INTRODUCTION
Parasites by definition negatively impact host fitness, potentially affecting a wide range of fitness components. For instance, infections can induce physiological stress in the host (Williams et al. Reference Williams, Poulin and Sinclair2004), reduce host reproductive success (Bollache et al. Reference Bollache, Gambade and Cézilly2001; Fredensborg and Poulin, Reference Fredensborg and Poulin2006), impair the host's anti-predator responses (Moore, Reference Moore2002; Babirat et al. Reference Babirat, Mouritsen and Poulin2004; Poulin, Reference Poulin2010), or increase its chances of mortality directly (Rousset et al. Reference Rousset, Thomas, De Meeus and Renaud1996). These individual-level effects often translate into population-level impacts, with parasites widely recognized as important determinants of host population dynamics (Smith et al. Reference Smith, Acevedo-Whitehouse and Pedersen2009; Tompkins et al. Reference Tompkins, Dunn, Smith and Telfer2011). However, while host individuals are usually infected by multiple parasite species, studies of host-parasite interactions have until recently generally focused on single-host/single-parasite systems (see tables in Tompkins and Begon, Reference Tompkins and Begon1999; Moore, Reference Moore2002). This approach, in turn, has led to the often unstated assumption that effects of parasites on host individuals and populations are generally additive (i.e. they do not interact), with this assumption forming the basis of much of current parasitological and epidemiological thinking (e.g. Hudson et al. Reference Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2002; Moore, Reference Moore2002; Keeling and Rohani, Reference Keeling and Rohani2007).
Multiple authors have recently highlighted that, due to often high degrees of concomitant infections, this general assumption of additive effects may commonly not hold. Rather, such sharing of the same ‘environment’ could potentially influence how parasites affect their hosts, through either competition or facilitation between parasites (Thomas et al. Reference Thomas, Renaud and Poulin1998; Poulin et al. Reference Poulin, Nichol and Latham2003; Lello et al. Reference Lello, Boag, Fenton, Stevenson and Hudson2004; Behnke et al. Reference Behnke, Eira, Rogan, Gilbert, Torres, Miquel and Lewis2009), or alteration of their host interactions by different stages of the same species (Sparkes et al. Reference Sparkes, Wright, Renwick, Weil, Talkington and Milhalyov2004) or by other parasite species present (Thomas et al. Reference Thomas, Fauchier and Lafferty2002; Beldomenico and Begon, Reference Beldomenico and Begon2010). Indeed, with a large body of literature in the last decade exploring the implications of parasite community interactions on parasite fitness and distributions (see summaries in Poulin, Reference Poulin2001a; Tompkins et al. Reference Tompkins, Dunn, Smith and Telfer2011), such implications for host fitness also seem likely. Hence, with the vast majority of studies to date considering only one focal parasite species out of the array of parasite species infecting any given host species, our current models of parasite impact on hosts, based on this literature, may not be realistic.
Thus there is a clear gap in our understanding of the relative and/or interactive effects of different parasite species on the same host population, mediated by each parasite's influence on each other, and on one or more facets of individual host fitness. This can only be remedied by broadening the focus of studies to include all parasites infecting a given host population, with concurrent assessments of both the frequency and implications of co-infection allowing the validity of the current model of additive parasite effects to be tested. Here we examine the effects of all the macroparasite species occurring in a population of the freshwater amphipod Paracalliope fluviatilis (Eusiridae), on several components of individual host fitness. Understanding such interactions in aquatic invertebrates is important since they commonly act as intermediate hosts for parasites of vertebrates (Combes, Reference Combes2001), are often highly sensitive to environmental stress (Ebert, Reference Ebert2011), and with their parasites often form key components of food webs (Lafferty et al. Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, McLaughlin, Mordecai, Pascual, Poulin and Thieltges2008).
