Introduction
The planthopper Hyalesthes obsoletus Signoret (Hemiptera: Auchenorrhyncha: Cixiidae) plays an important role as a vector of stolbur phytoplasma (Candidatus Phytoplasma solani, 16SrXII-A phylogenetic group), which is associated with the grapevine yellow known as Bois noir (BN) (Maixner, Reference Maixner1994). Among cixiids collected in vineyards, Reptalus panzeri (Löw), R. quinquecostatus (Dufour) and Pentastiridius sp. have also been found positive for stolbur phytoplasma (Palermo et al., Reference Palermo, Elekes, Botti, Ember, Alma, Orosz, Bertaccini and Kölber2004; Botti et al., Reference Botti, Paltrinieri, Mori, Milanesi, Bondavalli and Bertaccini2005; Trivellone et al., Reference Trivellone, Pinzauti and Bagnoli2005; Riedle-Bauer et al., Reference Riedle-Bauer, Tiefenbrunner, Otreba, Hanak, Schildberger and Regner2006), but currently H. obsoletus is the only confirmed vector of this pathogen to grapevines (Maixner, Reference Maixner, Weintraub and Jones2010).
In Europe, H. obsoletus is univoltinous on mainly herbaceous wild plants. Females lay eggs during the summer in the soil near the roots of host plants on which the nymphs feed and overwinter (Brčak, Reference Brčak, Maramorosch and Harris1979). Grapevines represent an occasional host for adults (Alma et al., Reference Alma, Arnò, Arzone, Vidano, Vidano and Arzone1988; Bressan et al., Reference Bressan, Turata, Maixner, Spiazzi, Boudon-Padieu and Girolami2007; Lessio et al., Reference Lessio, Tedeschi and Alma2007). The occurrence and the spread of BN in vineyards are associated with the presence, in the vineyard and in the surrounding areas, of plants that could be host of both the vector and the phytoplasma. Among these, Urtica dioica L. (stinging nettle) and Convolvulus arvensis L. (field bindweed) are the main host plants and play an important role in BN epidemiology (Alma et al., Reference Alma, Arnò, Arzone, Vidano, Vidano and Arzone1988, Reference Alma, Soldi, Tedeschi and Marzachì2002; Maixner, Reference Maixner1994; Langer et al., Reference Langer, Darimont and Maixner2003; Bressan et al., Reference Bressan, Turata, Maixner, Spiazzi, Boudon-Padieu and Girolami2007; Mori et al., Reference Mori, Pavan, Bondavalli, Reggiani, Paltrinieri and Bertaccini2008b; Forte et al., Reference Forte, Angelini, Maixner and Borgo2010; Kessler et al., Reference Kessler, Schaerer, Delabays, Turlings, Trivellone and Kehrli2011).
Five nymphal instars are reported for H. obsoletus (Musil, Reference Musil1956; Alma et al., Reference Alma, Arnò, Arzone, Vidano, Vidano and Arzone1988; Güclü & Ozbek, Reference Güclü and Ozbek1988; Sforza et al., Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999). Currently, a dichotomous key for their identification is available, based on nymphs reared in the laboratory on Lavandula angustifolia Miller (Sforza et al., Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999). In relation to different areas, different overwintering nymphal instars have been reported. In France, Germany and northern Italy, the planthopper overwinters as second-third instars (Alma et al., Reference Alma, Arnò, Arzone, Vidano, Vidano and Arzone1988; Sforza et al., Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999; Maixner, Reference Maixner2007), in Slovakia as third instars (Musil, Reference Musil1956), in Bulgaria as fourth instars (Brčak, Reference Brčak, Maramorosch and Harris1979) and in Turkey as first-second instars (Güclü & Ozbek, Reference Güclü and Ozbek1988).
An earlier phenology of H. obsoletus adults on C. arvensis than on U. dioica was observed both in Germany (Maixner, Reference Maixner2007) and in northern Italy (Mori et al., Reference Mori, Pavan, Bacchiavini, Reggiani, Bonomi and Bertaccini2008a). This would suggest an influence of host plant species on nymphal development time (Maixner, Reference Maixner2007). Based on this hypothesis, the initial aim of the present research was to compare the phenology of the nymphal instars of H. obsoletus on U. dioica and C. arvensis in northern Italy. However, because the preliminary survey carried out on cixiid nymphs collected on the two plants had highlighted some problems in the identification of the instars using the dichotomous key of Sforza et al. (Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999), the correct identification of nymphs to both species and instar level became a necessary and primary aim of this research. Indeed, only certain identification could permit a proper answer to the initial question, i.e. if the phenology of nymphal instars on U. dioica and C. arvensis is different.
