary production as well as the relative importance of planktonic and benthic carbon sources to higher trophic levels (Bruschetti et al. Reference Bruschetti, Luppi, Fanjul, Rosenthal and Iribarne2008) and modifies flow and sedimentation patterns (Schwindt et al. Reference Schwindt, Bortolus and Iribarne2001). The reef-like aggregates of F. enigmaticus also provide refuge for other species of invertebrates, including crabs, amphipods and mollusks (Schwindt et al. Reference Schwindt, Bortolus and Iribarne2001; Luppi and Bas, Reference Luppi and Bas2002; Obenat et al. Reference Obenat, Spivak and Garrido2006; Bruschetti et al. Reference Bruschetti, Bazterrica, Luppi and Iribarne2009), serve as resting and feeding areas for migratory and local breeding species of shorebirds that use the lagoon as stopover site (Botto et al. Reference Botto, Iribarne and Martínez1998; Ferrero, Reference Ferrero and Iribarne2001; Bruschetti et al. Reference Bruschetti, Bazterrica, Luppi and Iribarne2009), and affect abundance and richness of associated fauna (Bruschetti et al. Reference Bruschetti, Bazterrica, Luppi and Iribarne2009).
The gastropods Heleobia conexa (Gaillard, 1974) and Heleobia australis (d'Orbigny, 1835) (Cochliopidae) can be considered as the most abundant among the species of mollusks associated with the reef-like aggregates of F. enigmaticus. Heleobia conexa is usually found in the middle part of the lagoon, where water and air temperatures fluctuate widely and tides are negligible. Generally, H. conexa lives mainly on hard substrata (submerged stones) while in the lagoon this species lives only in the aggregates of F. enigmaticus (De Francesco and Isla, Reference De Francesco and Isla2003; Bruschetti et al. Reference Bruschetti, Bazterrica, Luppi and Iribarne2009). Heleobia australis inhabits the estuarine zone of the lagoon and can be found living in the mud and in the aggregates (Parietti, Reference Parietti2011).
Combined, these two species of gastropods serve as first intermediate hosts for at least 24 species of digeneans (Trematoda: Digenea) (Etchegoin, Reference Etchegoin1997; Merlo, Reference Merlo2009; Merlo and Etchegoin, Reference Merlo and Etchegoin2011; Parietti, Reference Parietti2011). The digeneans are parasites with complex life cycles with different larval stages that parasitize first and second intermediate hosts (mainly mollusks and crustaceans, respectively) to finally mature as adults in vertebrate definitive hosts.
As mentioned earlier, the reef-like aggregates of F. enigmaticus serve as concentration areas for invertebrates and vertebrates, increasing the probability of contact between the intermediate and definitive hosts of digeneans. As a consequence, these areas were considered by Merlo and Etchegoin (Reference Merlo and Etchegoin2011) to be potential foci of parasite transmission.
As a continuation of the study of Merlo and Etchegoin (Reference Merlo and Etchegoin2011) the objectives of the present study were to analyse the role of F. enigmaticus as a facilitator of digenean transmission in Mar Chiquita coastal lagoon, and to evaluate the influence of the habitats selected by 2 species of mollusks (H. conexa and H. australis) on the richness and the prevalence of the digenean assemblages that parasitize them.
MATERIALS AND METHODS
The Mar Chiquita coastal lagoon can be divided into a freshwater zone, characterized by continental water discharge without tidal effects, and an estuarine zone that communicates with the open sea. The estuarine zone is characterized by mixo-euryhaline waters and is greatly influenced by marine water (Reta et al. Reference Reta, Martos, Perillo, Piccolo, Ferrante and Iribarne2001) while the freshwater zone receives freshwater and sediment from a drainage basin of approximately 10 000 km2 (Fasano et al. Reference Fasano, Hernández, Isla and Schnack1982) (Fig. 1). Nevertheless, the limits between the two zones and the levels of salinity are extremely variable (Reta et al. Reference Reta, Martos, Perillo, Piccolo, Ferrante and Iribarne2001).
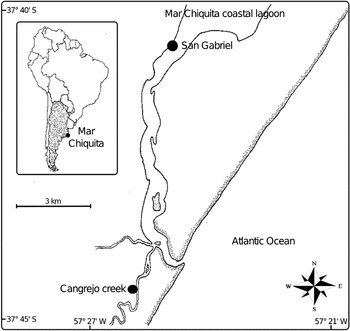
Fig. 1. Map of Mar Chiquita coastal lagoon, showing sampling sites.
