Surgery is integral to the management of CHD, and pain is an expected component of the postoperative period. Pain exposure early in life can make children more susceptible to prolonged pain following subsequent injuries. Reference Schwaller and Fitzgerald1,Reference Pollak and Serraf2 Pain also impacts haemodynamics through heart rate and blood pressure changes, and adequate postoperative pain control reduces the risk of bleeding and cardiac events. Reference Fuller, Kumar and Roy3 For these reasons, dedicated pain management strategies for children and neonates are warranted. Despite the importance of pain management and the ubiquity of postoperative pain, no national or society guidelines currently exist specific to pain in the CHD population. Efforts directed towards enhanced recovery after surgery have addressed pain management, but are not comprehensive in their scope. While numerous review articles have been published on pharmacotherapy strategies, they do not provide actionable guidance for practitioners. Reference Pollak and Serraf2–Reference Wolf and Jackman9 An international survey of 15 centres found significant practice variation in choice of analgesics and dosing. Reference Zeilmaker-Roest, Wildschut and Van Dijk10 Furthermore, a national survey of heart centres conducted by the Paediatric Acute Care Cardiology Collaborative in 2021 revealed only 6 of 34 respondents reported having an institutional written policy regarding pain management, three reported standardised practice among providers but no written policy, and the remaining 25 said there was no standardisation among providers within their institution.
Challenges to creating management guidelines for children with CHD include the current variability in practice, the relative infrequency combined with wide heterogeneity of lesions, changes in baseline physiology with different ages of childhood, and the lack of consensus regarding meaningful endpoints in study design. For pain, specifically, another challenge is presented by the inherent impact of analgesic agents on haemodynamics. In the face of these challenges, it has been suggested that collaborative learning networks such as Paediatric Acute Care Cardiology Collaborative are well suited to undertake the task of creating and disseminating management guidelines. Reference Thompson, Foote and King8 Clinical pathways and clinical practice guidelines have been shown to reduce practice variation, facilitate incorporation of up-to-date research into clinical practice, and improve healthcare quality and outcomes. Reference Graham, Mancher, Miller Wolman, Greenfield and Steinberg11–Reference Neame, Chacko, Surace, Sinha and Hawcutt14 To confer these benefits to children undergoing cardiac surgery, we aimed to create an evidence-based clinical practice guidelines that could be utilised broadly to improve quality of pain management among children undergoing cardiac surgery and reduce practice variability.
Methods and results
Panel composition
Paediatric Acute Care Cardiology Collaborative convened a 19-member panel with expertise in paediatric cardiology and postoperative care including paediatric cardiologists, advanced practice practitioners, pharmacists, a paediatric cardiothoracic surgeon, and a paediatric cardiac anaesthesiologist. This panel was tasked with reviewing the literature and evidence as well as existing site-specific pain management protocols and formulating recommendations.
Target audience and scope
The intent of this clinical practice guidelines is to provide guidance on management of routine postoperative pain among children undergoing cardiac surgery. It is intended to address term gestational infants, children, and adolescents undergoing cardiac surgery who are extubated within 24 hours of surgery. The intended audience is any healthcare provider who manages pain in such patients. Sedation, delirium, chronic pain, pain related to cardiac procedures but not surgery such as catheterisation or electrophysiology studies, habituation, and withdrawal are outside of the scope of this clinical practice guidelines.
Evidence review
The panel collectively determined the scope and key questions as well as inclusion criteria to guide the evidence review. The literature was searched using PubMed, SCOPUS, EMBASE, and the Agency for Healthcare Research and Quality (formerly National Guideline Clearinghouse) (Supplemental Table). Articles were excluded if they were published prior to the year 2000 or not available in English. Case reports, editorials, letters, and commentaries were also excluded. Search results and article retrieval are detailed in Figure 1. Searches were conducted on 9/18/21 and 9/28/21. References from searched articles were also considered and included if they met above criteria. Search criteria included terms related to the paediatric population but adult data were included if the studies offered reliable, robust data, and strength of recommendation was downgraded if based on adult data exclusively. Articles were categorised by broad topics and each article was reviewed by two-panel members from different centres. If the two-panel members disagreed about the quality of the evidence or strength of recommendation to be generated from the article, a third-panel member from a third centre also read the article and adjudicated the decision.

