Introduction
The parasite Trypanosoma cruzi, the aetiological agent of Chagas disease, is currently classified into seven discrete typing units (DTUs), TcI-TcVI and Tcbat (Zingales et al., Reference Zingales, Miles, Campbell, Tibayrenc, Macedo, Teixeira, Schijman, Llewellyn, Lages-Silva, Machado, Andrade and Sturm2012). In many endemic areas, mixed infections with different DTUs are common in patients (Rodrigues Dos Santos et al., Reference Rodrigues Dos Santos, Melo, de Castro, Hasslocher-Moreno, do Brasil, Silvestre de Sousa, Britto and Moreira2018; Bizai et al., Reference Bizai, Peralta, Simonetto, Olivera, Arias, Dalla Costa, Manattini, Sione, Fabbro and Diez2020) and reservoirs (Barros et al., Reference Barros, Xavier, Bilac, Lima, Dario and Jansen2017). The parasite genetic variability and the genetic background of the host could determine the clinical course of the infection (Messenger et al., Reference Messenger, Miles and Bern2015) and the treatment effectiveness (Cencig et al., Reference Cencig, Coltel, Truyens and Carlier2012; Díaz et al., Reference Díaz, Leal, Mantilla, Molina-Berríosa, López-Muñoz, Solari, Escobar and González Rugeles2015). The approved treatments for this infection consist of two compounds, benznidazole (BZ) and nifurtimox. These drugs have a variable efficacy regarding the stage of the infection (acute or chronic phase), patient's age, geographical area and T. cruzi strains; and they also can cause undesired side effects (Perez-Molina and Molina, Reference Perez-Molina and Molina2018; Ribeiro et al., Reference Ribeiro, Dias, Paiva, Hagström-Bex, Nitz, Pratesi and Hecht2020; Vela et al., Reference Vela, Coral-Almeida, Sereno, Costales, Barnabe´ and Brenière2021).
For these reasons, many efforts are being made in the search for new therapies. Combined treatments, mainly with BZ and other compounds, have been the most promising (Cunha et al., Reference Cunha, Torchelsen, Cunha, Oliveira, Reis, Fonseca, Vieira, Carneiro and Lana2019; Echeverría et al., Reference Echeverría, González, Hernandez, Díaz, Nieto, López-Romero, Rivera, Suárez, Ochoa, Rojas and Morillo2020), due to the synergistic mechanisms underlying combinatorial therapy (Zhang and Yan, Reference Zhang and Yan2019).
Clomipramine (CLO) is a tricyclic antidepressant that has been shown to competitively inhibit the enzyme trypanothione reductase, which provides the parasite with the ability to counteract the oxidizing environment generated by the cellular immune response of the host (Thomson et al., Reference Thomson, Denicola and Radi2003). CLO + BZ showed synergistic activity in vitro against the clinically relevant life stages of two strains of T. cruzi (the most susceptible forms being the intracellular amastigotes), with no toxicity upon mammalian cells; and the combination of both drugs, in vivo, significantly diminished the circulating parasites and the mortality rate of the infected animals (García et al., Reference García, Ponce, Sanmarco, Manzo, Jimenez-Kairuz and Aoki2016; Strauss et al., Reference Strauss, Rodrigues, Lo Presti, Bazán, Báez, Paglini-Oliva, Nakamura, Bustamante and Rivarola2018a).
