INTRODUCTION
Squat lobsters (Decapoda: Anomura: Galatheidae) comprise more than 400 small-sized species distributed worldwide, ranging from sublittoral to abyssal environments (Baba, Reference Baba2005; Osawa et al., Reference Osawa, Lin and Chan2006). Some species form dense aggregations in mid latitudes, as for example Pleuroncodes monodon, (up to 100 ind m−2) on the coast of central Chile (Arana & Ziller, 1990 in Tapella, Reference Tapella2002), Pleuroncodes planipes (up to 40 individuals m−2) on the Mexican Pacific coast (Aurioles-Gamboa, Reference Aurioles-Gamboa, Aurioles-Gamboa and Balart1995) and the juvenile pelagic (individuals m−3) and adult benthic (3–30 individuals m−2) phases of Munida gregaria off New Zealand (Zeldis, Reference Zeldis1985). Because of their high abundance, some galatheids represent an important food source, supporting large-scale fisheries (Longhurst, Reference Longhurst1967; Roa et al., Reference Roa, Gallardo, Ernst, Baltazar, Cañete and Enríquez-Brionnes1995) and populations of several marine resources such as crabs, squids, fish, whales, albatrosses and penguins (Romero, Reference Romero2003; Longhurst, Reference Longhurst2004).
Munida (Leach, 1980) is the only galatheid genus present in the south-western Atlantic. Fourteen species live between the equator and the La Plata River (Melo-Filho & Melo Reference Melo-Filho and Melo2001), and only three, Munida spinosa (Henderson, 1885), Munida gregaria (Fabricius, 1793) and Munida subrugosa (White, 1847) (Spivak, Reference Spivak1997), have been reported to occur on the continental shelf from the La Plata River (Uruguay, 35°S) to the southern tip of South America (Cape Horn, 55°S) (Spivak, Reference Spivak1997). Although the taxonomic status of M. gregaria and M. subrugosa is not completely resolved (Ahyong & Poore, Reference Ahyong and Poore2004; Tapella & Lovrich, Reference Tapella and Lovrich2006), recent molecular evidence (Perez Barros et al., in press) and the results of interbreeding experiments (Perez Barros, Reference Pérez-Barros2007) support the hypothesis that both are different morphs of the same species. Therefore, in the present work ‘subrugosa’ and ‘gregaria’ will be considered different morphs of M. gregaria.
Munida gregaria has a wide distribution in the southern hemisphere, being present not only in the south-west Atlantic but also in the south-east Pacific from Cape Horn to Chiloe Island (41°S) (Boschi et al., Reference Boschi, Fischbach and Iorio1992), Eastern Indian Island (off Tasmania) (Zeldis, Reference Zeldis1985), south-western Pacific (off Tasmania and Eastern New Zealand and its subantarctic islands) (Zeldis, Reference Zeldis1985). Its reported bathymetric distribution ranges from the sublittoral down to a depth of 1137 m (Arntz et al., Reference Arntz, Gorny, Soto, Lardies, Retamal and Wehrtmann1999). In the southern limits of its South American range of distribution, aggregations can attain densities of up to 27 individuals m−2 and carapace length (CL) and wet weight can reach up to 30 mm and 15–20 g respectively (Tapella, Reference Tapella2002). In contrast, on the Atlantic coast of northern Patagonia the species grows up to 22 mm in CL and 8 g in wet weight, attaining densities of up to 16 individuals m−2 (Dellatorre & Galván, unpublished data) at depths ranging from 5 to 100 m.
Munida gregaria is simultaneously a deposit feeder and a predator on components of the phyto and zoo-benthos, and is considered a key species in the Patagonian shelf marine community representing ‘the direct link between the detritus and the top predators’ (Romero et al., Reference Romero, Lovrich, Tapella and Thatje2004), including most commercial fish, crustaceans, squids, marine birds and mammals from the continental shelf off Patagonia (Thompson, Reference Thompson1993; Isla & San Roman, Reference Isla and San Román1995; Sanchez & Prensky, Reference Sanchez and Prensky1996; Croxall & Wood, Reference Croxall and Wood2000; Phillips et al., Reference Phillips, Nichols and Jackson2003), and a potentially exploitable fisheries resource (Lovrich et al., Reference Lovrich, Casalinuovo, Molina, Cárcamo and Pierotti1998; Wyngaard et al., Reference Wyngaard, Iorio and Boschi2001). On the other hand, larvae of M. gregaria constitute an important fraction of the zooplankton biomass during late winter and spring (Lovrich, Reference Lovrich1999) and could have a great influence in the trophic dynamic of the plankton community.