Figure 1 Results of literature search. Dates of searches: PubMed 9/18/21, EMBASE and SCOPUS 9/28/21.
Searching of the databases resulted in 1196 articles, 531 of which were duplicates. Further screening resulted in 113 eligible articles which were reviewed in full for inclusion. Articles addressing preoperative and intraoperative topics were included if they also studied impact of interventions on postoperative pain.
In addition to review of the published literature, Paediatric Acute Care Cardiology Collaborative sites were contacted and asked to share any existing site-specific protocols. These were reviewed after completion of the literature review and panel members had the opportunity to generate recommendations based on expert opinion of these protocols if not otherwise addressed in the literature.
Grading of the evidence and recommendations
The panel rated the quality of the evidence and strength of recommendation using methods described by the Grading of Recommendations Assessment, Development, and Evaluation) Working Group. Reference Guyatt, Oxman and Kunz15–Reference Guyatt, Oxman and Vist17 The quality of the evidence was rated as high, moderate, low, or very low, and the strength of recommendation was rated as strong or weak. Generally, quality of the evidence assessment is based on the study design, study limitations, and criticality of outcomes. Recommendations based on high-quality evidence are unlikely to change in the future, even if new research is published. In contrast, recommendations based on low- or very low-quality evidence have a high likelihood of changing in the future based on new evidence. The strength of recommendation is largely based on how clearly the potential benefits of following the recommendation outweigh the potential risks or harms of not following the recommendation. For weak recommendations, choosing whether to follow it must be based on consideration of individual circumstances including personal preferences and values of patients and clinicians. A strong strength of recommendation does not necessarily correlate with high quality of the evidence and vice versa. If a recommendation is supported by multiple articles with differing levels of quality of the evidence or strength of recommendation, the highest quality of the evidence and strength of recommendation are reported. For recommendations based on expert opinion, on which there is no available peer-reviewed literature, quality of the evidence is denoted as not applicable (N/A) and strength of recommendation is based on the panel consensus. Specific age ranges in recommendations reflect the populations in the supporting studies.
Guideline development process
The panel met virtually in September 2021. The scope and key definitions were agreed upon during that meeting. A modified Delphi technique was then employed. Reference Hasson, Keeney and McKenna18 For Round 1 of the Delphi, panel members reviewed a subset of the identified literature (as described above) and created an initial set of potential recommendations. Of the 113 included articles (8 included adult data), 110 served as the basis for potential recommendations which were voted on in Delphi Round 2. After analysis of the results of Delphi Round 2, site-specific protocols were distributed to panel members who then had the opportunity to submit recommendations based on expert opinion addressing any topics for which peer-reviewed literature was unavailable. This was intentionally completed after evidence review to limit bias when assessing the literature. Round 3 of the Delphi was focused on these expert recommendations, as well as literature-based recommendations which had not previously achieved consensus. In total, 4 Delphi rounds were completed and 63 recommendations were accepted (Fig 2). Two recommendations were based solely on adult data. In subsequent review, 3 recommendations were felt to be repetitive or non-contributory and were removed. For all Delphi rounds subsequent to Round 1, consensus was defined as greater than two-thirds majority and was required for approval or rejection of recommendations, but the panel aimed for unanimity or near unanimity. Except in one specific case, there were no reported conflicts of interest, and all panellists voted on all recommendations. One panellist reported a conflict of interest related to review of an article they had authored and therefore recused themselves from voting on that specific recommendation.

Figure 2 Results of Delphi Rounds. Three recommendations were removed after completion of the Delphi rounds as they were felt to be repetitive or non-contributory.
The panel finalised the recommendations in April 2022. Once recommendations were finalised, a guideline was drafted and reviewed by all panellists for editing. Once panellists agreed upon the written guideline, it was shared with the Paediatric Acute Care Cardiology Collaborative Executive Committee, comprised of 30 members from 16 centres in North America plus two patient family representatives, for peer review. After another round of revisions among the clinical practice guidelines panel, the guideline was finalised and submitted for external peer review.