It has been demonstrated that T. cruzi is capable of persisting in the host even after prolonged treatment (Sánchez-Valdéz et al., Reference Sánchez-Valdéz, Padilla, Wang, Orr and Tarleton2018). Additionally, it has been previously described that parasite bloodstream populations are different from those found in the tissues of the same host (Burgos et al., Reference Burgos, Diez, Vigliano, Bisio, Risso, Duffy, Cura, Brusses, Favaloro, Leguizamon, Lucero, Laguens, Levin, Favaloro and Schijman2010; Lo Presti et al., Reference Lo Presti, Esteves, Moya, Bazán, Strauss, Báez, Pizzi, Quispe Ricalde, Valladares, Rivarola and Paglini-Oliva2014; Strauss et al., Reference Strauss, Velázquez López, Moya, Bazán, Báez, Rivarola, Paglini-Oliva and Lo Presti2018b). To date, parasitological, serological and molecular methods have been used to evaluate treatment efficacy and verify cure (Blanchet et al., Reference Blanchet, Brenière, Schijman, Bisio, Simon, Véron, Mayence, Demar-Pierre, Djossou and Aznar2014; Moscatelli et al., Reference Moscatelli, Moroni, García Bournissen, González, Ballering, Schijman, Corral, Bisio, Freilij and Altcheh2019; Alonso-Padilla et al., Reference Alonso-Padilla, Abril, Alarcón de Noya, Almeida, Angheben, Araujo Jorge, Chatelain, Esteva, Gascón, Grijalva, Guhl, Hasslocher-Moreno, López, Luquetti, Noya, Pinazo, Ramsey, Ribeiro, Ruiz, Schijman, Sosa-Estani, Thomas, Torrico, Zrein and Picado2020; Sulleiro et al., Reference Sulleiro, Silgado, Serre-Delcor, Salvador, Tavares de Oliveira, Moure, Sao-Aviles, Oliveira, Treviño, Goterris, Sánchez-Montalvá, Pou, Molina and Pumarola2020). The persistence of the parasite after a specific treatment may be due to natural drug resistance of some clones present in the infecting parasite population. The aim of the present work was to evaluate the distribution of the different clones of the parasite prevailing after the treatment with a combination of BZ and CLO, in mice infected with a T. cruzi isolate (Casibla) which consists of a mixture of DTUs (Lo Presti et al., Reference Lo Presti, Esteves, Moya, Bazán, Strauss, Báez, Pizzi, Quispe Ricalde, Valladares, Rivarola and Paglini-Oliva2014; Strauss et al., Reference Strauss, Velázquez López, Moya, Bazán, Báez, Rivarola, Paglini-Oliva and Lo Presti2018b).
Materials and methods
T. cruzi isolate
The Casibla isolate was originally obtained from the umbilical cord blood of a congenitally infected newborn from an endemic area in Argentina. It consists of a mixture of TcII and TcVI DTUs (Lo Presti et al., Reference Lo Presti, Esteves, Moya, Bazán, Strauss, Báez, Pizzi, Quispe Ricalde, Valladares, Rivarola and Paglini-Oliva2014; Strauss et al., Reference Strauss, Velázquez López, Moya, Bazán, Báez, Rivarola, Paglini-Oliva and Lo Presti2018b). This isolate has been maintained in the laboratory by successive infections of new mice every 15 days.
In vitro activity of BZ and CLO against the Casibla isolate
Trypomastigotes from Casibla isolate (1 × 107 parasites/mL) were inoculated in DMEM (Gibco Invitrogen, pH 7.4, supplemented with 2 mm L-glutamine, 10% FBS, and 50 mg/L gentamicin) in 96-well plates in the presence or absence of increasing concentrations of CLO and BZ (BZ: 0.125; 0.25; 0.5; 1; 2; 4 and 6 μg/mL and CLO: 0.243; 0.487; 0.975; 1.95; 3.9; 7.8; 15.6 and 31 μg/mL) and the combination of the different concentrations of each drug. Parasites were incubated for 24 h at 37°C in a 5% CO2 atmosphere; then their viability was examined under light microscope (Olympus CX31) using the Pizzi-Brener method (an aliquot of 5 μL was immediately counted using an optical microscope, 50 fields were analysed). The potency and efficacy of each drug were measured (Moraes et al., Reference Moraes, Giardini, Kim, Franco, Araujo-Junior, Schenkman, Chatelain and Freitas-Junior2014) and the effect of the combination of BZ and CLO was evaluated through the combination index (CI) method [CI = (IC50BZ combined/IC50BZ alone) + (IC50CLO combined/IC50CLO alone); where the numerators are the concentrations of each drug that in combination are active against 50% of the cells, and the denominators are the concentrations that have this same effect for each drug alone]. CI values less than, equal to, and more than 1 indicate synergism, addition and antagonism, respectively (Chou and Talalay, Reference Chou and Talalay1984). The data were also graphically expressed as isobolograms.
Animals and experimental design
Adult male and female albino Swiss mice, weighing 25 ± 3 g, were inoculated, by intraperitoneal injection, with 50 trypomastigotes from the Casibla isolate. Mice were kept in controlled housing conditions (12 h light period, 23 ± 3°C, with food and water ad libitum).