The reproduction of M. gregaria has been studied in the central and southern regions of its South American distribution (Rodríguez & Bahamonde, Reference Rodríguez, Bahamonde and Arana1986; Tapella et al., Reference Tapella, Romero, Lovrich, Chizzini, Paul, Dawe, Elner, Jamieson, Kruse, Otto, Sainte-Marie, Shirley and Woodby2002a, Reference Tapella, Lovrich, Romero and Thatjeb; Vinuesa, Reference Vinuesa2007). In the Beagle Channel (55ºS) females reach their physiological maturity at approximately 10 mm CL, fecundity, estimated as eggs carried by female at a given time, ranges from 124 to 10750 eggs per female with an average of 4332, egg extrusion and fertilization extends from May to August, and ovigerous females are present until November (Tapella et al., Reference Tapella, Lovrich, Romero and Thatje2002b). In San Jorge Gulf (46ºS), Vinuesa (Reference Vinuesa2007) reports that female maturity is reached at 9.3 mm CL, fecundity varies between 5 and 7545 eggs per female and ovigerous females are present between June and November.
Estimations of individual fecundity must rely on the knowledge of two types of data: (1) clutch size; and (2) number of clutches produced by a female during the breeding season. Clutch size of M. gregaria is related to CL (Tapella et al., Reference Tapella, Lovrich, Romero and Thatje2002b) but this relationship is highly variable between individuals (Vinuesa, Reference Vinuesa2007). The number of clutches produced by female per breeding season is constrained both by length of this season and duration of embryonic development. Based on the observation of monthly changes in the proportions of ovigerous females, and relative frequencies of stages of ovary maturity and embryo development, Tapella et al. (Reference Tapella, Lovrich, Romero and Thatje2002b) and Vinuesa (Reference Vinuesa2007) reported that the species produces one clutch per female per year in the Beagle Channel and two in San Jorge Gulf. Even though, the length of embryonic development has not been determined, Tapella et al. (Reference Tapella, Lovrich, Romero and Thatje2002b) and Vinuesa (Reference Vinuesa2007) respectively hypothesize that embryonic development of the species may last 90–120 days in Beagle Channel and 70–90 days in San Jorge Gulf. Such differences probably reflect contrasting environmental conditions at both geographical locations (Beagle Channel monthly average sea surface temperature (SST) range: 4.2°–9.8°C, Balestrini et al., Reference Balestrini, Manzella and Lovrich1998; Central San Jorge Gulf monthly average SST range: 6.8°–14.2°C; SST data obtained from satellite estimations for the period 1987–1998, AVHRR Oceans Pathfinder NOAA–NASA), encouraging study of the reproductive ecology of M. gregaria in the northern range of its distribution to fully understand its adaptations to local temperature regimes.
The objectives of this work are to determine: (1) the breeding season span of M. gregaria in northern Patagonia; (2) the potential of the species to produce multiple clutches of eggs; (3) the length of its embryonic development; and (4) based on the previous objectives, to estimate the potential number of clutches produced by M. gregaria by breeding season.
MATERIALS AND METHODS
Study area
This study was conducted in Nuevo Gulf, northern Patagonia (42.75ºS 65.00ºW, Chubut, Argentina), a 184-m deep elliptical basin, 2440-km2 in surface area communicating with the south-western Atlantic through a 17-km wide strait (Mouzo et al., Reference Mouzo, Garza, Izquierdo and Zibecchi1978). SST monthly averages range from a maximum of 17.2ºC in February to a minimum of 9.6ºC in September (SST data obtained from satellite estimations for the period 1987–1998, AVHRR Oceans Pathfinder NOAA–NASA) and salinity varies seasonally within 33.5–33.9 ppt (Rivas & Ripa, Reference Rivas and Ripa1989).