The present study was performed during November 2010. The sampling sites were selected according to the existence of stable populations of both H. conexa and H. australis and only 2 locations fulfilled this requirement: (a) San Gabriel, and (b) the Cangrejo creek (Fig. 1). In San Gabriel the abundant reef-like aggregates of the serpulid F. enigmaticus serve as a unique habitat of the snail H. conexa while in Cangrejo creek, H. australis can be found inhabiting soft substrate (mud) and also the aggregates of F. enigmaticus. During the study, the two species of mollusks did not share the same area of distribution. In fact, H. conexa was the only species of mollusks present in San Gabriel and H. australis is the only species in Cangrejo creek.
In the sampling sites, snails were collected using random cores (15 cm diameter × 15 cm deep), and then placed into plastic cups filled with water from the lagoon for transportation. In the laboratory, snails were removed from reefs and mud, counted and measured with a Vernier caliper (precision: 0·1 mm). Each snail was isolated in 45 ml vol. plastic cups and exposed to a 100 W incandescent lamp for 48 h to stimulate shedding of cercariae (patent infections). Finally, all gastropods were dissected under a stereomicroscope in order to detect the presence of sporocysts, rediae and immature cercariae (pre-patent infections) (Curtis and Hubbard, Reference Curtis and Hubbard1990). Shed cercariae, sporocysts, rediae and immature cercariae were identified according to Etchegoin and Martorelli (Reference Etchegoin and Martorelli1997, Reference Etchegoin and Martorelli1998), Martorelli, (Reference Martorelli1986, Reference Martorelli1988, Reference Martorelli1989, Reference Martorelli1990 and Reference Martorelli1991) and Martorelli and Etchegoin (Reference Martorelli and Etchegoin1996).
With reference to host minimum sample sizes, the criteria proposed by Merlo et al. (Reference Merlo, Parietti and Etchegoin2010) were followed. These authors concluded that approximately 300 individuals of H. conexa or H. australis from Mar Chiquita represent a good minimum sample size to estimate total diversity of its larval digenean community (including rare species). In this study, all the samples including 300 or more snails were considered for further comparisons of the diversities and prevalences of larval digenean communities in both snail host species. According to the criteria of Merlo et al. (Reference Merlo, Parietti and Etchegoin2010) a total of 9 cores were finally selected: 3 cores from the mud plus 3 cores from the aggregates at Cangrejo creek and 3 cores from the aggregates at San Gabriel.
In order to analyse possible differences in abundance and size of specimens of H. australis, collected in ‘Cangrejo’ creek from soft substrates (mud) and reefs (hard substrates), a t-test was used (Zar, Reference Zar2009).
To analyse and to compare the composition of the community of larval digeneans in H. australis and H. conexa in the different habitats (mud and reef-like aggregates in Cangrejo creek and reef-like aggregates in San Gabriel), the following indices were computed for each sample: (a) Species richness (S) which represents the total number of species in a sample (Ludwig and Reynolds, Reference Ludwig and Reynolds1988; Magurran, 1988) and (b) Overall prevalence = the number of parasitized snails/ the number of collected snails × 100 (Lafferty et al. Reference Lafferty, Sammond and Kuris1994).
According to Etchegoin (Reference Etchegoin1997), Merlo (Reference Merlo2009), Merlo and Etchegoin (Reference Merlo and Etchegoin2011) and Parietti (Reference Parietti2011), the diversity of larval digeneans in H. conexa and in H. australis is strongly influenced by the presence of bird and fish definitive hosts. To estimate the contribution of these two groups of vertebrates to the diversity and prevalence of larval digeneans parasitizing H. conexa and H. australis, the following indices were used: (c) Species richness contributed by bird (Sbird) which represents the total number of species of digenean that use birds as definitive host; (d) Species richness contributed by fish (Sfish) which represents the total number of species of digenean that use fish as definitive host; (e) Prevalence contributed by birds (Pbird) = the number of snails parasitized by larval digeneans that used birds as definitive host/the number of collected snails × 100, and (f) Prevalence contributed by fish (Pfish) = the number of snails parasitized by larval digeneans that used fish as definitive host/the number of collected snails × 100.
The diversity (S) and the prevalence of larval digeneans parasitizing H. conexa and H. australis, estimated for the different habitats and for the two groups of vertebrate definitive hosts, were analysed and evaluated using a one-way ANOVA. Tukey tests were performed for post-hoc comparisons (Zar, Reference Zar2009). To analyse the homogeneity of variances and normality, the Levene and Shapiro-Wilks tests were used, respectively (Zar, Reference Zar2009).
RESULTS
A total of 1645 specimens of H. australis were examined from Cangrejo creek (743 snails collected from reef-like aggregates of F. enigmaticus, and 913 collected from soft substrate). In San Gabriel, 300 specimens of H. conexa were collected from the reef-like aggregates.
