A recurrent theme during discussions at previous HTAi Asia Policy Forums (HAPFs) is the need for effective tools that enable decision makers to prioritize technologies when investing in new items for benefit packages. Like most global health policy makers, those in the Asia region are tasked with the challenge of ensuring the long-term sustainability of universal health care (UHC), balancing the need to reduce health inequalities whilst facilitating universal access to safe and effective healthcare that also represents value for money, all within a limited budget. Globally, health budgets are affected by major drivers of rising healthcare costs that include changes in population structure (an aging population in part resulting from a decline in fertility rate concomitant with increased longevity (Reference Bloom1)), the increased utilization of health care alongside the increasing prevalence of chronic disease such as diabetes, and a demand-driven investment in new devices, diagnostic tests, medical procedures, and pharmaceuticals (Reference Dieleman, Squires, Bui, Campbell, Chapin and Hamavid2). Although it has been estimated that up to 48 percent of health-spending growth is due to health technologies, this is a result of not only the investment in new but also an increase in the use of existing health technologies (Reference Harris, Green, Ramsey, King and Green3).
In resource-limited environments, tools such as HTA may increase the efficiency of the health system, ensuring it provides value for money, is fit for purpose, enabling provision of optimum care for all patients. HTA assists policy makers in the evidence-based decision-making process around priority setting, informing on the quality, safety, and cost-effectiveness of new technologies. Whilst investment in new healthcare technologies is important, continuous investment is not sustainable and a concurrent process for reassessing existing healthcare services is needed. Reassessment can identify technologies that have been superseded or found to be unsafe, ineffective, or costly when used in real-world clinical practice (4), but importantly may also identify new, more effective uses for existing technologies.
As such, the 2019 HAPF examined what role, if any, that horizon scanning (HS) for technologies that have yet to become part of established healthcare practice, and health technology reassessment (HTR) of existing technologies may play in the sustainability of health systems in the Asia region.
Life Cycle of a Health Technology
Health systems usually consider the introduction of a new health technology as a binary process: new technologies in, old technologies out, with the life cycle of a technology depicted as an S curve (Figure 1). Emerging technologies may be detected by HS before widespread use, with these technologies picked up and used by early adopters as they begin to slowly diffuse throughout the health system. Depending on the technology, regulatory approval may occur at the inflection point of the technology curve, after which diffusion of the technology may increase. A full HTA is usually only conducted when approaching maximum growth and diffusion of the technology, toward the top of the curve, when the effectiveness and cost-effectiveness evidence base has matured sufficiently to inform and formulate health policy. The curve then moves into a period of technology management, where use of the technology plateaus (Reference Smith, Tarricone and Vella5).

Figure 1. The typical life cycle of a health technology in a health system.
Opportunities for HTR may occur when established technologies become outdated, inefficient or no-longer-effective, or new uses for the technology have been identified. Investment in new clinical practices and technologies may passively render existing ones obsolete through natural attrition, although new and old technologies often co-exist together in the system for some time. Passive HTR may be achieved simply by education and the dissemination of information without the need for direct intervention from policy makers. Clinicians often stop using a technology when a better alternative becomes available, as is often the case with surgical procedures (Reference Harris, Green, Ramsey, Allen and King6). The overlapping S curve model in Figure 2 more accurately describes the reality of health systems, where the introduction of new technologies is a continuous, overlapping process. Technologies reach their limit of diffusion and plateau, or worse, begin to reduce value and patient outcomes. At the same time, new technologies are introduced into the system and the process begins again, with an “in-between” period between the old and new offering an opportunity for HTR (Reference Babaian7).

Figure 2. The overlapping S curves of health technology's life cycle in a health system (adapted from (Reference Babaian7)).
The challenge in healthcare is identifying the right time to invest in innovative and potentially disruptive technologies in order to value add to the existing health system, and to avoid using technologies that may be outdated, inefficient, or ineffective. A continual process of HS integrated with a parallel process of HTR may be one way of achieving the overlapping S curve technology life cycle.