Sampling and biological determinations
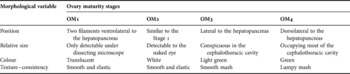
Sampling was performed on a monthly basis from January 2005 to March 2006. Munida gregaria specimens were captured with cylindrical expandable crayfish traps deployed for 1–2 days on the muddy sea bottom at depths ranging from 18 to 25 m. After sampling all crabs were fixed in 6% formalin in seawater. In the laboratory, M. gregaria specimens were sorted by morph (all of the specimens belonged to the ‘subrugosa’ morph), sex and spawning condition (ovigerous or non-ovigerous). Carapace length (CL), taken from the posterior edge of the orbital arch to the mid-dorsal posterior margin of the carapace, was measured with a digital caliper to the nearest 0.1 mm. Physiological maturity of adult females (13–21 mm CL) was assessed by direct visual observation of ovary morphology, size and colour under a dissecting microscope. Based on these observations, adult females were classified into four categories of ovary maturity (OM) (Table 1). Oocyte size–frequency distributions were obtained by measuring the diameter (OD) of 80–150 randomly chosen oocytes from 37 females sub-sampled in May, August, September, October and November. For this purpose, oocytes were spread on a Petri dish, and those above the minimum detectable OD at 50X magnification (45 µm) were measured with an ocular micrometer under a dissecting microscope. Females sampled in November, December and February displayed vitellogenic oocytes with signs of athresia, making it impossible to spread intact oocytes and subsequently measure their diameter. Vas deferens smears from 8–20 males 15–23 mm in CL were examined every month under a dissecting microscope at 50X magnification. The presence of spermatophores in the vasa deferentia was considered as evidence of advanced sexual maturity.
Table 1. Macroscopic scale of ovary maturity of Munida gregaria.
Based on the observation of size, pigmentation and yolk proportion, embryos were classified into five categories of embryonic development from egg extrusion to larvae hatching (ED) (Table 2) (modified from Pinheiro & Hattori, Reference Pinheiro and Hattori2003). Length of the embryonic development was estimated by incubation of 17 ovigerous females. Experiments were conducted in 10-l plastic aquaria filled with seawater and maintained with aeration in a temperature controlled chamber at 11ºC constant temperature. Females were fed and seawater was replaced every 4 days. Every two days, a sample of at least 10 developing embryos were removed from the pleopods of each female, examined under a dissecting microscope at 25X magnification, and classified into stages described in Table 2. Since only three M. gregaria females incubated their eggs from an early stage of development (from 8-cell to 64-cell blastula) until hatching, only the data corresponding to these individuals are reported in the present work.
Table 2. Scale of embryonic development of Munida gregaria (modified from Pinheiro & Hattori, Reference Pinheiro and Hattori2003).

Data analysis
Proportions of ovigerous females and mature males in monthly samples were analysed to determine the span of the breeding season. To verify whether or not ovigerous female ovaries re-mature while embryos develop, the relative frequencies of the stages of ovary maturity were compared between females grouped according to the embryonic development stage of the eggs they carried at the time of sampling. Additionally, to establish whether or not ovary re-maturation and embryonic development occur synchronously among individuals, stages of ovary maturity and embryonic development were examined in ovigerous females from four additional samples taken at regular intervals through a period equivalent to the estimated length of embryonic development. To simplify the interpretation of the last analysis results, ovary maturity and embryonic development stages were grouped in two categories: early (OM stages 1 and 2; ED stages 1 and 2) and advanced (OM stages 3 and 4, ED stages 3 to 5). Finally, to validate the macroscopic classification of ovarian maturity, the relationship between oocyte size and ovarian maturity was analysed. Since oocyte size–frequency distributions usually showed more than one modal component it was necessary to separate the modal components in an objective way. Therefore, the number of modal components of oocyte size–frequency distributions of individual females, and their mean, variance and mixing proportion were estimated by running the modal component separation routine in Mclust library (Fraley & Raftery, Reference Fraley and Raftery2007) on R programming language version R-2.4.1 (http://www.r-project.org/; R Development Core Team, 2006). Estimated means of the largest modal component of OD frequency distributions from each female were compared between OM stages using the Jonckheere–Tersptra test (Jonckheere, Reference Jonckheere1954; Siegel & Castellan, Reference Siegel and Castellan1988) for distribution-free samples.
RESULTS
Monthly percentages of spawning conditions of adult M. gregaria in the study area showed that ovigerous individuals occur from June to December, representing more than 80% of sampled females from July to October (Figure 1). Data obtained in some months showed that mature males occurred at high proportions from autumn to spring (Figure 1).