The treatment scheme previously proposed (Strauss et al., Reference Strauss, Rodrigues, Lo Presti, Bazán, Báez, Paglini-Oliva, Nakamura, Bustamante and Rivarola2018a) was used; briefly, infected animals (INF) were divided into: infected and non-treated (INT); infected and treated with BZ: 100 mg/kg (BZ100) and 6.25 mg/kg (BZ6.25); infected and treated with CLO: 5 mg/ kg (CLO5) and 1.25 mg/kg (CLO1.25); and infected and treated with the co-administration of BZ6.25 + CLO1.25; n = 6 for each group (3 females and 3 males). The same scheme of treatment was used for non-infected animals (NI), n = 3 for each group. In all treated groups the drugs were administered orally, every day, for 30 days, using the appearance of parasites in blood as criteria for initiation of treatment in the infected mice (parasites began to be detected around the third and fourth day after infection and treatment began on the fifth day for all treated mice). The experimental procedures were carried out according to the procedures approved by the Institutional Committee for the Care and Use of Laboratory Animals from the Faculty of Medical Sciences, National University of Córdoba, Argentina (Tolosa de Talamoni et al., Reference Tolosa de Talamoni, Moya, Martini, López, Gallará and Ponzio2010).
Parasitaemia and survival
Parasitaemia was determined in a Neubauer haemocytometer using blood samples obtained from the tail of the mice once a week until day 42 post infection (p.i.). Survival of the different groups was monitored daily.
Histopathological studies
By day 42 p.i., all mice were sacrificed by decapitation, using Ketamine ClH (Ketalar®, Parke Davis, Warner Lambert Co, USA) anaesthesia (10 mg/kg).
Samples from liver, kidney and intestine were extracted from three mice of each group (from all the groups tested), in order to evaluate the structural alterations due to possible toxic effects of the drugs. The tissues were fixed in 10% buffered formalin (pH 7.0) and embedded in paraffin. Sections (5 μ m thick) were stained with haematoxylin−eosin (n = 108 samples) and analysed under a microscope (200× magnification). Ten different fields were analysed for each tissue section, and three different tissue sections were examined for each mouse. The samples were classified using a numerical scale: (0 = normal) without alterations; (25 = mild): mild inflammatory infiltrates, ⩽1 inflammatory focus; (50 = moderate) moderate inflammatory infiltration, ⩽2 inflammatory foci; (75 = severe) extensive inflammation, >3 inflammatory foci; and (100 = intense) extensive inflammation and necrosis. The mean value of this scale was graphed for each treatment group.
Real-time polymerase chain reaction assay
T. cruzi detection and quantification was performed by real-time PCR (qPCR) using blood (from jugular vein), cardiac and skeletal muscle samples from three mice of each group on day 42 p.i. DNA extraction was performed using the traditional phenol−chloroform method (Lachaud et al., Reference Lachaud, Chabbert, Dubessay, Reynes, Lamothe and Bastien2001). For blood and tissue DNA extraction, 500 μL and 30 mg of sample were used, respectively.
Parasite DNA detection was carried out by the amplification of a 188 bp fragment of T. cruzi satellite DNA (Virreira et al., Reference Virreira, Torrico, Truyens, Alonso-Vega, Solano, Carlier and Svoboda2003), and was performed as previously described (Strauss et al., Reference Strauss, Rodrigues, Lo Presti, Bazán, Báez, Paglini-Oliva, Nakamura, Bustamante and Rivarola2018a). The quantification was based on a standard curve built using DNA extracted from culture T. cruzi epimastigotes, Y strain (Wong and Medrano, Reference Wong and Medrano2005).