Paracalliope fluviatilis is a widespread and abundant species in New Zealand freshwater systems (Sutherland et al. Reference Sutherland, Hogg and Waas2007). It serves as intermediate host for 4 species of helminth parasite. The trematode Coitocaecum parvum is acquired by amphipods through penetration of the cuticle by infective cercariae; most parasites then encyst as metacercariae within the body cavity of the amphipod, awaiting ingestion by fish definitive hosts (Macfarlane, Reference Macfarlane1939), with a few metacercariae growing much larger than others and developing precociously into egg-producing adult worms (Lagrue and Poulin, Reference Lagrue and Poulin2007). A second trematode, an un-described species of Microphallus, also infects amphipods by penetration and subsequently encysts within their body cavity (Lefebvre et al. Reference Lefebvre, Fredensborg, Armstrong, Hansen and Poulin2005); this parasite completes its life cycle when ingested by an avian definitive host. The acanthocephalan Acanthocephalus galaxii infects an amphipod when its egg is accidentally ingested; it then grows within the amphipod's body cavity, awaiting ingestion by fish definitive hosts (Hine, Reference Hine1977). Finally, a newly-found and un-described larval cestode of the order Cyclophyllidea also infects the amphipod; its definitive host is unknown, but very likely to be a bird (unpublished data). Since all these larval helminths are likely trophically transmitted to their next host, any parasite-induced alterations in amphipod behaviour that would increase predation risk could be of benefit to their fitness (Poulin, Reference Poulin2010). Also, since all 4 parasites reach relatively large sizes within their amphipod intermediate hosts (unpublished data), the energetic demands that they impose on their host very likely translate into lower host reproduction and survival. The specific objectives of this study were thus to quantify the frequency of co-infection, and the separate and combined effect of these 4 helminth species on aspects of host behaviour, reproduction and survival.
MATERIALS AND METHODS
Sample collection
Naturally infected amphipods were collected from Swin Burn Stream (55°59′N–22°98′E), South Island, New Zealand in September 2010 (Austral spring). Amphipods were caught using a dip-net and transported (in water from the stream) to the laboratory within 3 h post-capture, where they were maintained at room temperature (14°C±1°C) in a 20 litre freshwater tank with aquatic plants for food, supplementary aeration, and controlled photo-period (12 h dark:12 h light).
Parasite burdens and relative survival rates
Within 24 h of capture, 670 amphipods in one subset of those collected were individually separated into the cells of tissue culture micro-test plates, with 400 μl of freshwater per cell, and maintained under previous temperature and photo-period conditions but without food or supplementary aeration. Only single amphipods were used in this subset. Plates were checked daily under a dissecting microscope, with days survived recorded for dead amphipods, which were preserved in 70% ethanol. The head length and sex of each amphipod was assessed prior to identifying and counting all macroparasites present. Because egg-producing metacercariae of C. parvum were very rare in this population, no distinction was made between them and non-egg-producing individuals when parasites were counted.
Mating success
A second subset of 488 pairs (976 amphipods) was identified within 24 h of capture. These amphipods consisted of a male clasping a female in pre-copula, with all pairs formed under natural conditions; they were also preserved in 70% ethanol and processed as detailed above.
Behavioural trials
Using trials adapted from Cézilly et al. (Reference Cézilly, Gregoire and Bertin2000) and Poulin (Reference Poulin2001b), relative measures of amphipod phototaxis and activity were recorded from 990 individual specimens (717 female and 273 male) in a third subset. Light avoidance and activity patterns are widely recognized as being important factors in the anti-predatory response of crustaceans and parasites are known to alter the behaviour of infected individuals (Bethel and Holmes, Reference Bethel and Holmes1973, Reference Bethel and Holmes1977; Moore, Reference Moore2002). Each amphipod was placed in a 17 ml glass tube (1·5 cm diameter by 12·5 cm length) filled with freshwater and positioned on its side 20 cm beneath a 40 watt lamp.
To measure relative photo-taxis, light and dark zones were made available to each amphipod by covering half of each glass tube with an aluminium tube. After being acclimatized in position for 5 min, location was scored for each amphipod (1 for light and 0 for dark) at 30-sec intervals over a further 5 min. Total scores, summed across the photo-taxis trials, hence ranged from 0 for extremely photophobic amphipods to 10 for extremely photophilic amphipods. To measure relative activity, amphipods were acclimatized for 5 min after the light trial, with the aluminium tube removed and each glass tube placed above a grid dividing it into 4 equal zones. Location was then again scored for each amphipod (zone 1, 2, 3 or 4) at 30-sec intervals. Change in position was calculated between each adjacent pair of location records, and summed across the activity trial to result in a total score of 0 for an extremely non-active amphipod to 27 for an extremely active amphipod.