The morphological identification of the three Hyalesthes species known in Italy (i.e. H. luteipes Fieber, H. obsoletus and H. scotti Ferrari) is possible for the adults (Holzinger et al., Reference Holzinger, Kammerlander and Nickel2003) but not for the nymphs. Therefore, only the nymphs obtained from eggs laid by identified adults of H. obsoletus can be confidently attributed to this species. Recently, molecular keys were proposed as an alternative method to identify the Italian species of Hyaleshtes sp. (Bertin et al., Reference Bertin, Picciau, Àcs, Alma and Bosco2010b) and those of Reptalus sp. (Bertin et al., Reference Bertin, Picciau, Àcs, Alma and Bosco2010a), whose nymphs also feed on roots of herbaceous plants (Holzinger, Reference Holzinger, Kammerlander and Nickel2003). Therefore, using specific molecular markers, nymphs can be identified with certainty at species level without knowing the identity of parents.
Materials and methods
Field collection of H. obsoletus nymphs and adults
Nymphs and adults attributable to H. obsoletus were collected during 2008–2010 in three flatland vineyard habitats of northern Italy.
In the first vineyard habitat, named locality 1 (Cormons, Friuli Venezia Giulia region, 45°57′ latitude N, 13°27′ longitude E, 55 m a.s.l., loam soil according to USDA classification), planthopper individuals were collected on U. dioica, growing along a ditch located about 6–8 m from a vineyard border, and Artemisia verlotorum Lamotte, growing in an uncultivated field bordering a vineyard. In this habitat, grapevines of the cultivar Chardonnay were affected by BN tuf-a type. On stinging nettle, nymphs were collected from March to November 2008 and adults in June 2009 and July 2010. On A. verlotorum, nymphs and adults were collected during June 2009 and July 2010.
In the second vineyard habitat, named locality 2 (Carpi, Emilia Romagna region, 44°45′ latitude N, 10°49′ longitude E, 26 m a.s.l., clay soil according to USDA classification), planthopper individuals were collected on U. dioica, growing along a ditch located 3–5 m from a vineyard border. In this habitat, grapevines of the cultivar Lambrusco were affected by BN tuf-a type. Nymphs were collected from February to October 2008 and adults in June–July 2008.
In the third vineyard habitat, named locality 3 (Sesso, Reggio Emilia, Emilia Romagna region, 44°46′ latitude N, 10°37′ longitude E, 27 m a.s.l., clay soil according to USDA classification), planthopper individuals were collected on C. arvensis, growing inside two vineyards. In this habitat, grapevines of the cultivar Lambrusco were affected by BN tuf-b type. Nymphs were collected from February to June 2008 and adults in June 2008.
Nymphs were picked up from roots with a small paint brush and preserved in 70% ethanol, while adults were captured alive with a sweep net or by a manual suction device and frozen at –20°C. The collected individuals were used for different purposes (table 1). Nymphs were studied morphologically and morphometrically after mounting on slides in Berlese's medium.
Table 1. Adults and nymphs of Hyalesthes obsoletus, collected in the field or obtained in laboratory rearing (lab), submitted to morphological or molecular identification, and used in morphological and morphometric studies.

1, locality 1; 2, locality 2; 3, locality 3; A, Artemisia verlotorum; C, Convolvulus arvensis; U, Urtica dioica.
* 20 just-hatched and two older individuals.
Laboratory rearings of H. obsoletus
In late June 2009 in locality 1, last instar nymphs (attributable to H. obsoletus) and the U. dioica plants they fed on were collected. The plants with nymphs and soil were placed in a 30 cm-diameter pot within transparent plexiglass cages (50×50×100 cm) and maintained outdoors. The emerged adults were collected a few days after mating and egg laying (mid-July). From mid-August the soil was searched for eggs and hatched nymphs, which were picked up and mounted on slides (table 1).
In early July 2010 in locality 1, last instar nymphs (attributable to H. obsoletus) and the A. verlotorum plants they fed on were collected and managed as above. The emerged adults were collected a few days after mating and egg laying (mid-July) (table 1). At the same time, more than 30 eggs, found near the roots under the soil surface, were picked up and placed into glass Petri's dishes to obtain just-hatched nymphs to mount on slides (table 1). In late August the soil was searched for nymphs, which were picked up and mounted on slides (table 1).