Fig. 1. Monthly variation in the percentage of non-ovigerous (grey bars) and ovigerous females (black bars) (top) and immature (grey bars) and mature (black bars) males (bottom) of Munida gregaria. Samples sizes are indicated above bars. Months with no samples are bare.
Throughout the study period, ovaries of most females carrying recently extruded eggs (ED1) were at immature or early maturity stages (OM1 and OM2) (Figure 2). Ovigerous females carrying embryos at more advanced ED stages showed higher proportions of ovaries in intermediate or advanced maturity stages (OM3 and OM4). Seventy-six per cent of females carrying embryos ready to hatch (ED5) showed ovaries at advanced maturity (OM4) (Figure 2). During the breeding season, only 3% of the females with OM4 were not incubating eggs in their pleopods. Relative frequencies of females at early and advanced OM and ED stages varied synchronously (Figure 3).

Fig. 2. Relative frequency of ovary maturity stages (OM) in ovigerous females with embryos in different embryonic development (ED) stages.

Fig. 3. Chronological changes in relative frequencies of ovary maturity (OM) (top) and embryonic development (ED) (bottom) stages of Munida gregaria in samples taken periodically throughout a period equivalent to the length of embryonic development.
In most females, two modal components of oocyte diameter were detected in the ovaries (Figure 4). Almost all ovaries analysed presented small oocytes (modal components with mean OD ranging within 60–200 µm). Ovaries at OM1 showed two or three modal components with means ranging within 63–196 µm, except for one with a low proportion (3%) of large oocytes (modal component with 617-µm mean OD). Ovaries at OM2 presented a modal component of small oocytes and another intermediate size, ranging between 137–303 µm. Three of this OM2 ovaries presented a few (less than 5% of the total number) large oocytes (means ranging within 447–783 µm). Most ovaries at OM3 and OM4 presented two clearly different modal components, one of smaller-sized oocytes (means ranging within 80–296 µm) and another of larger-sized ones (means respectively ranging within 303–488 µm in OM3 and 435–620 µm in OM4 ovaries) (Figure 4). The larger oocyte-diameter modal component in the ovaries increases significantly with increasing maturity stage (Jonckheere–Terpstra test, P < 0.001).

Fig. 4. Oocyte diameter distribution in ovaries of Munida gregaria. Empty, grey and black dots represent the mean oocyte diameter of the modal components with smaller, intermediate and larger oocytes respectively. Vertical bars represent standard deviations. Dot size-classes represent four ranges for the percentage of oocytes in each modal component relative to total oocytes.
The complete embryonic development of M. gregaria lasted 26, 27 and 29 days in three incubation experiments. During the incubation experiments it was observed that: (1) ovigerous females are able to actively swim similarly to non-ovigerous females or males; (2) grooming the egg masses with the fifth pereiopod and abdomen flapping are frequent behaviours; and (3) egg chorion and funiculus rests detach from the pleopods immediately after hatching.
DISCUSSION
Duration of different phases of the reproductive cycles may vary among populations of crustacean species (Sastry, Reference Sastry, Bliss, Vernberg and Vernberg1983; Brante et al., Reference Brante, Cifuentes, Pörtner, Arntz and Fernández2004; Ituarte et al., Reference Ituarte, Bas, Luppi and Spivak2006). Notwithstanding the contrasting temperature regimes in the southern and northern limits of M. gregaria coastal distribution along the Atlantic coast of South America, the breeding season is quite similar at both extremes: mating and egg extrusion occurs in early winter and ovigerous females are present in high proportions until late spring. However, in northern Patagonia the breeding season seems to be slightly longer and temporally displaced towards late spring than in southern and central Patagonia (Tapella et al., Reference Tapella, Lovrich, Romero and Thatje2002b; Vinuesa, Reference Vinuesa2007). Moreover, in New Zealand, approximately at 46ºS larvae of M. gregaria are present from June/July to mid-October (Zeldis, Reference Zeldis1985) suggesting that the breeding season at this location is synchronous with that of the South American populations. Also, other galatheids display similar reproductive seasonalities: P. monodon spawns in central Chile (south-eastern Pacific, 37ºS) from May to October–November (Palma & Arana, Reference Palma and Arana1997), and P. planipes breeds in California (north-eastern Pacific 33ºN) from December to March (northern hemisphere winter) (Boyd & Johnson, Reference Boyd and Johnson1963).