Recommendations
Clinical practice guidelines use and general information
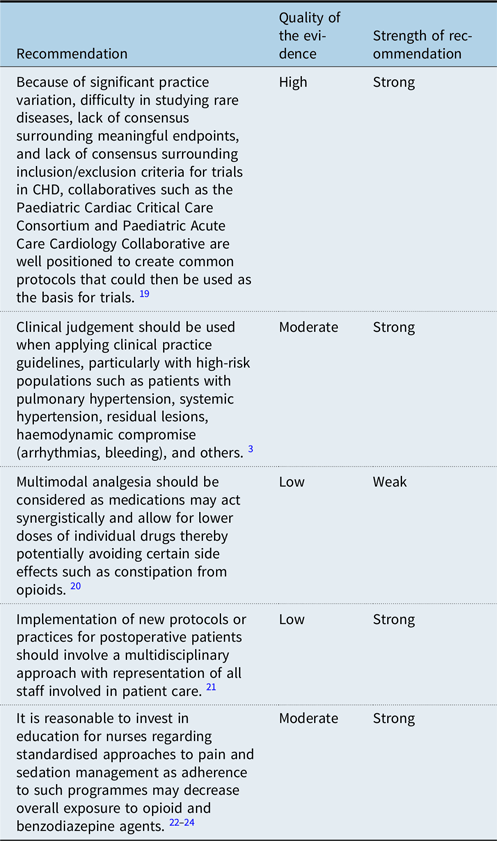
One challenge to developing protocols and clinical practice guidelines in paediatric cardiology is the significant heterogeneity of disease. When addressing postoperative pain management, the wide variety of underlying disease and various surgical options challenges the ability to decrease practice variability. Collaboratives offer the opportunity to amass multi-centre data and experiences, thereby increasing understanding of a given topic, such as postoperative pain management. Reference Thompson, Foote and King19 While clinical practice guidelines are helpful in providing guidance to clinicians, it is important to recognise that they cannot address all patient populations and judgement must be used when applying them. Individual patients and high-risk populations may not benefit from application of clinical practice guidelines. Reference Fuller, Kumar and Roy3 Involvement of all stakeholders and participants in clinical care is important when developing protocols. Bedside nurses, in particular, are well positioned to improve associated processes as they frequently assess and treat pain. Reference Bastero, DiNardo, Pratap, Schwartz and Sivarajan21–Reference Magner, Valkenburg and Doherty24
Pain assessment

Undertreatment or reluctance to treat pain among children with cardiac disease is likely common and often related to concerns that analgesics may have unfavourable haemodynamic effects. However, undertreatment may negatively result in increased stress response which may have negative long- and short-term consequences. Reference Diaz6,Reference Hehir, Easley and Byrnes7 A variety of tools have been developed to assess pain in infants and children, utilising both subjective and objective information. There is insufficient evidence to suggest that any of these tools is superior, however consistent use and unit-wide acceptance of a given tool improves consistency of pain assessment and subsequent treatment. Reference Diaz6,Reference Hehir, Easley and Byrnes7,Reference Franck, Ridout, Howard, Peters and Honour25–Reference Bai, Hsu, Tang and Van Dijk27 Pain assessment among infants and children, particularly those who are non-verbal, relies on a combination of factors including objective data such as vital sign changes and subjective information such as patient-reported symptoms or family assessment. Reference Huth, Broome, Mussatto and Morgan29 There are currently efforts to develop additional tools to objectively measure pain; however, these efforts have been primarily limited to adult populations. Reference Pollak, Bronicki, Achuff and Checchia5 Children would benefit from such tools, especially non-verbal children or children who are developmentally unable to express their pain. It is important to recognise that social and cultural factors may influence the experience of pain and treatment should be adjusted based on individual response. Reference Pollak, Bronicki, Achuff and Checchia5,Reference Diaz6,Reference Bai and Hsu28 Though we did not identify literature examining the impact of implicit bias or racism on the treatment of pain among children undergoing congenital heart surgery, this is a known problem in medicine Reference Sabin and Greenwald30–Reference Fanta, Ladzekpo and Unaka32 and pain should never be managed differentially based solely on a patient’s race or ethnicity.