Morphological and molecular identification of studied cixiids
The adults of planthoppers, collected in the field and obtained in confined rearing (table 1), were identified morphologically (Holzinger et al., Reference Holzinger, Kammerlander and Nickel2003; Bertin et al., Reference Bertin, Picciau, Àcs, Alma and Bosco2010a,Reference Bertin, Picciau, Àcs, Alma and Boscob).
Eighteen nymphs collected in the three localities, two males of H. obsoletus and two males of Reptalus cuspidatus (Fieber) were identified using molecular techniques (table 1). The adults had been previously identified on the basis of genitalia traits. The molecular analysis was carried out in order to compare the results with molecular keys of Hyalesthes and Reptalus species, proposed by Bertin et al. (Reference Bertin, Picciau, Àcs, Alma and Bosco2010a,Reference Bertin, Picciau, Àcs, Alma and Boscob).
Genomic DNA was individually extracted from the planthoppers using a salting-out protocol (Patwary et al., Reference Patwary, Kenchington, Bird and Zouros1994). PCR analysis was performed following Bertin et al. (Reference Bertin, Picciau, Àcs, Alma and Bosco2010a,Reference Bertin, Picciau, Àcs, Alma and Boscob) with slight modifications. A fragment of the COI mitochondrial gene was amplified using the primers, C1-J-2195 (5′-TTGATTTTTTGGTCATCCAGAAGT-3′) and TL2-N-3014 (5′-TCCAATGCACTAATCTGCCATATTA-3′) (Simon et al., Reference Simon, Frati, Beckenbach, Crespi, Liu and Flook1994). Amplifications were performed with 25 μl reactions containing 1×PCR buffer, 1.8 mM MgCl2, 200 μM each of the four dNTPs, 0.4 μM of each primer, 0.75 units of GoTaq Flexi DNA Polymerase (Promega, Madison, WI, USA) and about 20 ng of template DNA. The PCR program consisted of 42 cycles: denaturation at 94°C for 1 min (3 min for the first cycle), annealing at 56°C for 50 s, and extension at 72°C for 1 min (10 min for the last cycle). PCR products were separated by electrophoresis on a 1% (w/v) agarose gel, stained in ethidium bromide and visualised on a UV transilluminator. The COI amplicons from all tested planthoppers were digested with the TaqI restriction enzyme (Bertin et al., Reference Bertin, Picciau, Àcs, Alma and Bosco2010b) following the manufacturer's instructions (Fermentas, Vilnius, Lithuania). The restriction fragments were separated by electrophoresis on a 2% (w/v) 1×TBE agarose gel at 100 V for 1.5 h and stained and visualised as described above.
Morphometry and morphology of nymphs
The 715 individuals mounted on slides (table 1) were observed under an optical microscope to measure body parameters, with a precision of 1.25 μm using a calibrated ocular micrometer, and to count morphological characteristics. The measurements concerned body length, head-thoracic length, mesonotal wingpad length (W1), length of the part of metanotum not covered by the mesonotal wingpad (W2), head width, thoracic width, metatibia length and metatarsomeres (T1 the proximal to metatibia, T2, T3) length (fig. 1). Furthermore, the following ratios were calculated: (i) between the length of metatarsomeres (T1/T2) and (ii) between the lengths of the metanotal wingpad not covered by the mesonotal wingpad and the mesonotal wingpad (W2/W1). Measurements and ratios were grouped in frequency classes and plotted to verify the existence of a series of discrete size classes. The mean and standard deviation (SD) of measurements belonging to each size class were calculated for the parameters for which it was possible to individuate discrete size classes. The ratios between the means of successive size classes were calculated for each parameter to verify if they agreed with Dyar's rule (Dyar, Reference Dyar1890). The linear growth regressions between the size class number and the logarithmic mean of the measurements for each size class were also calculated (Daly, Reference Daly1985).
The morphological characteristics counted were: sensory pits on thoracic plates, sensory pits on abdominal tergites (only for the last two instars), metatarsomeres, apical spines on metatibia and apical spines on metatarsomeres. Body coloration, and eye and antennal traits were also annotated.
Drawings of nymphs were made using photographs taken under an optical microscope.