The densities of the snails H. australis were 0·96 ind/cm3 (± 0·29) and 1·37 ind/cm3 (±0·15) in hard and soft substratum respectively, although these values were not significantly different (t = − 2·15, d.f. = 4, P=0·097). In the same way, sizes of the snails were 5·53 mm (±0·74) in reef-like aggregates and 5·57 mm (±0·77) in mud. These values, again, were not different (t= − 1·01 d.f. = 1642, P = 0·30).
The species richness of larval digeneans was similar between the three sampling habitats: San Gabriel (reefs), Cangrejo creek (mud and reefs) (one way ANOVA, F2,6 = 0·5, P = 0·63) (Fig. 2). In total, 15 digenean species, belonging to 8 families, were observed. Furthermore, only 6 species of digeneans were observed in all sampling sites and the remaining species were recorded in only one or two sites (Table 1). From the 15 digeneans species, 26·7% use fish, 66·7% use birds and/or mammals and 6·7% use reptiles as definitive hosts. The Sbird and Sfish were similar between the three sampling habitats (Sbird one way ANOVA, F2,6 = 4·11, P = 0·07 and Sfish one way ANOVA, F2,6 = 1·46, P = 0·30).
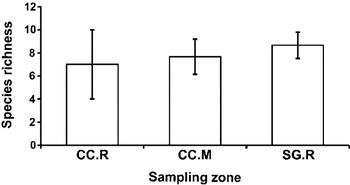
Fig. 2. Values of species richness (±s.d.) of the larval digenean community parasitizing Heleobia australis and Heleobia conexa in the three habitats considered. CC.R: Cangrejo creek, reefs; CC.M: Cangrejo creek, mud and SG.R: San Gabriel, reefs.
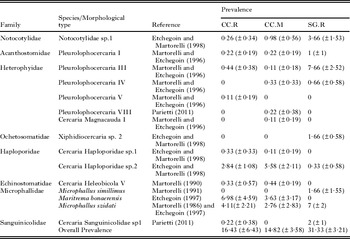
Table 1. Prevalence and detailed list of species or morphological types of larval digeneans parasitizing Heleobia australis and Heleobia conexa in the three habitats considered
The overall prevalence of larval digeneans was higher in reef-like aggregates from San Gabriel than in reef-like aggregates and mud from Cangrejo creek (one-way ANOVA, F2,6 = 9·25, P < 0·05, Tukey test P < 0·05) (Fig. 3). However, the overall prevalences of larval digeneans in hard and soft substratum in Cangrejo creek were similar (Tukey test P = 0·94). The comparison of Pbird revealed higher values in reefs from San Gabriel than in reefs and mud of Cangrejo creek (one-way ANOVA, F2,6 = 17·64, P < 0·05, Tukey test P < 0·05). In contrast, the values of Pfish were similar between the three sampling habitats (one-way ANOVA, F2,6 = 3·62, P = 0·09).
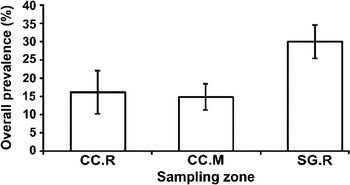
Fig. 3. Overall prevalences (±s.d.) of the larval digenean community parasitizing Heleobia australis and Heleobia conexa in the three habitats considered. CC.R: Cangrejo creek, reefs; CC.M: Cangrejo creek, mud and SG.R: San Gabriel, reefs.
DISCUSSION
In general, the larval digenean communities in snails hosts are characterized by their spatial and temporal variability in species composition (e.g. Curtis and Hurd, Reference Curtis and Hurd1983; Sousa, Reference Sousa, Esch, Bush and Aho1991; Esch and Fernández, Reference Esch and Fernández1994; Jokela and Lively, Reference Jokela and Lively1995; Curtis, Reference Curtis1997; Kube et al. Reference Kube, Kube and Dierschke2002; Merlo and Etchegoin, Reference Merlo and Etchegoin2011). This variability, and the temporal and spatial fluctuations in larval digenean prevalences, have been related to the biology of intermediate and definitive hosts, to abiotic environmental factors and to interspecific interactions among parasite species (e.g. Kuris, Reference Kuris, Esch, Bush and Aho1991; Skirnisson et al. Reference Skirnisson, Galaktionov and Kozminsky2004; Byers et al. Reference Byers, Blakeslee, Linder, Cooper and Maguire2008; Faltýnková et al. Reference Faltýnková, Valtonen and Karvonen2008).
According to our results, the larval digenean community of H. australis in Cangrejo creek did not reveal significant variations in diversity (S, Sbird and Sfish) and in prevalence (overall prevalence, Pbird and Pfish) in the two habitats considered (mud and reefs). The similarity in diversity and prevalence may be due to the closeness of the sampling sites and to the homogeneity in host sample composition (snail densities and sizes did not differ between mud and reefs).