General principles

Pain in the postoperative period should be considered in the context of the individual patient and their risk factors for ongoing pain. Specifically, the incision type and presence of chest tubes may predispose patients to ongoing pain, requiring additional analgesia. Reference Karuppiah, Pehora, Haller and Taylor33 Additionally, timing within a hospitalisation should be taken into account given the temporally associated reduction of pain as patients' progress from their surgery. As patients’ pain improves and their need for analgesia diminishes, it is most appropriate to first discontinue or reduce frequency of administration of medications with the highest risk for side effects and continue those medications which have fewer side effects. It is appropriate to utilise additional medications to treat known and common side effects of commonly used analgesics. In particular, a bowel regimen should be implemented for patients taking opioid medications as opioids are associated with constipation.
Pain that does not follow the anticipated course over time, or that is inconsistent in type or location, or is associated with other signs and symptoms such as vital sign changes or respiratory distress is cause for rapid and thorough evaluation for other causes. Similarly, additional evaluation is needed when patient or family reports of pain are discrepant from provider’s assessment of pain.
Preoperative considerations
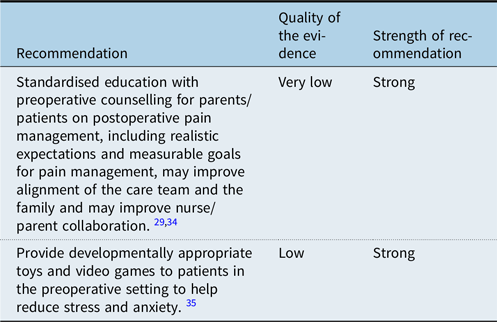
Families and providers often have discrepant expectations for postoperative pain level and management which can challenge the therapeutic relationship needed to assist children in their recovery. Preoperative counselling should therefore address realistic expectations for postoperative pain and aim to establish understanding with families regarding what their child may experience and how it will be addressed. Reference Huth, Broome, Mussatto and Morgan29,Reference Harvey and Kovalesky34 Discussions related to goals of pain management should emphasise functionality rather than complete elimination of pain. Additionally, management of stress and anxiety in the preoperative setting has been associated with reduced postoperative pain. Reference Kumar, Das, Chauhan, Kiran and Satapathy35
Intraoperative considerations

It has been suggested that multimodal pain management during surgery, including use of acetaminophen, local anaesthesia and postoperative nausea and vomiting treatments, may reduce the need for intraoperative opioids and improve postoperative pain outcomes. Reference Roy, Parra and Brown4 There have been a number of studies examining the use of intraoperative methadone and its impact on postoperative pain but based on the current evidence, this panel does not currently have a recommendation related to its use. Reference Iguidbashian, Chang, Iguidbashian, Lines and Maxwell36–Reference Robinson, Caruso, Wu, Kleiman and Kwiatkowski38
Regional anaesthesia

The use of regional anaesthesia has gained interest and acceptance as a means of reducing intraoperative and postoperative opioid requirements. Currently, centre-specific considerations are required for use of regional anaesthesia-based experience of anaesthesiologists and availability of providers to manage such modalities outside of the operating room such as consultative pain services. Multiple studies have examined specific types of regional anaesthesia in the context of paediatric congenital heart surgery and it has widely been found to be safe. Specifically for transversus thoracis plane blocks, studies have shown improved pain scores, decreased intraoperative and postoperative opioid requirements, shorter time to extubation and shorter ICU length of stay compared to patients not receiving nerve blocks. Reference Abdelbaser and Mageed39–Reference Cakmak and Isik41 Similar endpoints have been used in studies of paravertebral block and have shown promise though the body of evidence is insufficient currently to uniformly recommend paravertebral block. Reference Türköz, Balcı and Can Güner42,Reference Sahajanandan, Varsha and Kumar43 Additionally, erector spinae plane blocks and parasternal intercostal blocks using continuous infusions shown to be safe but there is inconclusive evidence to suggest uniform adoption of such approaches. Reference Macaire, Ho and Nguyen45–Reference Kaushal, Chauhan and Saini51 No specific anaesthetic agent has shown superiority in these studies. Caudal analgesia has been shown to be safe with minimal harm. However, there is controversy regarding its impact on postoperative pain as studies comparing use of caudal analgesia to children not receiving regional anaesthesia have not consistently demonstrated improved outcomes; therefore it is reasonable to consider caudal analgesia but as it does not have clear benefits, it is not uniformly recommended. Reference Samantaray, Trehan, Chowdhry and Reedy52–Reference Maharramova and Taylor56 Spinal anaesthesia may improve pain scores and reduce postoperative opioid requirement however only one study with a small sample size of 20 patients has demonstrated this. Reference Hammer, Ramamoorthy and Cao57 While many forms of regional anaesthesia have conflicting evidence, continuous infusion of local anaesthetic at incision sites has been shown to reduce postoperative pain scores and opioid requirements and should be considered, though some evidence to support this comes from adult studies. Reference Nasr and Abdelhamid54,Reference Tirotta, Munro and Salvaggio58,Reference Paladini, Di Carlo and Musella59 Further research is needed to explore the efficacy of these various regional anaesthetic techniques in order to identify which patients might benefit from regional anaesthesia.