Horizon Scanning
HS is a risk management tool that is not unique to healthcare and is used in many fields to reduce uncertainty, anticipate and inform on future trends, enable planning to facilitate appropriate adoption, and in so doing, provide a degree of future proofing (Reference Kennedy-Martin, Mitchell, Boye, Chen, Curtis and Flynn8). HS provides advance warning and intelligence of the risks and benefits of not only new and emerging technologies but new uses of existing, health technologies and possible targets for HTR (Reference Mundy9;Reference Packer, Gutierrez-Ibarluzea and Simpson10). Early intelligence can guide future investments and assist in healthcare planning including financial capacity, infrastructure requirements (physical structures, IT requirements, etc.), workforce capability, and training prerequisites. HS helps build organizational resilience by assessing the implications of a new technology to ensure that health systems can embrace innovation in a sustainable way before technologies enter the system. HS may prove to be an invaluable tool as health systems embrace high-cost health technologies such as gene technology, cellular therapies and oncological pharmaceuticals, wearables, 3D-printing, implants, and E-health technologies (Reference Daruwalla, Kambhammettu, Chainani, McKeering and Bhattacharjee11).
The type of HS framework adopted and the value of HS is dependent on the health system it will support, varying with different financing and reimbursement policies, the degree of public subsidization, the level of health insurance, the types of services offered, and the size and strength of the private sector (Reference Packer, Fung and Stevens12). Not every country will, like Malaysia, want to conduct HS across the board for all health technologies (see later survey results). Some countries may choose to focus on highly innovative and high-cost technologies such as cellular and gene therapies targeted by Singapore's newly developed HS system (HSS), whilst Taipei's Center for Drug Evaluation (CDE) only conducts HS for pharmaceuticals.
The HS process will not be discussed in-depth in this paper—for a broader overview, see the 2019 HAPF background paper published on the Health Technology Assessment International (HTAi) Web site (Reference Mundy, Kearney and Trowman13). All HSSs use three essential elements as per the EuroScan methodology toolkit (14): identification, filtration, and prioritization. HSSs also need to define and identify their primary stakeholders (e.g., policy makers, regulatory agencies, clinicians, or patients); the time horizon, usually defined by stakeholders needs; technologies of interest (devices, diagnostics, procedures, drugs, programs), whether they should focus on single technologies, all technologies relating to a specific disease, or all technologies in a clinical care pathway (prevention, diagnosis, treatment, and even rehabilitation); the sources used to identify technologies, which should be pertinent and relevant to local health system needs; the type of assessment (rapid or more in-depth depending on stakeholders needs); and lastly, how assessments are disseminated (freely available, remain confidential).
Health Technology Reassessment
In an ideal health system, existing clinical practices and technologies should be subject to ongoing review and continuous evaluation in order to identify the instances of poorly coordinated care, duplication or gaps in service delivery, practices that encourage overtreatment or overdiagnosis, ineffective practices or systemic waste (Reference Harris, Green, Ramsey, Allen and King6), or new uses for existing technologies (Reference Harris, Green, Ramsey, King and Green3).
There are many reasons why HTR should be routinely conducted, including addressing political priorities or organizational concerns; meeting regulatory, legal, or ethical requirements; or used to placate social pressures for a more equitable health system. Economic reasons may be cited in order to maintain quality without extra expenditure, or patient-focused reasons such as improving patient flow and reduce waiting times. HTR may be needed purely for financial reasons such as finding funds to spend elsewhere or support investment in new technologies, or to meet budget cuts (Reference Harris, Green, Ramsey, Allen and King6).
HTR is not an easy process, as, unlike the investment in healthcare, there is no validated methodology or framework for health systems or HTA agencies to follow. The lack of methodology may explain the difficulty that the HTR process has in gaining traction and “buy in” at all levels of the health system. An explicit, transparent process may be more acceptable to stakeholders rather than the ad hoc, seemingly random process of HTR currently operating in many health systems.
Other challenges and issues around the implementation of HTR include difficulty identifying low-value services due to a lack of evidence; resources and HTA capacity; political, clinical, and administrative will to evaluate established technologies; and data, data linkage, and postmarket surveillance. Factors such as multiple stakeholders and stakeholder resistance; vested interests from manufacturers and clinicians; and clinical practices associated with sensitivities (e.g., those used to care for pediatric, cancer, or palliative care patients) also make successful HTR challenging (Reference Mundy, Harris, Hewson and Jacobsen15–Reference Henshall, Schuller and Mardhani-Bayne17).
HTR can be driven by a combination of evidence, incentives (not always financial) and high-level support. The identification and fostering of “clinical champions,” key clinical groups, or influential experts is pivotal in driving the acceptance of change, with change more likely to be accepted by both clinicians and consumers if the focus is firmly placed on quality and safety (Reference Mundy, Harris, Hewson and Jacobsen15).