Amphipods were stored in 70% ethanol at the termination of activity trials, and processed as detailed above with the addition of egg presence or absence in the marsupium of females (gravid or non-gravid – a measure of female maturity) also being recorded. Because egg-producing metacercariae of C. parvum or young inmature A. galaxii (acanthellae) were very rare in this subset, no distinction was made between them and non-egg-producing C. parvum or mature cystacanths of A. galaxii when parasites were counted.
Statistics
All tests were conducted in Statistica version 9 (Statsoft, Tulsa), using a significance level of 95%. The influence of the number of parasites per amphipod on amphipod survival, mating status, female maturity, and behaviour was analysed using General Linear Models (GLMs), which included the following predictor variables; amphipod head length, sex, and infection (numbers per host) by Microphallus sp., A. galaxii, C. parvum, and Cyclophyllidea sp. and their second order interactions where significant (excluding Cyclophyllidea sp. because of their low abundance).
Since all of the amphipods in the ‘behavioural trials’ subset were unpaired and freshly killed, they were used as the control group against which to evaluate infection differences with paired individuals. Normally distributed error structures were specified for the tests of the survival and behavioural measures, while binomial error structures (i.e. logistic regressions) were specified when testing for infection differences between males versus females, gravid females versus non-gravid females, and paired versus unpaired amphipods. Kruskal-Wallis Mean Rank was used as a post-hoc test to evaluate differences in behavioural scores of single-infection amphipods and mixed-infection amphipods.
Spearman's Rank Correlation was used to test for relationships between the intensities of different parasite species, and between measures of both photo-taxis and activity across infected and uninfected amphipods.
RESULTS
A total of 2636 amphipods was used in this study. Prevalence and mean intensity of infection were 42·5% and 1·5 for Microphallus sp., 7·4% and 1·2 for A. galaxii, 3·3% and 1·0 for C. parvum, and 1·3% and 2·1 for Cyclophyllidea sp. The highest intensity of infection was 14 for Microphallus sp., 4 for A. galaxii, 2 for C. parvum, and 8 for Cyclophyllidea sp. Amphipod sex ratio was 1·6 females per male (female-biased sex ratios are common for amphipods; e.g. Kruschwitz, Reference Kruschwitz1978; Rauque, Reference Rauque2007). The intensities of different parasite species were not correlated among each other across the entire sample of amphipods (P>0·25). The frequency distribution of co-infected amphipods was: 101 A. galaxii+ Microphallus sp., 42 Microphallus sp.+C. parvum, 13 Microphallus sp.+Cyclophyllidea sp., 9 Microphallus sp.+A. galaxii+C. parvum, 1 Microphallus sp.+A. galaxii+Cyclophyllidea sp., and 3 A. galaxii+C. parvum.
Relative survival and mating success
In an analysis of the subset of amphipods used in the relative survival trial, 2 significant predictors of amphipod survival were identified (Table 1), with survival increasing with amphipod head length and decreasing with Microphallus sp. infection (Fig. 1). No second-order interactions between parasite species were significant.

Fig. 1. The relationships between amphipod survival and (A) mean intensity of Microphallus sp. and (B) mean amphipod head length (mm). To compare groups with similar numbers of amphipods, scales of days of survival are unequal. Sample sizes are given at the base of bars.