Opioids
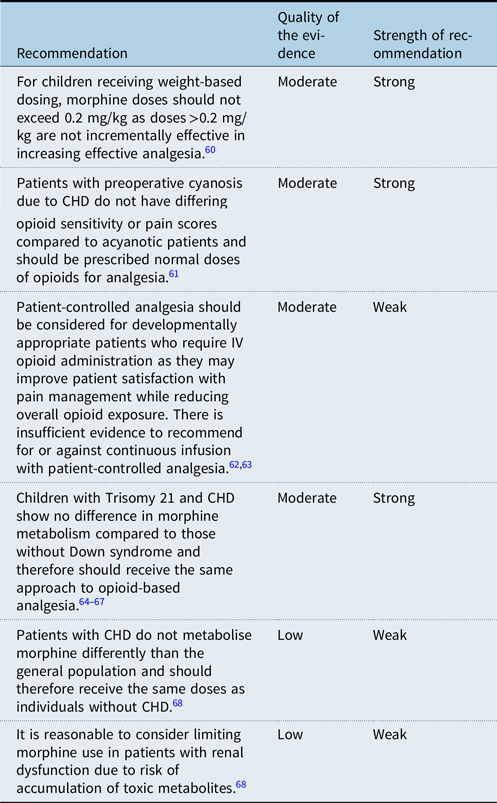
Despite side effects and risk for habituation, opioids remain a mainstay of postoperative pain management and offer effective analgesia. Reference Chou, Gordon and de Leon-Casasola69 There are some specific considerations regarding opioid use among children undergoing cardiothoracic surgery. In animal studies, chronic hypoxaemia has been associated with increased sensitivity to opioid medications. Studies among humans with obstructive sleep apnoea and chronic hypoxaemia have shown similar results prompting consideration of whether children with cyanotic CHD may also have variable sensitivity to opioids. When studied, however, children with cyanotic lesions were not found to have differing sensitivity to opioids and therefore should be prescribed typical doses. Reference Murray-Torres, Tobias and Winch61
Another specific consideration relates to children with Trisomy 21 and CHD. Previous reports have suggested possible altered pharmacokinetics or pharmacodynamics among children with Trisomy 21; however, multiple studies have found no difference among children with and without Trisomy 21, and these children should therefore receive opioids at typical doses. Reference Goot, Kaufman and Pan64–Reference Van Driest, Shah and Marshall67
There have been limited published reports related to decreased opioid clearance among individuals with decreased systemic blood flow prompting consideration for need to dose opioids differentially among individuals with CHD. Reference Elkomy, Drover and Glotzbach68,Reference Dagan, Klein, Bohn, Barker and Koren70 Despite these reports, modelling of pharmacokinetics has shown that children with CHD do not metabolise morphine significantly differently from children without CHD and should therefore be treated with conventional doses. Reference Elkomy, Drover and Glotzbach68 Patients with renal dysfunction, a common issue following cardiac surgery, are at risk for toxic accumulation of metabolites of morphine. Reference Elkomy, Drover and Glotzbach68
Among certain populations, patient-controlled analgesias have been shown to have increased effectiveness of analgesia compared to provider-initiated intermittent boluses. Reference Chou, Gordon and de Leon-Casasola69 Limited studies among children undergoing cardiac surgery have shown similar findings. Reference Mota, Marcolan, Pereira, Milanez, Dallan and Diccini62,Reference Epstein63 Because of the potential for better analgesia, higher satisfaction, and overall reduction in opioid exposure, patient-controlled analgesias should be considered for children who require intravenous opioids and are developmentally appropriate.