Methods
The seventh HAPF was held from 6–8 November 2019, in Hanoi, Vietnam, with fifty-nine invited experts in attendance comprising delegates from public sector HTA agencies, most of whom are embedded within, or funded by the health departments of fifteen countries in the Asia region; twenty-seven for-profit industry delegates from fourteen companies with interest and experience in Asia; leaders from HTAi; senior public officials from the Vietnamese Ministry of Health; and representatives from the World Health Organization, the Gates Foundation, and the World Bank.
To inform discussions, a background paper (Reference Mundy, Kearney and Trowman13) was developed comprising several elements: firstly, a literature review that described the basic components of, and challenges associated with, HS and HTR. Secondly, a short survey of health system payers (HTA agencies) participating in the 2019 HAPF was undertaken to capture their experience of HS and HTR, noting that responses were provided by individuals and may not accurately reflect the full situation in each agency or country. For the survey, a series of questions were developed around HS (n = 16) and HTR (n = 6) using an online survey tool (please see 2019 HAPF background paper published on the HTAi Web site for survey structure (Reference Mundy, Kearney and Trowman13)). Agencies indicated whether they were actively conducting HS and HTR, with only the active agencies required to complete the entire survey. Lastly, case studies of several agencies were conducted describing pathways and issues in establishing HSSs and HTR.
The HAPF is designed to promote open and constructive dialogue, without fear or favor. As such, meetings are conducted under the Chatham House Rule in which participants are free to share information obtained during the meeting but the identity or affiliation of the person providing the information cannot be revealed (18). This study provides the authors' summary of the premeeting surveys and discussions among participants during the 2019 HAPF and does not necessarily represent the consensus view of those attending the meeting, or those of the organizations they represent.
Results
Results From the Premeeting Horizon Scanning Survey
The response rate for the HTA agency survey was 100 percent, being completed by fourteen public sector agencies from eleven countries representing China (Beijing, Shanghai, and Hong Kong), India, Indonesia, Japan, Malaysia, the Philippines, Singapore, South Korea, Taipei (two agencies), Thailand, and Vietnam. Results of the survey are summarized in Table 1. Five of the fourteen agencies have either established, or are developing, an HSS. The three established systems have conducted HS for at least 3 years (South Korea, Malaysia, and Taipei-CDE) with Singapore and Shanghai in the process of developing HS. Except for Hong Kong, eight of the remaining nine agencies are interested in developing an HSS. Of the agencies actively engaging in HS, four were interested in forming an information-sharing HS network similar to the EuroScan network in order to provide a forum for information sharing, reduce duplication of effort, and importantly, provide a platform for collaboration with industry in the region. Five of the eight agencies interested in developing an HS capacity also expressed an interest in forming and participating in such a network.
Table 1. Horizon Scanning Survey Results from Payer Agencies

HS, horizon scanning; EuroScan, EuroScan International Network; CADTH, Canadian Agency for Drugs and Technologies in Health; HTA, heath technology assessment.
a Medical device authority and National Pharmaceutical Regulatory Agency.
b Rapid brief = brief overview of the technology, limited if any evidence but important to alert policy makers, typically 1–3 pages produced in <1 week.
c Assessment brief = in-depth assessment covering safety, effectiveness, cost, and impact, typically 8–12 pages produced in 1–4 weeks.
Of the established HSSs, Malaysia has the most comprehensive, aiming to identify all types of new and emerging healthcare technologies that may impact on the Malaysian health system. South Korea identifies new devices, diagnostics, and medical procedures, whilst Taipei's CDE only identifies new pharmaceuticals. Singapore began developing its HSS in 2019 in order to target innovative, disruptive high-cost technologies with the potential for considerable growth in clinical need and expenditure, with an initial focus on scanning for gene therapies.
All four HSSs are primarily proactive, actively identifying new and emerging health technologies from a range of sources; however, at times they may be reactive, responding to requests for information from stakeholders such as clinicians or policy makers. This is especially important when used to identify new uses for existing technologies. In addition, all agencies currently identify single technologies rather than a range of technologies involved in a clinical care pathway or those addressing a specific disease. Both Malaysia and Taipei would consider using their HS methodology to identify potential HTR targets.