Table 1. General Linear Models for relative amphipod survival and phototaxis with respect to parasite infection, controlling for the effects of sex and head length of amphipods
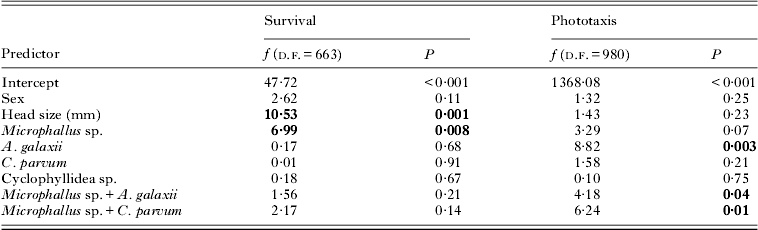
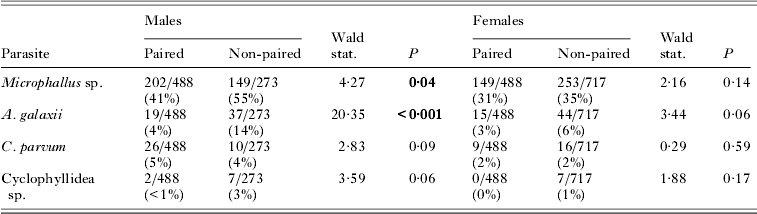
When the paired versus unpaired status of amphipods was analysed with respect to parasite infection (in an analysis including pairs and the individual amphipods from the behavioural trials), infection by any of the 4 parasite species was associated with a decreased probability of being paired for both male and female amphipods, although this effect was only significant for male amphipods with respect to Microphallus sp. and A. galaxii (Table 2). Again, no second-order interactions between parasite species were significant.
Table 2. Parasite infection in paired and unpaired male and female amphipods

Behaviour and female maturity
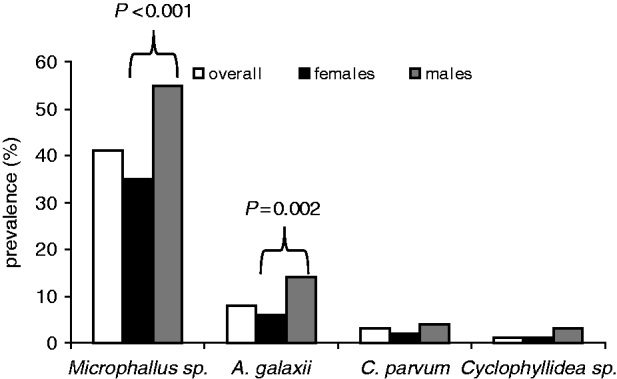
In an analysis of the subset of amphipods used in the behavioural trials, males were proportionally more infected by all 4 parasite species than were females (Fig. 2), with these differences being significant for both Microphallus sp. (Wald stat.=29·6; P<0·001) and A. galaxii (Wald stat.=9·2; P=0·002). No second-order interactions between parasite species were significant.
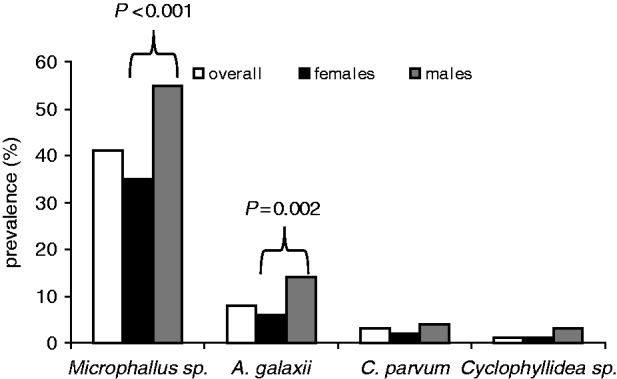
Fig. 2. Prevalence of parasites by sex and in the overall sample of amphipods in the behavioural group.
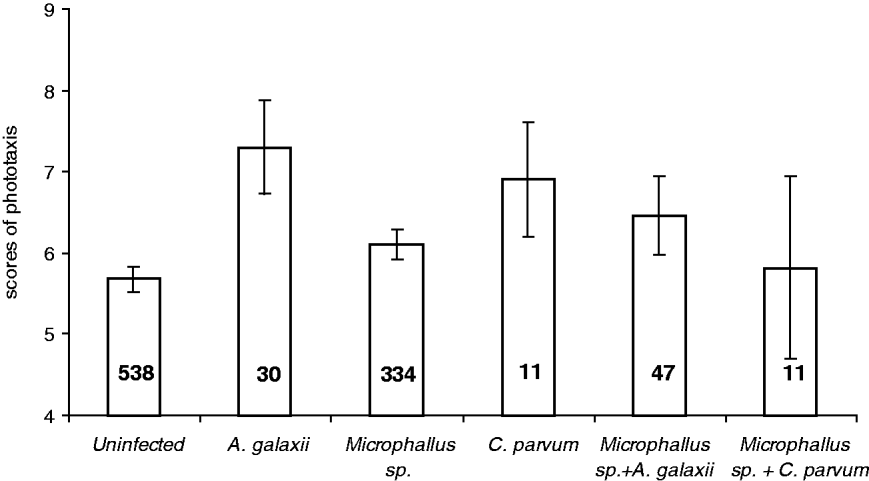
In the phototaxis trials, a significant positive relationship between relative photophilia (higher scores) and infection by A. galaxii was observed (Table 1; Fig. 3). In addition, significant second-order interactions between Microphallus sp. and both A. galaxii and C. parvum were also related to levels of photophilia (Table 1; Fig. 3). Post-hoc tests showed that while the behaviour of amphipods infected with A. galaxii alone was significantly different from uninfected amphipods (H=10·4; P=0·02), the behaviour of those infected with either Microphallus sp. alone or Microphallus sp. and A. galaxii was not. Post-hoc tests for the interaction between Microphallus sp. and C. parvum were not conducted due to an insufficient sample of amphipods with such co-infections.