Opioid-sparing
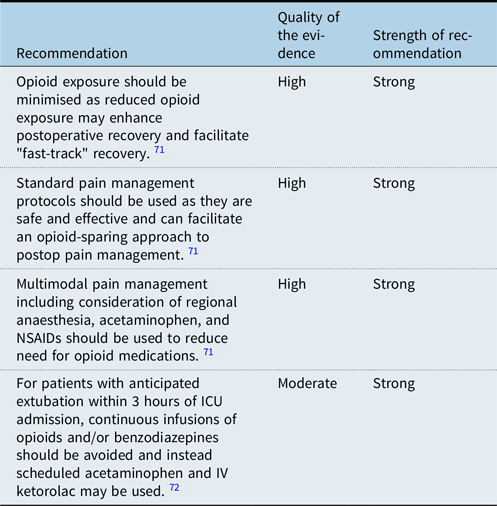
Due to increased awareness of risks associated with opioid medications including habituation, withdrawal, and side effects including constipation and respiratory depression, as well as evidence that opioid-sparing approaches may facilitate postoperative recovery, there is significant interest in developing protocols and approaches to postoperative pain management which minimise opioid exposure. Reference Chou, Gordon and de Leon-Casasola69,Reference Frankel, Maul and Chrysostomou71 Multimodal pain management has been utilised successfully to reduce opioid exposure postoperatively. Reference Roy, Parra and Brown4 Additionally, increased awareness of the likely trajectory of patients in the immediate postoperative period, particularly those who are likely to tolerate extubation soon after arrival in the ICU, has facilitated opioid-sparing approaches. Reference Frankel, Maul and Chrysostomou71 In a randomised controlled trial of children recovering from cardiothoracic surgery, there was no difference in pain control among those who received continuous versus intermittent opioids but use of continuous infusions resulted higher total doses of opioids and longer length of stay. Reference Penk, Lefaiver, Brady, Steffensen and Wittmayer72 Increased examination of whether continuous infusions are needed for individual patients may result in decreased reflexive use of them and improved patient outcomes.
Non-opioid medications

Multimodal pain management relies largely, though not exclusively, upon NSAIDs and acetaminophen. While NSAIDs are used in neonates and infants for indications such as closure of the patent ductus arteriosus, retinopathy of prematurity, and prevention of intraventricular haemorrhage, there has been reluctance to use NSAIDs as a primary means of analgesia among infants less than 6 months of age. Reference Aranda, Salomone, Valencia and Beharry89,Reference Ziesenitz, Zutter, Erb and van den Anker90 Additionally, there is significant variation in practice related to NSAID and acetaminophen or paracetamol use in children. A systematic review of guidelines for anti-pyresis among children identified 55 guidelines addressing use of ibuprofen and 69 addressing use of paracetamol. There was minimal agreement between guidelines regarding appropriate population, dosing, frequency, duration of treatment, or conditions in which to avoid or use caution prior to initiating the drug. Reference Green, Krafft, Guyatt and Martin91 Additionally, for many of the age-based recommendations against use of NSAIDs or acetaminophen in young children, there was either no published evidence or low quality of evidence to support those recommendations. Reference Green, Krafft, Guyatt and Martin91 More recent publications have examined use of NSAIDs in younger children and found them to be safe. Reference Ziesenitz, Zutter, Erb and van den Anker90
Multiple studies have examined use of ibuprofen or ketorolac in postoperative infants and children, some as young as 28 days. In addition to focusing on achieving analgesia, these studies have largely used creatinine or blood urea nitrogen as endpoints. Reference Saini, Maher and Deshpande74–Reference Dawkins, Barclay, Gardiner and Krawczeski76 These are known to be imperfect markers of true renal function and, while they were found to be increased among patients receiving NSAIDs, the clinical implication of that rise is unclear, particularly as the values did not always fall outside of the range of normal. Acute kidney injury following cardiac surgery is common but is multifactorial in aetiology and not directly related to nephrotoxin exposure, including ibuprofen and ketorolac. Reference Uber, Montez-Rath, Kwiatkowski, Krawczeski and Sutherland92 Additionally, studies examining the risk of bleeding and changes to platelet function while receiving NSAIDs have found platelet function is altered but there is no increase in clinically significant bleeding. Reference Savva, Kishk, Morgan, Biggs, Seung and Bauer73–Reference Dawkins, Barclay, Gardiner and Krawczeski76,Reference Gupta, Daggett, Ludwick, Wells and Lewis79,Reference Kim, Kaufman, Patel, Manco-Johnson, Di Paola and da Cruz88 It is unclear what the implication of changed platelet function is, and this warrants further research, particularly considering the additional risk factors in this population including cardiopulmonary bypass, presence of chest tubes, and surgical wounds.