T. cruzi DTU genotyping
DTU genotyping was performed on positive T. cruzi DNA samples from mice infected and non-treated, and treated with BZ100, CLO5 and BZ6.25 + CLO1.25; n = 3 for each group. In order to confirm the DTUs present in the Casibla isolate, a multiplex real-time PCR algorithm (MTq-PCR) based on TaqMan probes targeted to the Spliced Leader Intergenic Region (SL-IR), 18S rDNA (18S), Cytochrome Oxidase II (COII), and 24Sα rDNA (24Sα) was used to detect TcI-TcVI DTUs (Fig. S1) (Cura et al., Reference Cura, Duffy, Lucero, Bisio, Péneau, Jimenez-Coello, Calabuig, Gimenez, Valencia Ayala, Kjos, Santalla, Mahaney, Cayo, Nagel, Barcán, Málaga Machaca, Acosta Viana, Brutus, Ocampo, Aznar, Cuba Cuba, Gürtler, Ramsey, Ribeiro, VandeBerg, Yadon, Osuna and Schijman2015). In order to confirm the presence or absence of TcII and TcVI in the samples tested, the A10 nuclear fragment was amplified in a hemi-nested endpoint polymerase chain reaction (PCR), allowing the discrimination between both DTUs based on amplicon size, 580 bp for TcII and 525 bp for TcVI (Burgos et al., Reference Burgos, Altcheh, Bisio, Duffy, Valadares, Seidenstein, Piccinali, Freitas, Levin, Macchi, Macedo, Freilij and Schijman2007).
Statistical analysis
Statistical analyses were performed using SigmaPlot v12.0 (Systat Software Inc., San Jose, CA) and InfoStat v2018 (FCA-UNC, Córdoba, Argentina). Parasitaemia was analysed by multivariate analysis (MANOVA/Hotelling's test). Survival data were analysed by Kaplan–Meier survival test. The parasite load was compared using analysis of variance and multiple comparisons by the Fisher test. The histological alterations were analysed using Kruskal–Wallis test and pair-wise comparisons. P < 0.05 was considered statistically significant.
Results
Bz and CLO in vitro activity
When administered separately upon Casibla trypomastigotes, BZ caused a mortality of 100% of the parasites at a lower concentration than CLO (Fig. S2A). The effect of the combination of BZ and CLO showed a CI = 0.96, which is consistent with an almost additive effect of the drugs, whereas the isobolographic analysis showed a moderate synergistic effect (Fig. S2B).
Parasite load
The evolution of bloodstream parasite loads in mice infected with T. cruzi Casibla isolate is shown in Fig. 1A. All treatment schemes determined significantly lower parasite levels than the INT group (Hotelling test: P < 0.05). The groups treated with BZ6.25 and BZ6.25 + CLO1.25 had similar parasite loads throughout the studied period. By day 42 p.i., the parasites were no longer detectable by light microscopy. However, qPCR results indicated the presence of parasite DNA in all the tissues analysed (blood, and skeletal and cardiac muscles) in all groups, including mice treated with BZ100, although this group had the lowest parasite DNA load (Fisher's test: P < 0.05) (Fig. 1B).

Fig. 1. A: Evolution of parasitaemia in albino Swiss mice inoculated with T. cruzi, Casibla isolate (n = 6, each group). All treatments began on the fifth day after the infection; the arrow indicates the start of the treatment. B: Parasite load in blood, and skeletal and cardiac muscles by day 42 post infection in Swiss mice infected and treated (n = 3, each group). *Parasite load in the BZ100 group was significantly lower when compared to all the other groups (P < 0.05). Bars indicate standard error. The insert represents the dynamic range of the real-time PCR. DNA from epimastigotes (Y strain) was amplified in triplicates; 10-fold serial dilutions were used. The limit of detection was 1.25 fg/μL T. cruzi genomic DNA. Regression coefficient (R 2): 0.998. Slope: −3.196. Efficiency: 108%. C: Kaplan–Meier survival curves for infected and treated mice. *Indicates significant differences between BZ100 and all the other groups tested (log-rank test–Chi square, P < 0.05); BZ6.25 and BZ6.25 + CLO1.25 showed the same survival percentage as well as CLO5 and CLO1.25.
Survival
By day 42 p.i., 66.67% of the BZ6.25 + CLO1.25 and BZ6.25 murine groups were alive, while the survival rate was 33.34% for INT mice and 50% for both groups treated with CLO, all of them being significantly lower than the survival of mice treated with BZ100 (100%) (Fig. 1C).