Results
Morphological and molecular identification
All adults collected in the field and those emerged from nymphal rearing belonged morphologically to H. obsoletus (table 1). Therefore, the eggs and the hatched nymphs, obtained in rearing from H. obsoletus adults, can be attributed with certainty to this species.
The amplification of the mitochondrial COI gene resulted in an 890-bp fragment, typical of Hyalesthes sp. (Bertin et al., Reference Bertin, Picciau, Àcs, Alma and Bosco2010b), from all tested nymphs and the two males of H. obsoletus. The COI amplicons of the two males of R. cuspidatus (Fieber) were about 920 bp as reported in the literature for Reptalus sp. (Bertin et al., Reference Bertin, Picciau, Àcs, Alma and Bosco2010a). The RFLP analyses with TaqI confirmed that the two adult males of H. obsoletus belonged to this species, in agreement with Bertin et al. (Reference Bertin, Picciau, Àcs, Alma and Bosco2010b). All the tested nymphs shared the same RFLP profile, consisting of two restriction fragments of about 210 bp and 680 bp respectively, with the two adult males of H. obsoletus. Therefore, all the analysed nymphs belonged to this species.
Morphometric parameters of nymphs
In locality 1 (U. dioica), five discrete size classes were individuated for the following parameters: body and head-thoracic lengths, and head and thoracic widths (fig. 1a–d). Each individual belonged to the same size class for all the above parameters. Therefore, frequency distributions of these parameters were in agreement with five nymphal instars. The means±SD of each instar are reported in table 2. The correspondence between size classes and nymphal instars was also supported by linear growth regressions. In locality 1, the means±SD of morphometric parameters of nymphs collected on A. verlotorum agreed with those reported for the fifth-instar nymphs collected on U. dioica.

Fig. 1. Frequency distribution of body parameters measured on Hyalesthes obsoletus nymphs from locality 1 (U. dioica), separated into five discrete size classes (□, 1st; ![]() , 2nd;
, 2nd; ![]() , 3rd;
, 3rd; ![]() , 4th; ■, 5th).
, 4th; ■, 5th).
Table 2. Measurements (expressed in μm) of body parameters (mean±SD) of Hyalesthes obsoletus nymphs (except newly hatched nymphs).

In locality 2 (U. dioica), four discrete size classes were observed for the following parameters: body and head-thoracic lengths, and head and thoracic widths (table 2). For these parameters, the means of each size class were similar, even if always lower, to those calculated for the second to fifth instars of locality 1. However, the means±SD of body length of the fourth and fifth instars of the two localities were not overlapped.
In locality 3 (C. arvensis), three discrete size classes were recorded for the following parameters: body and head-thoracic lengths, and head and thoracic widths (table 2). For head width, these size classes were in agreement with the third to fifth instars of locality 1. For the other parameters, overlap between the two localities was always observed in the third instar and sometimes in the others. In the geographically close localities 2 (on U. dioica) and 3 (on C. arvensis), the means±SD of the same instars overlapped for all parameters.
The Dyar's ratios varied in relation to both the nymphal instars and the measured body parameters (table 3). In particular, on U. dioica the 4th/3rd ratio was the highest for most of the parameters. On C. arvensis, in contrast to U. dioica, the 4th/3rd and 5th/4th ratios were similar for the same parameter. On U. dioica, the average value of Dyar's ratios was always higher for thoracic width (1.45 and 1.46 in localities 1 and 2, respectively) and metatibia length (1.53 and 1.55 in localities 1 and 2, respectively) than for the other parameters (ranged between 1.36 and 1.41 in locality 1 and between 1.33 and 1.36 in locality 2).
Table 3. Dyar's ratios (ratios between the means of successive size classes) of body parameters calculated for Hyalesthes obsoletus nymphs.

Of the total individuals submitted to the morphometric and morphological analysis (table 1), 34 were not attributed to a specific instar because undergoing moulting (locality 1: 1st/2nd=4 nymphs, 2nd/3rd=2 nymphs, 4th/5th=15 nymphs; locality 2: 1st/2nd=1 nymph, 2nd/3rd=3 nymphs, 4th/5th=2 nymphs; locality 3: 2nd/3rd=1 nymph, 3rd/4th=6 nymphs).