The typical time horizon used to identify technologies was 1–3 years before health system entry or prior to regulatory approval, with only Taipei considering technologies 12 months away from health system entry. The primary purpose of all HS agencies was to inform policy makers at the health department level. HS is also used to inform hospital policy makers and clinicians (South Korea, Malaysia, and Singapore), patients (South Korea and Malaysia), and regulatory bodies (Malaysia).
There was great variation between the agencies regarding HS sources with Malaysia having the most comprehensive range of primary and secondary sources (see Table 1). There was, however, a great deal of consistency between agencies regarding the criteria used to prioritize identified technologies, with all agencies considering the burden of disease a priority, as well technologies associated with safety concerns. All agencies considered the potential impact of the technology on costs, whether that be increased costs or savings to the health system, technologies that require a large capital outlay, or technologies that might require out-of-pocket payments for patients. Aligned with these concerns, all agencies prioritized those technologies that would have an impact on existing services that may require reorganization or structural changes, have staff training requirements, be associated with learning curves, or require quality assurance procedures. In addition, technologies that would impact on patient morbidity, mortality, and quality of life were also a priority for all agencies. Identified technologies are prioritized either by the in-house HS team (South Korea, Singapore, and Taipei) and/or by a panel of clinical experts (Malaysia and Singapore).
All agencies produce in-depth assessment briefs that typically consist of a description of the technology, regulatory information, and include an assessment of the evidence on the safety and effectiveness of the new technology from several studies. Cost-effectiveness analyses are rarely identified due to the newness of technologies; however, a summary of basic costs including infrastructure and training requirements would be presented. Assessments are typically 8–12 pages long, taking 1–4 weeks to produce. Malaysia and Singapore have a Rapid Brief product that is approximately 1–3 pages produced in <1 week, providing a brief overview of the technology including costs and any safety and effectiveness evidence if available. These rapid briefs may not have enough evidence for investment decision making but are important to alert policy makers to potential disruptive technologies that may impact on the health system in the future, providing a basis for the ongoing monitoring of a technology.
Results from the HS Case Studies
The case studies of an established (Malaysia) and a developing (Singapore) HSS asked about the challenges of setting up and maintaining an HS unit. The greatest challenge identified by Malaysia was the investment in human resources, and the difficulty of training and maintaining skilled staff in the face of high staff turnover. In addition, getting “buy in” from stakeholders, especially regulators, was challenging at first but over time these relationships have improved. Singapore identified several challenges, the first being that a great deal of clinician engagement and expertise was required to prioritize identified technologies, especially the complex gene therapies currently targeted by their HS. Consultations with clinicians to understand local patient numbers, current treatment practices, and the potential disruptive impact of new technologies to the healthcare system were required to inform prioritization scores for most of the identified technologies. Singapore's early experience emphasized the need and value of defining the scope of the HSS at the outset, with their remit currently being too broad and ill-defined. Lastly, the value of the HS outputs to key stakeholders remains to be seen, with limited evidence making it difficult to assess the impact of a technology and uncertainties around whether manufacturers would launch these innovative, high-cost technologies in the relatively small Singapore market.
Results from the Premeeting Health Technology Reassessment Survey
Fourteen agencies responded to the HTR activity survey with the results summarized in Table 2. Six (42.9 percent) agencies reported having some experience with the reassessment of technology with a view to either its partial, or complete, removal (China-Shanghai, India, Malaysia, South Korea, Taipei, and Vietnam). Although Japan reported no experience with reassessment thus far, the new HTA system introduced in 2019 has an expectation that technologies will be reassessed in the future if new clinical evidence is published in the peer-reviewed literature, or importantly, if costs of the technology are higher than expected once the technology is in routine clinical practice. Most agencies reported that they would be interested in forming a regional information-sharing network around reassessment activities, which may operate in tandem with an HS network.