Histological studies
Figure 2 shows representative liver, kidney and intestine histological sections from the studied animals. No structural alterations were observed in the intestine of any of the tested groups. Diffuse inflammation was observed in the liver and kidney from the treated NI mice, with an intensity that varied according to the treatment dose, except for the NI CLO1.25 group that showed no structural alterations (Fig. 2J and K). In all cases, the INF animals showed greater alterations compared to the NI group under the same treatment (P < 0.05). In the NI animals, BZ6.25 produced mild alterations in the liver which were similar to the ones found when using CLO5 and BZ6.25 + CLO1.25; BZ6.25 also produced alterations in the kidneys. The livers and kidneys from the groups treated with BZ100 showed mild to moderate intensity alterations, similar to those from the INT group (Fig. 2F and I).

Fig. 2. Histological sections of liver, kidney and intestine. Haematoxylin−eosin stain 42 days post infection 200×. A, B and C: Liver, kidney and intestine, respectively, from a non-infected and non-treated mouse. D: Liver from a non-infected and CLO5-treated mouse. E: Liver from a non-infected and BZ6.25 + CLO1.25-treated mouse. F: Kidney from an infected and BZ100-treated mouse. G: Liver from an infected and BZ100-treated mouse. H: Liver from an infected and BZ6.25 + CLO1.25-treated mouse. I: Kidney from an infected and non-treated mouse. Circles show inflammatory infiltrates foci and the arrows show foci of infiltrates of moderate intensity. Bars correspond to 200 μ m. J and K: Structural alterations in liver and kidney in mice infected (INF) and non-infected (NI), respectively, after being treated (n = 3, each group). Data are shown as mean ± standard error and were analysed by non parametric Kruskal–Wallis test; groups with different letters show significant differences between them (P < 0.05).
Parasite genotyping
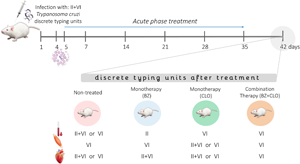
While some samples from the mice infected and non-treated presented both DTUs (TcII + TcVI), TcVI was the predominant DTU found in this group. This was also the case for samples from mice treated with CLO5 (Table 1). However, in mice treated with BZ100 and BZ6.25 + CLO1.25 only one DTU was found in each sample. The mice treated with BZ100 presented samples with TcII, TcVI or non-detectable results, whereas mice treated with BZ6.25 + CLO1.25 showed only TcVI in all the samples tested.
Table 1. T. cruzi discrete typing units detected by day 42 p.i. in blood, skeletal (SM) and cardiac muscles (CM) from three mice, after treatment during the acute phase of the infection

INT, infected non-treated; BZ, benznidazole; CLO, clomipramine; ND, non-detectable.
Discussion
Compared to the same treatment scheme reported previously using a different parasite strain (Strauss et al., Reference Strauss, Rodrigues, Lo Presti, Bazán, Báez, Paglini-Oliva, Nakamura, Bustamante and Rivarola2018a), in the present work, the drug combination did not produce the same effect. Previous in vitro studies, using Tulahuen and Y strains of T. cruzi, showed a synergistic effect of BZ + CLO upon trypomastigotes, with CIs of 0.38 and 0.60, respectively (García et al., Reference García, Ponce, Sanmarco, Manzo, Jimenez-Kairuz and Aoki2016; Strauss et al., Reference Strauss, Rodrigues, Lo Presti, Bazán, Báez, Paglini-Oliva, Nakamura, Bustamante and Rivarola2018a). In contrast, the in vitro study carried out with the Casibla isolate in this work showed an additive/moderate synergistic effect (CI = 0.96). Interestingly, both parasite strains mentioned above (Tulahuen and Y) are BZ-susceptible and BZ-partially resistant strains, respectively. As mentioned, Casibla isolate is composed of multiclonal parasite populations with different genetic composition (Lo Presti et al., Reference Lo Presti, Esteves, Moya, Bazán, Strauss, Báez, Pizzi, Quispe Ricalde, Valladares, Rivarola and Paglini-Oliva2014; Strauss et al., Reference Strauss, Velázquez López, Moya, Bazán, Báez, Rivarola, Paglini-Oliva and Lo Presti2018b) and these different populations probably have distinct susceptibility to drugs. Our in vitro results show that Casibla is a BZ-susceptible isolate, showing susceptibility to CLO as well.