Morphological characteristics of nymphs
The numbers of metatarsomeres, thoracic sensory pits, and apical spines of metatibia and metatarsomeres were discriminating characteristics for identifying nymphal instars (table 4). The position of thoracic pits can be observed in fig. 2a–f. Under the optical microscope, the thoracic pits of metanotal plates were easily countable and allowed different instars to be distinguished (table 4). No morphological difference among localities and host plants was observed in the same instar, except for the number of mesonotal pits of the third instar.

Fig. 2. Nymphal instars of Hyalesthes obsoletes drawn from photographs taken under an optical microscope: (a) first instar, newly hatched; (b) first instar, older; (c) second instar; (d) third instar; (e) fourth instar; (f) fifth instar; (T1, T2 and T3) metatarsomeres; W1, length of mesonotal wingpad; W2, length of metanotal wingpad not covered by mesonotal wingpad.
Table 4. The numbers of morphological characteristics for all studied nymphs of Hyalesthes obsoletus.

T1, metatarsomere proximal to metatibia. Numbers in bold are discriminant between instars with the same number of metatarsomeres. Numbers in brackets indicate rare cases.
* for fourth and fifth instars the number of pits refers to areas not covered by the mesonotal wingpad.
Metaleg and wingpad morphometric parameters of nymphs
Metatibia length was discriminant among all nymphal instars (table 5). The ratio T1/T2 allowed the second (T1 about 1/2 of T2) and the third instars (T1 about 2/3 of T2) to be distinguished, but not the fourth and the fifth instars. No difference among localities and host plants was observed.
Table 5. Measurements of metaleg parts (μm) (mean±SD) of Hyalesthes obsoletus nymphs.

T1, metatarsomere proximal to metatibia. Means±SD in bold are discriminant between second and third instars.
Mesonotal wingpad length and the W2/W1 ratio were discriminant between the fourth and fifth instars (table 6). No difference among localities and host plants was observed.
Table 6. Measurements of wingpad parts (μm) (mean±SD) of Hyalesthes obsoletus nymphs.

Means±SD in bold are discriminant between the instars.
Comparison between newly hatched and older first-instar nymphs
Significant differences in body length were observed for first-instar nymphs when comparing 20 newly hatched individuals (from eggs laid in the laboratory rearing on A. verlotorum) and 40 older individuals (nymphs feeding on U. dioica roots in the laboratory rearing) (table 7) (P<0.0001 with the Mann-Whitney test). Significant differences also resulted when considering the head-thoracic and abdomen lengths separately (P<0.0001, Mann-Whitney test). In particular, the length increase was proportionally greater for the abdomen, as is evident when comparing fig. 2a, b. Significant differences (P<0.001 at Mann-Whitney test) were observed also for the head and thoracic widths, and the metatibia and metatarsomere lengths. However, the ratio between the means of the newly hatched and older first-instar nymphs was much lower for these latter parameters (ranging from 1.05 to 1.14) than for the body length (1.42). Comparing this ratio with the Dyar's ratio reported in table 3, similar values were observed only with regard to the body length, so two different instars could be presumed. However, on the basis of discriminant morphological characteristics, both forms belonged to the first instar.
Table 7. Comparison of different body measurements (μm) (mean±SD) between newly hatched and older first-instar nymphs of Hyalesthes obsoletus.

The size measurements of the two nymphs collected from A. verlotorum roots, and belonging to the same population of nymphs hatched in Petri's dishes, were: 1050 and 1100 μm for body length, 540 and 550 μm for head-thoracic length, 510 and 550 μm for abdomen length, 250 and 260 μm for head width, 430 and 435 μm for thoracic width. These measurements were much higher than those of newly hatched nymphs and similar to those of first-instar nymphs collected from stinging nettle roots both in the field and in the rearing (tables 2 and 7).
Synthetic description of the eggs and the five instars and proposed dichotomous key
Eggs
Laid in groups; each egg elongated (about 550 μm), white, chorion translucent and covered with wax.
First instar (fig. 2a, b)
Measurements and morphological characteristics in tables 2, 4, 5 and 7. Other traits: body white; eyespots never visible; antennae three-segmented, scape and pedicel cylindrical and subequal, flagellum bulbous basally and filamentous distally, basal swelling of the flagellum subequal in size to pedicel and with Bourgoin's organ and tubular second projection (Shih & Yang, Reference Shih and Yang1996); thoracic nota longitudinally divided into three pairs of plates, each plate subrectangular; wingpad not developed; on the one-segmented metatarsus visible, a weak transverse line in the middle of the plantar surface; abdomen 9-segmented.