In addition to NSAIDs and acetaminophen, there have been a few studies examining use of other non-opioid analgesics among adults and there have been suggestions to study these among children. Perioperative administration of magnesium has been shown to decrease postoperative pain and opioid consumption among adults. Reference Pollak, Bronicki, Achuff and Checchia5 While there are no data, to our knowledge, among children, it is a worthwhile consideration given the relative ease of monitoring magnesium levels (something that is often already occurring in postoperative patients) and the low risk of side effects when administered enterally or slowly if parenterally. Additionally, gabapentinoids have been identified as potentially useful in children recovering from cardiac surgery. Pregabalin, in particular, appears to be effective for acute pain and has been studied among adults recovering from cardiac surgery. Reference Pollak, Bronicki, Achuff and Checchia5 We could not identify any data related to spasmolytic medications but in practice, these are commonly utilised after CHD surgery, particularly in adolescents and young adults. Further research is warranted to explore these, and other, non-opioid medications for treatment of pain in children.
Non-pharmaceutical pain management

Non-pharmacologic approaches to analgesia are widely accepted as an important component of postoperative care, but may be underutilised among children undergoing and recovering from cardiac surgery. Reference Chou, Gordon and de Leon-Casasola69,Reference Miller, Lisanti and Witte93 Reasons for underutilisation include lack of resources and inadequate awareness of benefits but services such as music therapy, massage therapy, acupuncture, and others have been shown to improve postoperative pain scores in this population. Reference Miller, Lisanti and Witte93–Reference Huang, Lei, Liu, Cao, Yu and Chen95,Reference Kakar, Billar, van Rosmalen, Klimek, Takkenberg and Jeekel97–Reference Albert, Gillinov, Lytle, Feng, Cwynar and Blackstone100,Reference Mayan, Alvadj, Punja, Jou, Wildgen and Vohra103 Additionally, not all non-pharmacologic approaches are resource intensive – indeed, skin-to-skin contact has been shown to improve comfort among infants undergoing cardiac surgery and increase physiologic stability. Reference Lisanti, Demianczyk and Costarino104 Other interventions as simple as providing developmentally appropriate toys and optimising environmental factors such as lighting and noise can also improve postoperative outcomes related to pain. Reference Diaz6,Reference Chen, Lei, Liu, Cao, Yu and Chen102 Other non-pharmacologic analgesia modalities have been shown to be effective among adults undergoing thoracic surgery, chiefly transcutaneous electrical nerve stimulation. Reference Chou, Gordon and de Leon-Casasola69 Transcutaneous electrical nerve stimulation has also been shown to be effective in reducing procedural pain for children. Reference Lander and Fowler-Kerry105 Efforts should be made to determine if transcutaneous electrical nerve stimulation is effective among children undergoing cardiac surgery, particularly considering the lack of side effects and ease of use. Importantly, there is minimal risk associated with these non-pharmacologic modalities.