Many crab species produce multiple broods during an intermoult period (Morgan et al., Reference Morgan, Goy and Costlow1983; Dineen et al., Reference Dineen, Clark, Hines, Reed and Walton2001; Ituarte et al., Reference Ituarte, Spivak and Luppi2004). In some of them, a batch of oocytes start their vitellogenesis as soon as the previous egg clutch is extruded (Ituarte et al., Reference Ituarte, Spivak and Luppi2004). During this study, most M. gregaria females with recently extruded eggs had immature or recovering ovaries (Figure 2) with small (<200 µm mean diameter) oocytes (Figure 4). In some OM1 and OM2 ovaries a few large (>447 µm mean diameter) oocytes were detected, probably representing rests from the previous spawning. On the other hand, most females with developed embryos displayed OM4 ovaries ready for spawning. This pattern allows us to conclude that females start ovary re-maturation immediately after spawning and that this process completes before hatching, allowing females to produce multiple egg clutches during each breeding season. Considering that M. gregaria actively uses its abdomen to swim, and that increasing clutch volume could compromise mobility and embryonic survival, multiple spawning could be interpreted as a strategy to increase the reproductive output without compromising mobility or embryonic survival.
Temporal changes (peaks) in the proportion of ovigerous females have been used to detect single or multiple spawning events during the same reproductive season of several crab species (Mantelatto & Franzoso, Reference Mantelatto and Fransozo1999; Tapella et al., 2002; Vinuesa, Reference Vinuesa2007). The existence of peaks in the proportion of ovigerous females within the same reproductive season implies the existence of a temporal gap between hatching of larvae and the next egg extrusion. During this study: (1) most females showed fully mature ovaries (OM4) at hatching time (Figure 2); (2) it was observed in aquaria that egg chorion and funiculus completely detach from the pleopods immediately after hatching, allowing the attachment of the new egg mass; and (3) only a small proportion (3%) of the females with mature ovaries were not ovigerous. These observations suggest that M. gregaria is able to release newly hatched larvae, mate and spawn within a brief period (few days), making it difficult to detect the temporal gap between hatching and the next spawning. If this holds true, detecting spawning peaks by interpretation of monthly variations in the proportions of ovigerous females may lead to inaccurate conclusions.
Embryonic development was not studied for M. gregaria in the past. In a previous work, Rodríguez & Bahamonde (Reference Rodríguez, Bahamonde and Arana1986) speculated that embryogenesis of M. subrugosa (=M. gregaria) would last 8–9 months in the Magellan Strait (South America, 54ºS) and Tapella et al. (Reference Tapella, Lovrich, Romero and Thatje2002b) stated that females hold their eggs over a period of three to four months in the Beagle Channel (South America, 55ºS). Also, based on the monthly variations of the proportions of ovigerous females and stages of embryonic development, Vinuesa (Reference Vinuesa2007) proposed that embryonic development takes 90 days in females spawning in July and 70–80 days in females spawning in September. Our experiments show that the complete embryonic development of M. gregaria lasts 26–29 days at constant temperature of 11ºC. Since average SST in coastal waters off north-eastern Patagonia (43ºS) during the breeding season of M. gregaria is 11.5ºC (AVHRR Oceans Pathfinder NOAA–NASA), it can be concluded that embryogenesis lasts approximately one month in this region. Even considering that in the study areas of previous works average SST during the breeding season are lower than those typical from northern Patagonia (5.5ºC in Beagle Channel; San Jorge Gulf is 8.5ºC between June and August and 9.4ºC between September and November: AVHRR Oceans Pathfinder NOAA–NASA), and that these could delay the embryonic development, our experimental determinations of the length of embryogenesis differs markedly from estimations reported by other authors (Rodríguez & Bahamonde, 1989; Tapella et al., Reference Tapella, Lovrich, Romero and Thatje2002b; Vinuesa, 1997).
In this work, it is shown that in northern Patagonia: (1) the complete span of the breeding season is seven months (June–December), most adult M. gregaria females carrying developing embryos from July to October (4 months); (2) ovaries re-mature synchronically with embryonic development; and (3) embryonic development and ovary rematuration last approximately one month. Considering the last three points we conclude that in northern Patagonia the species has the potential to produce at least three or four egg clutches during the breeding season.