Table 2. Reassessment Survey Results from Payer Agencies
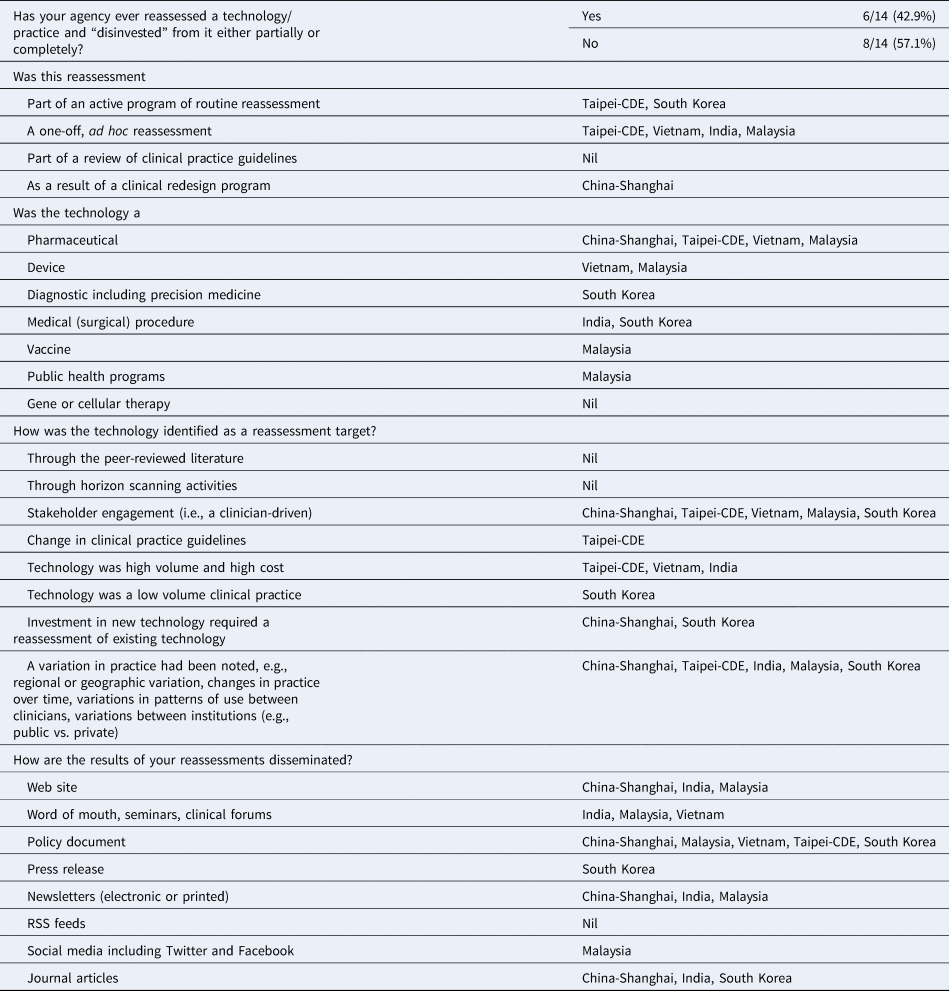
Of the six agencies with experience in HTR, Taipei and South Korea reported that their agencies had an active HTR program. Taipei's CDE routinely reassesses pharmaceuticals, whilst South Korea has conducted reassessments of diagnostics and medical procedures. Three countries (Vietnam, India, and Malaysia) had an experience of reassessment on an ad hoc basis, initiated either by clinician engagement or by noting a variation in clinical practice and China (Shanghai) conducted reassessment of a pharmaceutical as a result of a clinical redesign program. Malaysia has extensive experience in reassessment covering pharmaceuticals, devices, vaccines, and public health programs.
Most HTR was a result of clinician-driven identification of potential targets; however, it was interesting to note that five agencies identified reassessment targets due to a variation in practice. This practice may increase with the increasing use of data linkage and real-world data as discussed in previous HAPFs. Of note, three agencies conducted HTR of technologies that were identified as high volume and high cost.
Results from the HTR Case Study
From the case study, the South Korean HTA agency, NECA, is the most experienced agency in the region in terms of HTR. A key development of HTR in South Korea has been recognizing that a national registry for HTR should be created to investigate the influence of health technologies on health outcomes, as well as to analyze existing national health insurance claims data. In so doing, evidence to support the implementation of HTR of existing technologies could be generated (Reference Seo, Park and Lee16). NECA have developed an HTR model/methodology, the feasibility of which was trialed on two technologies: intestine capsule endoscopy and intradisc steroid injections. These pilot projects were used to develop the four steps of HTR: identification, prioritization, reassessment, and decision, similar to the steps described for HS. Within each step, several criteria with associated weighted values were developed to facilitate the practical implementation of HTR in South Korea (Reference Seo, Park and Lee16).