Discharge considerations

Children are likely to need ongoing analgesia after discharge home following cardiac surgery. Discharge teaching may even include consideration that pain can transiently worsen as children become more mobile in their home environments. Acetaminophen and NSAIDs should be used as the first-line medications for treatment of pain in the home environment – children still requiring frequent administration of opioids may not yet be ready for discharge. Reference Frankel, Maul and Chrysostomou71 To that end, Monitto et al studied the effectiveness of analgesia following discharge for a variety of paediatric surgeries including cardiac surgery and found that despite low levels of use of opioids at home, analgesia was largely good or excellent at 1–2 days following discharge and 10–14 days following discharge. Reference Monitto, Hsu and Gao107 Because so few patients require frequent opioid administration at home and the majority of prescribed doses are unused, it is reasonable to limit the number of prescribed doses and some patients may not require opioids at all. Reference Irfan, Martin and Canner106,Reference Monitto, Hsu and Gao107 In this study, atrial septal defects made up the majority of included patients making it easiest to apply their findings clinically to that population. However, it should be noted that much of the postoperative pain experienced by patients is related to incision sites (sternotomy, thoracotomy, chest tubes when in place) rather than the heart itself and therefore the specific form of CHD likely has little impact on postoperative pain and rather the extent of the incision, first time versus redo incision, incision healing, and features such as delayed sternal closure play heavily into the experience of pain.
Studies of opioid prescription at discharge among adults undergoing cardiac surgery have shown that the greatest predictor of amount of opioid prescribed is hospital, not patient or surgery-specific factors. Reference Holst, Dearani and Schaff108 There remains a need for research to better understand best practices surrounding opioid prescription at time of discharge for children undergoing cardiac surgery, particularly given the risks of undertreating pain in the home environment relative to the risks of overuse or misuse of opioid medications. Studies targeting opioid prescription variability for postoperative children have shown that institutional guidelines available to prescribing physicians can reduce variability and decrease overall opioid prescription at time of discharge, though these results have not been reproduced among children undergoing cardiac surgery. Reference Irfan, Martin and Canner106
Limitations
In aiming to address postoperative pain management, this guideline does not address sedation or delirium. It also does not address habituation or withdrawal for patients who may require prolonged infusions of narcotics. Agents such as dexmedetomidine, propofol, ketamine, and others often play an important role in postoperative pain management in the ICU setting but were not addressed in this guideline due to the lack of literature providing data on their use in extubated children. Additionally, because the scope of this work was limited to patients who extubate early, there are no intensive care providers on our panel. The panel was intentionally comprised of individuals from a large variety of centres geographically and with a variety of programme styles; however, recruitment did occur via Paediatric Acute Care Cardiology Collaborative which may introduce some bias. Panellists were not required to have recently published articles related to pain management and therefore may not be considered the most expert; however, this also may have limited bias in that no panellists were reviewing their own research.
Conclusions
Postoperative pain among children following cardiac surgery is currently an area of significant practice variability despite a large body of literature and the presence of centre-specific protocols. After a review of the evidence and these protocols, a panel of experts convened by Paediatric Acute Care Cardiology Collaborative created these recommendations, which were subsequently approved by the Paediatric Acute Care Cardiology Collaborative executive committee. Central to the recommendations is the concept that ideal pain management begins with preoperative counselling and continues through to patient discharge. While general principles may be applied, pain management must be tailored to individual patient experience and response to intervention. Standardised approaches to assessing and addressing pain are needed. Opioid medications remain a mainstay of pain management but multimodal approaches, non-opioid medications, and non-pharmacologic interventions are effective in minimising opioid exposure and improving postoperative pain measures.
The panel identified numerous gaps in the available evidence and found that the quality of existing evidence was overall low – only 9 of the 63 recommendations have a high quality of the evidence and many have a low or very low quality of the evidence. There is ongoing need for research, particularly in children, related to objective methods of pain assessment, the utility of regional anaesthesia, intraoperative pain management strategies, and non-opioid analgesics. The aim of this clinical practice guidelines is to address this variability and support providers in their development of more standard approaches to postoperative pain management.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122003559
Acknowledgements
We thank and appreciate the support of Paediatric Acute Care Cardiology Collaborative staff in assisting with logistical aspects of creating this work.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.