Regarding in vivo results, the efficacy of treatment was evaluated by comparing parasitaemia, survival and parasite DNA presence in the tissues (cardiac and skeletal muscles). The combined treatment (BZ6.25 + CLO1.25) had similar results than BZ6.25. Although BZ100 was the most effective treatment, it failed to reach parasite clearance. In this sense, the possibility of parasite DNA persistence (instead of the parasite itself) could be ruled out since it has been shown that T. cruzi DNA detected in tissues through PCR is not due to long-term persistence of DNA but to the persistence of the parasite in the tissues (Zhang and Tarleton, Reference Zhang and Tarleton1999; Cummings and Tarleton, Reference Cummings and Tarleton2003). Thus, a positive PCR result evidences failure to clear the parasite and consequent ineffective treatment; a negative PCR however, does not guarantee the absence of parasites and cannot confirm parasite clearance, since false negatives can occur due to fluctuations in parasitaemia, the retreat of the parasite to tissues or organs and the intrinsic limit of detection of PCR and qPCR techniques (Ruiz-Lancheros et al., Reference Ruiz-Lancheros, Chatelain, Ndao, Altcheh and Freilij2019).
Organs such as intestine, liver and kidney play a key role in the metabolism and elimination of endogenous and exogenous compounds (Klaassen and Aleksunes, Reference Klaassen and Aleksunes2010; Perdomo et al., Reference Perdomo, Rigalli, Villanueva, Ruiz, Luquita, Echenique and Catania2013). In this work, these organs were analysed to evaluate possible toxic effects of the drugs. The structure of the intestine was preserved in all the samples from all groups of treatment. In most cases, the alterations in the liver and kidney from the infected animals were greater than those from the non-infected ones under the same treatment, indicating that most of the structural alterations observed were due to the presence of the parasite rather than due to drug toxicity. Nevertheless, diffuse inflammation, with mild-to-moderate intensity according to the treatment dose, was observed in the liver and kidney from the different groups of treatment in the NI animals, being the mice treated with BZ100 (INF and NI) and those from the INT group the ones with the greatest structural alterations. Remarkably, the mice treated with the combination of drugs (BZ6.25 + CLO1.25) only showed mild inflammation in the liver (NI group) or in the liver and the kidney (INF group), and these alterations can be attributed to BZ since they were similar to the ones found in the BZ6.25 groups, and CLO1.25 alone did not produce any structural alteration in NI animals. The biotransformation of BZ by the liver enzymatic complex has been associated with mitochondrial functional alterations, which may be potentially related to BZ toxicity (Rendon, Reference Rendon2014). When the parasite infects the host, it triggers an intense inflammatory process that can be histologically detected in many tissues and leads to the control of the infection (Fabrino et al., Reference Fabrino, Ribeiro, Teixeira, Melo, Chiarini-Garcia and Melo2010; Perez-Molina and Molina, Reference Perez-Molina and Molina2018). It has been found that both, the inflammatory process and BZ administration, produce severe morpho-functional hepatic injury (Novaes et al., Reference Novaes, Santos, Cupertino, Bastos, Oliveira, Carvalho, Neves, Oliveira and Talvani2015). In this work, it was observed that the lowest dose of BZ or its combination with CLO induced less histological damage in liver and kidney than the higher dose of the drug. In agreement with our results, other authors have indicated that a high dose of BZ is toxic for the host without significant contribution to the therapeutic outcome (Castro et al., Reference Castro, De Mecca and Bartel2006; Guedes et al., Reference Guedes, Silva, Gutierrez and Silva2011; Novaes et al., Reference Novaes, Santos, Cupertino, Bastos, Oliveira, Carvalho, Neves, Oliveira and Talvani2015). For this reason, new clinical trials are being conducted to find the optimal dose of BZ (Cafferata et al., Reference Cafferata, Toscani, Althabe, Belizán, Bergel, Berrueta, Capparelli, Ciganda, Danesi, Dumonteil, Gibbons, Gulayin, Herrera, Momper, Rossi, Shaffer, Schijman, Sosa-Estani, Stella, Klein and Buekens2020; Molina-Morant et al., Reference Molina-Morant, Fernández, Bosch-Nicolau, Sulleiro, Bangher, Salvador, Sanchez-Montalva, Ribeiro, de Paula, Eloi, Correa-Oliveira, Villar, Sosa-Estani and Molina2020).