Discussion
One of the key issues raised during the meeting was that delineation between HS, HTR, and full HTA was unclear and caused confusion amongst delegates. Discussions concluded that all three are elements of HTA, using similar principles and methodologies that are applied at different time points in the life cycle of a health technology. It was agreed that it was possible to scan the current clinical landscape using HS methodologies to identify new, additional, or outmoded uses of clinical practices, which may result in HTR being undertaken. It was clear that an integrated approach to HS, HTA, and HTR is required.
Noting that different stakeholders may vary in their use of HS, it also seemed important to clarify the definition and purpose of HS, with the HTA Glossary definition representing the views of most HAPF members: “[HS is] The systematic identification of health technologies that are new, emerging or becoming obsolete and that have the potential to effect health, health services and/or society.” There was general agreement by members that the benefits of HS include the identification of incoming therapies and the rapid evaluation of their potential impact on a health system; the managed entry of technology into all aspects of the health system; and an informal opportunity for early dialogue between key stakeholders, especially industry and clinicians, in so doing, building trust. When discussing the advantages and disadvantages of HS for single versus a class of technologies or all technologies in a clinical pathway, it was agreed that although HS for all technologies in a clinical pathway is more effective than looking at individual technologies, this approach requires more resources and may not always be feasible, with the assessment of single technologies being faster, easier, and less resource-intensive. It was also acknowledged that there were distinct differences when HS was used to identify pharmaceuticals as compared to devices due to differences in development timelines, with HS for pharmaceuticals occurring much earlier in the development pipeline and almost acting in a gatekeeper role. Device development tends to be rapid and may be associated with learning curve considerations; therefore, HS for devices tends to be around monitoring the early uptake and diffusion of a new (or iteration) device.
From discussions during the HAPF and the results of the survey, it was clear that HTR is in its infancy in the region, with few agencies having HTR experience, mostly on an ad hoc basis. Attendees agreed that HTR rarely resulted in the complete removal of a service but usually lead to a restriction of a service to certain patients. Discussions stressed that HTR may also result in a widening of indications, with the removal of restrictions, the identification of a new use for an existing technology or increased prices, especially for those used in managed entry agreements. Delegates agreed that a structured rather than an ad hoc HTR approach should be encouraged and conducted whenever introducing new technologies in order to ensure the continued optimum use of existing technologies. Delegates acknowledged that HTR is difficult and challenging, especially around the lack of HTA capacity, data and clear processes, and requires early and extensive stakeholder engagement with policy makers, clinician, and patients.
It was acknowledged that collaboration with industry is challenging around all facets of HTA, be it HS, HTR, or full HTA for market entry. Attendees agreed that it is important to continue to build links and to foster open and transparent dialogue with industry.
From discussions, there was a clear appetite for a formal information-sharing HS and HTR network in Asia, similar to that of EuroScan. Although many challenges with setting up such a network were identified, especially the differences in priorities and health systems in the region, it was agreed that the potential benefits could outweigh these difficulties. As some members of the HAPF are existing members of EuroScan (South Korea, Malaysia with Singapore joining), there was some discussion as to whether EuroScan could accommodate more members from the region, or whether a new Asian network should be established.
Conclusions
With many countries in the Asia region developing UHC alongside increasing their HTA capacity and skill base, HS and HTR activity is in its infancy. Several countries in the region are, however, experienced in all facets of HTA, with expertise in HS and/or HTR. As such, there is an appetite for setting up a collaborative network in the region that can facilitate information sharing around HTA activities, and more specifically, HS and HTR methodologies, experiences, and assessments. This network may simply be an expansion of the global membership, or perhaps an Asian chapter, of EuroScan, or a standalone AsiaScan network. Until such a time a formal network can be established, HAPF members should work toward sharing information on an informal basis in addition to developing some rules of engagement, to foster relationships, and to build trust and transparency between industry and agencies.
Conflicts of Interest
Linda Mundy is the Scientific Secretary for the HTAi Asia Policy Forum and as such is paid for this role by HTAi. Rebecca Trowman was formerly the HTAi Secretariat for all HTAi Policy Forums and as such was paid for her role as the Asia Policy Forum Secretariat. Prof Kearney is the Chair of the HTAi Asia Policy Forum Organizing Committee and as such is paid for this role by HTAi.
Acknowledgments
The authors thank the members of the HTAi Asia Policy Forum, especially the members of the Policy Forum Organizing Committee and invited speakers who attended the 2019 meeting. This article is based on discussions at the HTAi 2019 Asia Policy Forum held 6–8 November in Hanoi, Vietnam.