Second instar (fig. 2c)
Measurements and morphological characteristics in tables 2, 4 and 5. Other traits: two red eyespots sometimes visible; antennal pedicel with three sensory-plate organs of the flattened star-shaped type (Bourgoin & Deiss, Reference Bourgoin and Deiss1994); bulbous portion of the flagellum about 2/3 of pedicel length.
Third instar (fig. 2d)
Measurements and morphological characteristics in tables 2, 4 and 5. Other traits: two red eyespots often visible; antennal pedicel with five plate organs, bulbous portion of the flagellum about 2/5 of pedicel length; mesonotal wingpad covering metanotal wingpad laterally at the base.
Fourth instar (fig. 2e)
Measurements and morphological characteristics in tables 2, 4, 5 and 6. Other traits: compound eyes, consisting of small reddish areas; antennal pedicel with about seven plate organs, bulbous portion of flagellum about 1/5 of pedicel length; abdominal segment III with three or four sensory pits on each tergite, abdominal segments IV and V with nine pits on each tergite.
Fifth instar (fig. 2f)
Measurements and morphological characteristics in tables 2, 4, 5 and 6. Other traits: body white, thoracic nota infused with brown; red compound eyes; antennal pedicel with about nine plate organs; mesonotal wingpads extending nearly to apex of metanotal wingpads; abdominal segment III with three or four sensory pits on each tergite, abdominal segment IV and V with 11 pits on each tergite.
Key to nymphal instars
Proposed for recognition of each instar:
1 Metatarsus with three tarsomeres; compound eye present2
– Metatarsus with fewer than three tarsomeres; no compound eye3
2 Mesonotal wingpad extending nearly to apex of metanotal wingpad (uncovered metanotal wingpad length about 1/10 of mesonotal wingpad length); red compound eye with many facetsfifth instar
– Mesonotal wingpad covering lateral half of metanotal wingpad (uncovered metanotal wingpad length about 1/3 of mesonotal wingpad length); red compound eye with few facetsfourth instar
3 Metatarsus with one tarsomerefirst instar
– Metatarsus with two tarsomeres4
4 Metatarsomere T1 about 1/2 of T2; mesonotal wingpad not developedsecond instar
– Metatarsomere T1 about 2/3 of T2; mesonotal wingpad weakly developedthird instar
Phenology of nymphal instars on U. dioica and C. arvensis
In 2008, an earlier phenology of nymphs was observed on C. arvensis (locality 3) than on U. dioica (localities 1 and 2) (fig. 3a–c). In particular, nymphs were observed to overwinter only as third instars on C. arvensis and mostly as second instars on U. dioica. The last instar was reached from early June on field bindweed and from mid-late June on stinging nettle. Comparing stinging nettle and field bindweed growing in contiguous areas (localities 2 and 3, respectively), the differences in nymphal phenology, observed in late winter/early spring, persisted during the spring and seemed to be even greater in June, when the fifth instar occurred on C. arvensis 20 days before it did on U. dioica. Among the nymphs sampled on U. dioica, an earlier occurrence of fifth instar nymphs was recorded in locality 1 than in locality 2.
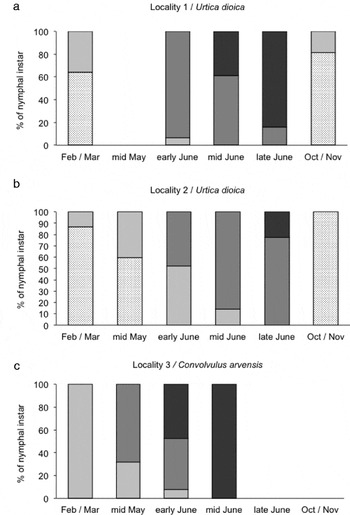
Fig. 3. Phenology of Hyalesthes obsoletus nymphs recorded in the three localities (a, b, c) in 2008 (![]() , 2nd;
, 2nd; ![]() , 3rd;
, 3rd; ![]() , 4th; ▪, 5th).
, 4th; ▪, 5th).
Discussion
H. obsoletus nymphal morphology in the present study and literature
Many analogies were observed with the study of Musil (Reference Musil1956), which described nymphs of H. obsoletus collected in Slovakia, probably on field bindweed. The body length reported for newly hatched first-instar nymphs (0.65 mm) is similar to that observed in our study (0.70 mm on average). The second instar was not described. The last three instars agree with our study in the metatarsomere number and wingpad features, but not in the body length (shorter than in our study).