The main purpose of T. cruzi genotyping should be directed towards its association with the clinical picture, the pathogenesis and the treatment of the disease (Guhl, Reference Guhl2013). Many studies have reported a lack of association between the different DTUs and differential susceptibility to BZ in vitro (Moreno et al., Reference Moreno, D'Avila, Silva, Galvão, Macedo, Chiari, Dias Gontijo and Zingales2010, Moraes et al., Reference Moraes, Giardini, Kim, Franco, Araujo-Junior, Schenkman, Chatelain and Freitas-Junior2014). For in vivo infections, many factors must be considered regarding T. cruzi susceptibility for available and experimental drugs, such as the virulence, histotropism, as well as drug characteristics (PK/PD properties) and the composition of the infecting parasite population (Revollo et al., Reference Revollo, Oury, Vela, Tibayrenc and Sereno2019). Regarding this later aspect, in the present work, in some samples from two groups of treatment (infected and non-treated, and treated with CLO5) both DTUs (TcII + TcVI) were detected, whereas TcVI was the predominant DTU in most other cases. The prevalence of TcVI over other DTUs was previously reported in blood and skeletal and cardiac muscle samples from mice infected with a mixture of DTUs (involving combinations of two isolates: TcIII + TcVI and TcV + TcVI, and three isolates: TcII + TcV + TcVI) during the acute phase of the disease (Ragone et al., Reference Ragone, Pérez Brandán, Monje Rumi, Tomasini, Lauthier, Cimino, Uncos, Ramos, Alberti, D'Amato, Basombrío and Diosque2015; Strauss et al., Reference Strauss, Velázquez López, Moya, Bazán, Báez, Rivarola, Paglini-Oliva and Lo Presti2018b). The detection of one DTU over others could be due to different factors, such as differential ability to escape the host immune system or the clonal histotropism of T. cruzi (Macedo et al., Reference Macedo, Oliveira and Pena2002). Intraspecific competition could also occur, as has been described for other parasitic infections (Pena et al., Reference Pena, Eger, Nogueira, Heck, Menin, Báfica and Steindel2011; Sales-Campos et al., Reference Sales-Campos, Kappel, Andrade, Lima, Mattos, de Castilho, Correia, Giraldo and Lages-Silva2014; Abkallo et al., Reference Abkallo, Tangena, Tang, Kobayashi, Inoue, Zoungrana, Colegrave and Culleton2015; Zhang and Buckling, Reference Zhang and Buckling2016). Another aspect to highlight for the BZ100 mice, is that this group presented the lowest parasite loads (in blood and tissues) for both DTUs and, in consequence, the variation observed in the parasite distribution, in this group at least, could be due to the parasite load levels that decreased below the limit of detection of the genotyping method used in this work.
Additionally, Resende et al. (Reference Resende, Oliveira, Guañabens, Repolês, Santana, Hiraiwa, Pena, Franco, Macedo, Tahara, Fragoso, Andrade and Machado2020) observed that different DTUs exhibit different rates of replication. In fact, Y strain (TcII) exhibited an increased rate of replication cycle when compared to CL Brener (TcVI), which is in line with their virulence in mice (Medeiros et al., Reference Medeiros, Araújo-Jorge, Batista, Da Silva and De Souza2010). Moreover, Sánchez-Valdéz et al. (Reference Sánchez-Valdéz, Padilla, Wang, Orr and Tarleton2018) demonstrated that T. cruzi enters a dormant state, as they observed that some amastigotes interrupted their cellular replication during an in vitro infection. They also found that different strains exhibited different degree of dormancy: CL Brener produced the highest percentages of dormant amastigotes, showing a negative correlation between dormancy and infectivity in T. cruzi, whereas Y strain showed lower degree of dormancy (Resende et al., Reference Resende, Oliveira, Guañabens, Repolês, Santana, Hiraiwa, Pena, Franco, Macedo, Tahara, Fragoso, Andrade and Machado2020). These features could also play a role in the differential distribution of TcII and TcVI DTUs in our infected mice. Furthermore, dormancy has been associated with drug resistance. Indeed, dormant parasites were resistant to doses of BZ 50-fold higher than the regular IC50 dose and recovered growth after 30 days post-infection (Sánchez-Valdéz et al., Reference Sánchez-Valdéz, Padilla, Wang, Orr and Tarleton2018). Interestingly, in our results, TcVI parasites were the most resistant to the treatments: the groups of mice treated with either drug alone presented both DTUs after the treatment, similar to the non-treated animals. In the BZ6.25 + CLO1.25 group, on the other hand, only one DTU was found after the treatment (TcVI). TcII clones present in Casibla isolate would then appear to be more susceptible to the combination of these compounds than TcVI ones, since they were found in the monotherapy-treated groups but were not detected when the combination therapy was applied, which supports the use of combined therapies for the treatment of this infection.