With reference to the comparisons between larval digenean communities parasitizing the two snail species (H. conexa and H. australis) in the three habitats, only the overall prevalence and the prevalence contributed by birds (Pbird) revealed significant differences. The higher values of prevalence registered in the larval digenean community of H. conexa from San Gabriel may be attributed to the influence of the reefs of F. enigmaticus on the abundance and richness of associated fauna (Bruschetti et al. Reference Bruschetti, Bazterrica, Luppi and Iribarne2009). As mentioned earlier, reefs serve as concentration areas for invertebrates and vertebrates (mainly birds) and, in consequence, could facilitate the contact between the intermediate and definitive hosts, increasing the number of hosts parasitized by digeneans. The influence of abiotic factors (mainly salinity) and biological factors related to differences in the biology of the two snail species could be discarded. In fact, in the lagoon (including the two sampling sites selected) H. conexa and H. australis are exposed and adapted to a similar range of salinity which fluctuates daily, and they share larval digenean species belonging to different families (meaning that both species are indistinctly used by the same species of digeneans as suitable first intermediate hosts) (Etchegoin, Reference Etchegoin1997; Merlo and Etchegoin, Reference Merlo and Etchegoin2011; Parietti, Reference Parietti2011).
Another factor that could influence the higher prevalences in San Gabriel is related to the time of establishment and colonization of F. enigmaticus. In the case of San Gabriel, the reefs of F. enigmaticus were detected a long time ago (approximately 3 decades) while the colonization of Cangrejo creek by the invasive polychaete is recent (approximately 4 years). Then, the period of time between the colonization of larval F. enigmaticus and the dissemination of the polychaete in a given area (determined by the increase of density and size of reefs), seems to be important for the establishment and development of digenean life cycles. During this period, the reefs of the exotic species would have been gradually ‘assimilated’ by the environment, becoming important rest, shelter and feeding areas for local species or, as in the case of H. conexa, the preferred habitat. The assimilation process also includes the creation of trophic links between invertebrates and vertebrates which would facilitate the transmission of larval stages and the completion of digenean life cycles. In fact, the extensive elimination of reefs in an open fishing area of the lagoon during 1999, resulted in a noticeable decrease of the abundance and diversity of the larval digenean community of H. conexa. Later, during the years 2004 and 2005, the gradual formation of new calcareous aggregates of the polychaete produced the restoration of links between intermediate and definitive hosts and, in consequence, the increase of the diversity and prevalence of larval digeneans in H. conexa (Merlo and Etchegoin, Reference Merlo and Etchegoin2011). Based on these observations, the authors suggested that the reefs of F. enigmaticus may serve as foci of parasite transmission. Our results support the suggestion of Merlo and Etchegoin (Reference Merlo and Etchegoin2011) only for H. conexa. Probably, the facilitation of parasite transmission by the polychaete could operate only when the reefs become the unique habitat of the snail host. In contrast, the homogeneous distribution of H. australis in hard and soft substrates observed in Cangrejo creek, would produce a dilution in the facilitation of digenean transmission mediated by F. enigmaticus. Nevertheless, the influence of F. enigmaticus on digenean transmission can be observed only in the values of prevalences, meaning that the reefs may increase the number of links between intermediate and definitive hosts. The diversity of larval digenean communities in H. conexa and H. australis may be influenced mainly by the presence and dispersion of the definitive hosts (birds and fish). The two study sites (San Gabriel and Cangrejo creek), separated by only 6 km, are visited by the same species of local and migratory birds and fish. The coincidence in vertebrate species, bearing adult digeneans, could determine the similarity in larval digenean diversity in both sites.
According to the results obtained during this study, the invasive polychaete F. enigmaticus could have an important role in facilitating the transmission of larval digeneans in the lagoon. Their reefs may act as foci of parasite transmission mainly when they are present in a given area during a long time, and when they become the unique or preferred habitat for the snail host species. To our knowledge, this is the first report of an exotic species acting as a facilitator of parasite transmission in an estuarine area. Taking into account the possible displacement of parasites and the emergence of parasites and diseases favoured by global climate change (Poulin, Reference Poulin2006, Reference Poulin2007; Brooks and Hoberg, Reference Brooks and Hoberg2007; Marcogliese, Reference Marcogliese2008) this report also highlights the importance of analysing the effects of the assimilation process of aggressive exotic species, like F. enigmaticus, by recently colonized environments (including the possible effects on the facilitation of parasite transmission).
ACKNOWLEDGMENTS
The authors gratefully thank Dr Robert Poulin (University of Otago, New Zealand) for his valuable suggestions on an earlier version of the manuscript.
FINANCIAL SUPPORT
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 114-200801-00001 to J.A.E); and Universidad Nacional de Mar del Plata (EXA 496/10 to J.A.E.). Jorge A. Etchegoin and Matías J. Merlo are members of CONICET. Manuela Parietti is member of CIC.