Many differences between H. obsoletus nymphs of the present study and those collected by Güclü & Ozbek (Reference Güclü and Ozbek1988) in Turkey on alfalfa were observed. In their study, the body length of the first three nymphal-instars was on average 1.6, 1.4 and 1.2× that of our study, respectively. Moreover, the features of wingpads in the drawings of the fourth and fifth instars do not agree with those observed in our study.
Important differences between the H. obsoletus nymphs of this study and those described by Sforza et al. (Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999) were observed for most of the instars. These latter authors reported for the first instar nymphs fewer sensory pits on thoracic plates (8, 4 and 2 instead of 10, 6 and 3, respectively, in pronotal, mesonotal and metanotal plates) and for the second instar nymphs one instead of two tarsomeres in metatarsus. The first and second instar individuals from the two studies differed also in the position of the thoracic pits. The third instar nymphs of both studies agree on the numbers of sensory pits and tarsomeres in metatarsus. The fourth and fifth instar nymphs of the two studies agree on metatarsi segmentation, but not always in the number of thoracic and abdominal pits and apical spines on T1. The fourth and fifth instar nymphs in the present study appeared with more pits on pronotal plates (24–26 and 28–29, respectively) than in the study of Sforza et al. (Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999) (about 14 and about 20, respectively). Also the number of pits on tergites of the fifth instar nymphs differed between our and their study (3–4 vs. 2 in the abdominal segment III, 11 vs. 6–11 in the abdominal segment IV and 11 vs. 8–10 in the abdominal segment V). Finally, in our study, more apical spines were observed on the metatibia of the fourth instar nymphs (6 vs. 5) and on the T1 of the fifth instar nymphs (7 vs. 6).
Comparison between nymphal morphology of H. obsoletus and other Cixiidae
The number of metatarsomeres observed in the five nymphal instars of H. obsoletus in the present study agrees with reports for the cixiid Myndus crudus Van Duzee (Wilson & Tsai, Reference Wilson and Tsai1982). The only difference is the way the first-instar metatarsi were described: 2-segmented with divisions between segments very obscure in Wilson & Tsay (Reference Wilson and Tsai1982) and 1-segmented with a weak transverse line in the middle of plantar surface in our study. Unlike H. obsoletus (this study) and M. crudus (Wilson & Tsay, Reference Wilson and Tsai1982), metatarsi in Pentastiridius pachyceps (Matsumura) are already clearly 2-segmented in the first instar (Chen & Yang, Reference Chen and Yang1995) and in Oecleus borealis Van Duzee they are already 3-segmented from the third instar (Wilson et al., Reference Wilson, Tsai and Thompson1983). Unlike H. obsoletus (our study), M. crudus and O. borealis, Sforza et al. (Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999) reported for H. obsoletus the presence of metatarsi with one tarsomere bearing a transverse line in the middle of the plantar surface for the second instar instead of the first instar.
The number and position of thoracic pits described for H. obsoletus in this study showed similarity with those reported for all instars of M. crudus and at least for the latest instars of O. borealis and Oliarus atkinsoni Myers (Cumber, Reference Cumber1952).
The number of sensory plate organs (Bourgoin & Deiss, Reference Bourgoin and Deiss1994) (sensory plaque organs sensu Shih & Yang, Reference Shih and Yang1996) on the surface of the antennal pedicel in this study increased with the nympal instar, as described for other species of Cixiidae and Delphacidae (Shih & Yang, Reference Shih and Yang1996).
Increase in body size of H. obsoletus first-instar nymphs after hatching
The body length of newly hatched first-instar nymphs (on average 706 μm), collected before the beginning of feeding activity, is almost similar to the egg length (about 550 μm), whereas that of older nymphs, collected on roots of host plants, is much longer (on average 1002 μm). This increase in body size during the first instar can be explained by distension of non-sclerotized parts of cuticle due to feeding activity after hatching. This hypothesis is supported by the fact that the size increase is greatest in the abdomen where more intersegmental sutures are present than in the head, thorax and legs. Supporting this concept, the abdomen of the newly hatched individuals appeared clearly retracted under the optical microscope (see fig. 2a). In agreement with these observations, the possibility of an increase of abdomen size during the same nymphal instar has been reported for other cixiids (Myers, Reference Myers1929; Cumber, Reference Cumber1952).