TcII clones however, could be present in other tissues that were not analysed in this study. Organs like the liver and the spleen are usually highly parasitized, and the T. cruzi clones present in these organs have been found to be different (even from a different DTU) to those present in the bloodstream of the same host (Burgos et al., Reference Burgos, Diez, Vigliano, Bisio, Risso, Duffy, Cura, Brusses, Favaloro, Leguizamon, Lucero, Laguens, Levin, Favaloro and Schijman2010; Lo Presti et al., Reference Lo Presti, Esteves, Moya, Bazán, Strauss, Báez, Pizzi, Quispe Ricalde, Valladares, Rivarola and Paglini-Oliva2014). Differential DTU distribution has also been found with respect to placental tropism in chronically infected mice (Solana et al., Reference Solana, Celentano, Tekiel, Jones and González Cappa2002; Juiz et al., Reference Juiz, Solana, Acevedo, Benatar, Ramirez, da Costa, Macedo, Longhi and Schijman2017). All this is in line with the histotropic-clonal model of T. cruzi infection proposed by Macedo et al. (Reference Macedo, Oliveira and Pena2002). Furthermore, different tissue distribution of parasite clones that may belong to the same or to a different DTU has also been found depending on the genetic background of the host mouse strain (Freitas et al., Reference Freitas, Andrade, Pires, Lima, Chiari, Santos, Soares, Machado, Franco, Pena and Macedo2009). In order to shed more light on the role of host and parasite genetic diversities and their interactions, with respect to treatment outcome, further studies with a greater number of animal models and tissues, as well as isolates from different DTUs, will be necessary. Even though the number of mice studied here was low, we believe that these results highlight the role of the genetic variability of the parasite within a given isolate in the response to a treatment and its possible failure. In this sense, the probable susceptibility/resistance of some of the parasites present in mixed infections, which are common in endemic areas, is a factor to consider in the response and the effectiveness of the treatments.
Conclusion
Animal models infected with T. cruzi isolates represent a more realistic picture of what is happening in endemic areas, but also reveal a complex scenario for the treatment of the infection and the evaluation of the efficacy of new therapies. For this reason, the combination of drugs with different mechanisms of action is a valid strategy to attempt to eliminate different infecting parasite populations (mixed infections), allowing the use of lower doses and reducing the undesirable side effects of drugs. Finally, the identification of DTUs has proved to be a valuable tool for monitoring the evolution of experimental infections and treatment outcomes in drug efficacy assessment studies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001281.
Acknowledgments
The authors wish to thank Clarisa Lagares for her assistance in the animal care, and the assistance of Universidad Nacional de Córdoba and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), who provided the facilities used in this investigation.
Author contributions
PAP, AGS and HWR conceived and designed the study. MS, JCR, DVL, ALB and PCB conducted data gathering. MS and MSL performed statistical analyses and wrote the article. All authors revised and approved the final version of the manuscript.
Financial support
This work was partially funded by PICT 2014-1188 (FONCyT, Argentina) to AGS and by Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba, Argentina (2012–2013 and 2014–2015) granted to HWR.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
The experimental procedures described were carried out according to the Guide for the Care and Use of Laboratory Animals from the Institutional Committee for the Care and Use of Laboratory Animals, Faculty of Medical Sciences, National University of Córdoba, Argentina (Tolosa de Talamoni et al., Reference Tolosa de Talamoni, Moya, Martini, López, Gallará and Ponzio2010).