Case report
A term, 4-kg, male neonate, with no prenatal cardiac diagnosis, was transferred to our hospital for evaluation of multiple congenital cardiac anomalies. On admission, the patient was in stable condition, with slight tachypnoea, well saturated, with no extracardiac malformations. The initial echocardiogram revealed tricuspid atresia type IC, with normally related great arteries, non-restrictive ventricular septal defect, and no pulmonary stenosis, and a hypoplastic right ventricle, with a functionally and anatomically normal pulmonary valve (Figs 1–3). A bedside balloon atrial septostomy was performed because of the presence of a restrictive atrial septal defect. At 8 days of age, however, the patient developed symptoms consistent with progressive pulmonary over-circulation and had to be placed on mechanical ventilation. The repeat echocardiogram documented retrograde flow in the ascending aorta and aortic arch, which together with low diastolic pressure raised suspicion for the presence of an important additional cardiac anomaly. An aortogram revealed a 6-mm-diameter aortopulmonary window at the pulmonary artery bifurcation, extending into the origin of the right pulmonary artery (Fig 4). At 10 days of age, the patient underwent division of the aortopulmonary window with patch closure of both the aorta and the pulmonary artery with two separate autologous pericardial patches. Subsequently, the pulmonary trunk was transacted, and a 4-mm modified Blalock–Taussig shunt was placed. The postoperative course was uneventful, and the patient was discharged home in a stable condition.
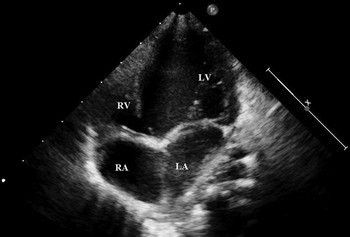
Figure 1 Transthoracal echocardiogram image, four-chamber view, demonstrating tricuspid atresia and the hypoplastic right ventricle: LA=left atrium; LV=left ventricle; RA=right atrium; RV=right ventricle.

Figure 2 Transthoracal echocardiogram image demonstrating unobstructed bulboventricular foramen – VSD. LV=left ventricle.

Figure 3 Transthoracal echocardiogram image demonstrating pulmonary outflow. LA=left atrium; PA=pulmonary artery; Ao=aorta.

Figure 4 Angiogram demonstrating the presence of an aortopulmonary window (APW).
The cardiac catheterisation, performed at 4 months of age, documented satisfactory haemodynamics for continuing with staged Fontan palliation. Subsequently, the patient underwent an uneventful superior cavopulmonary anastomosis with modified Blalock–Taussig shunt takedown.
Discussion
Tricuspid atresia occurs infrequently, with a prevalence of 0.3–3.7% in patients with CHD.Reference Fyler 1 Aortopulmonary window is rarer still, being found in no more than 0.2% of patients with CHD.Reference Jacobs, Quintessenza, Gaynor, Burke and Mavroudis 2 Coexistence of these anomalies in the same patient is extremely rare. To our knowledge, there have been only three reports of such cases in the literature.Reference Geva, Ott, Ludomirsky, Argyle and O’Laughlin 3 – Reference Hauck, da Cruz, Jaggers and Jone 5 Given the extreme rarity of this condition, it is clear that there are no established treatment guidelines. Geva et alReference Geva, Ott, Ludomirsky, Argyle and O’Laughlin 3 have described the case of type IB tricuspid atresia – normally related great arteries with pulmonary stenosis and hypoplasia – with an aortopulmonary window that was palliated with the closure of the aortopulmonary window using a fenestrated patch. The case of Peer et alReference Peer, Donofrio, Gaur and Sinha 4 reported similar morphology and underwent ligation of the aortopulmonary window, followed by placement of a 3.5-mm modified Blalock–Taussig shunt because of decreased pulmonary blood flow as a second operation, and, finally, creation of a superior cavopulmonary anastomosis as a third procedure. The patient described by Hauck et alReference Hauck, da Cruz, Jaggers and Jone 5 with type IIA tricuspid atresia – malposed great arteries with pulmonary atresia – and an aortopulmonary window was initially palliated by separation of the pulmonary artery from the aorta, atrial septectomy, and placement of a 3.5-mm modified Blalock–Taussig shunt as a first surgical procedure. This was followed by bidirectional cavopulmonary anastomosis as a second operation.
Patients with tricuspid atresia and normally related great vessels can have increased, balanced, or reduced pulmonary blood flow depending upon the size of the ventricular septal defect and/or the extent of pulmonary stenosis. In our case, pulmonary over-circulation was initially thought to be due to the presence of a non-restrictive ventricular septal defect and due to the absence of any pulmonary stenosis. Establishing an accurate diagnosis with echocardiography alone can be challenging, as also reported by Hauck et al.Reference Hauck, da Cruz, Jaggers and Jone 5 Physiological neonatal pulmonary hypertension initially causes the shunt at the level of the aortopulmonary window to be less prominent, thus reducing the chances of visualising the aortopulmonary window on the initial echocardiogram. With the physiological drop in pulmonary vascular resistance, the clinical signs and echocardiographic findings consistent with an aortopulmonary window became more obvious.
As far as we are aware, this is the first described case of tricuspid atresia and aortopulmonary window with pulmonary over-circulation due to not only the aortopulmonary window but also the lack of pulmonary stenosis at any level. All other reported cases had some degree of restriction of antegrade pulmonary blood flow due to a restrictive VSD, pulmonary stenosis, or pulmonary atresia.
Achieving a balanced pulmonary circulation in the treatment of these patients, as described by Peer et al,Reference Peer, Donofrio, Gaur and Sinha 4 can be quite challenging. At the time of repair of the aortopulmonary window, we decided to totally eliminate antegrade pulmonary blood flow and to leave the patient’s pulmonary blood flow shunt dependent. We felt that this approach was more predictable and reproducible than placing either an extraluminal or an intraluminal pulmonary artery band in the presence of the pulmonary artery patch angioplasty performed as part of the repair of the aortopulmonary window, although it was controversial. Partial closure of the aortopulmonary window with a fenestrated patch, as reported by Geva et al,Reference Geva, Ott, Ludomirsky, Argyle and O’Laughlin 3 is a less-predictable way to control pulmonary blood flow, seems more likely to produce pulmonary artery distortion over time, and will ultimately require definitive repair. Therefore, a full separation of the aorta from the pulmonary artery, with patch augmentation of both, in combination with placement of a systemic-to-pulmonary artery shunt seems to the best surgical approach for this condition.
In conclusion, tricuspid atresia type IC with aortopulmonary window is very rare. Its clinical signs and echocardiographic diagnosis can be less obvious in young neonates. We advocate full separation of the aorta from the pulmonary artery, with patch angioplasty of both, and placement of a systemic-to-pulmonary artery shunt as the initial surgical approach to ultimate Fontan palliation.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.