Influence of the host plant on H. obsoletus nymphal morphology
Large differences in host plant preference were observed for H. obsoletus when U. dioica and L. angustifolia were compared. Adults from stinging nettle placed on lavender died within 24 hours and did not lay eggs (Kessler & Kehrli, Reference Kessler, Kehrli, Kast, Stark-Urnau and Bleyer2009). Nymphs of different instars from stinging nettle placed on lavender did not develop to adult stage (Kessler et al., Reference Kessler, Schaerer, Delabays, Turlings, Trivellone and Kehrli2011). These differences in host preference are also associated with differences in nymphal morphology, as evidenced by the description of H. obsoletus from stinging nettle (this study) and from lavender (Sforza et al., Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999). Such marked differences in nymphal morphology between our results (stinging nettle and field bindweed) and the paper of Sforza et al. (Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999) (lavender) are not attributable to rearing conditions because our nymphs differed from those described by Sforza et al. (Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999) regardless of origin (i.e. field or rearing). Moreover, since the morphological differences occur already for the first instar, they can not be ascribed to the feeding on different plant species. The possible morphological differentiation of H. obsoletus populations in relation to host plant (i.e. lavender vs. stinging nettle/field bindweed) requires further research, also considering that genetic studies so far carried out on H. obsoletus individuals collected from different host plants have never been performed for lavender (Johannesen et al., Reference Johannesen, Lux, Michel, Seitz and Maixner2008; Bertin et al., Reference Bertin, Picciau, Àcs, Alma and Bosco2010b).
On the basis of this study, the differences in host preference by H. obsoletus presented with U. dioica and C. arvensis (Maixner, Reference Maixner2007; Mori et al., Reference Mori, Pavan, Bacchiavini, Reggiani, Bonomi and Bertaccini2008a; Kessler & Kehrli, Reference Kessler, Kehrli, Kast, Stark-Urnau and Bleyer2009; Kessler et al., Reference Kessler, Schaerer, Delabays, Turlings, Trivellone and Kehrli2011) are not associated with differences in nymphal morphology.
Influence of the host plant on H. obsoletus nymphal phenology
The differences in phenology of H. obsoletus nymphal instars can be ascribed almost exclusively to host plant species, i.e. U. dioica and C. arvensis, since localities 2 and 3 are placed in contiguous flatland areas, that have similar climatic and soil conditions, and samplings were carried out on the same dates.
The minor differences in phenology of H. obsoletus nymphal instars observed on U. dioica between localities 1 and 2 can be explained by differences in soil texture as suggested by Maixner & Langer (Reference Maixner and Langer2006). In fact, the soil texture of locality 1 (loam according to USDA textural class) could favour a more rapid increase in soil temperature during spring with respect to that of locality 2 (clay according to USDA textural class), even if the aerial temperatures are not significantly different between the two localities.
The differences between stinging nettle and field bindweed in the phenology of H. obsoletus nymphs are in agreement with those reported in the literature for adults. Even if the nymphs were observed to overwinter as third instars not only on C. arvensis (100% of individuals) but also on U. dioica (up to a third of individuals), the fifth-instar nymphs occurred on field bindweed about 20 days before than on stinging nettle. This can occur because on U. dioica: (i) the third-instar nymphs require more time to complete the stadium in spring, having spent less time in this instar in the previous autumn; (ii) the nymphal development in spring resumes later, or is slower, than on field bindweed. Due to the differences in the criteria for identifying nymphal instars of H. obsoletus, the phenological data reported in the literature by different authors (Musil, Reference Musil1956; Brčak, Reference Brčak, Maramorosch and Harris1979; Alma et al., Reference Alma, Arnò, Arzone, Vidano, Vidano and Arzone1988; Güclü & Ozbek, Reference Güclü and Ozbek1988; Sforza et al., Reference Sforza, Bourgoin, Wilson and Boudon-Padieu1999; Maixner, Reference Maixner2007; this study) are not comparable with certainty.
Acknowledgements
The authors would like to thank Nazzareno Reggiani who kindly helped in the field nymph collections and Nicola Tosone for helping in molecular analysis. The authors would like to thank the anonymous reviewers for their valuable comments and suggestions that improved the manuscript.