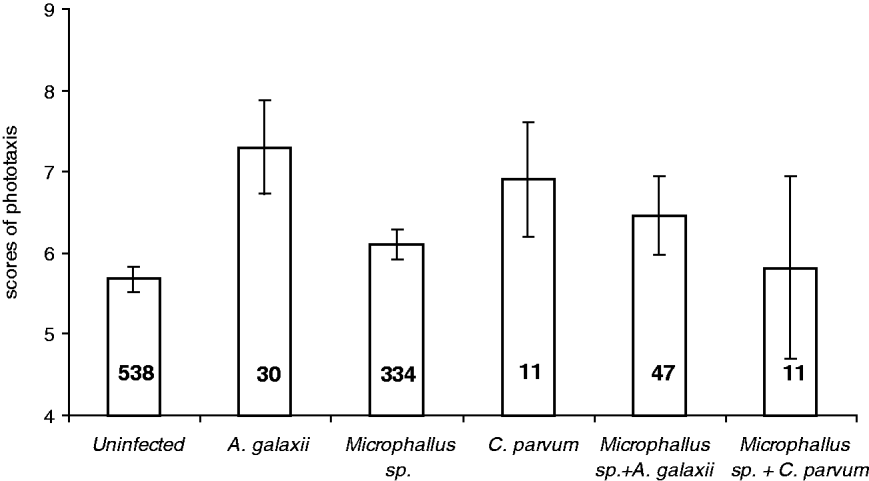
Fig. 3. Mean scores of phototaxis±s.e. of infected and uninfected amphipods. Sample sizes are given below bars.
In the activity trials, relative scores were only significantly related to amphipod sex, with males being more active than females (F=40·004; P<0·001). There were no significant correlations between light and activity scores in either uninfected amphipods or amphipods infected by the different species (P>0·07).
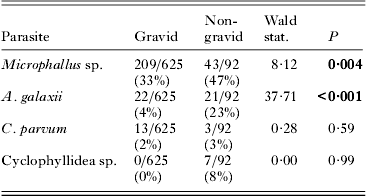
Of the 717 females in the behavioural group, 625 were gravid and 92 were non-gravid. Female amphipods infected by any of the 4 parasite species tended to be non-gravid, with these relationships being significant with respect to infection by Microphallus sp. and A. galaxii (Table 3). No second-order interactions between parasite species were significant.
Table 3. Parasite infection of gravid and non-gravid female amphipods in the behavioural group
DISCUSSION
Here we quantified the separate and combined effects of 4 helminth species on the behaviour, reproduction and survival of their amphipod host. Although our study was not based on experimental infections (but rather made use of existing natural variation in infection among host individuals), and hence cannot be used to confirm causality in any of the patterns observed, when combined with a background mechanistic understanding of host/parasite interactions this is a powerful approach for identifying the potential for different types of effects. That several of our more general findings agree with confirmed relationships well documented in the parasitological literature, such as greater infection of male versus female hosts potentially linked to greater activity levels of males increasing their infection risk (Lefebvre et al. Reference Lefebvre, Fredensborg, Armstrong, Hansen and Poulin2005; Rauque and Semenas, Reference Rauque and Semenas2007), testifies to the utility of this approach.
In addition to the significant relationships between specific parasites and measures of amphipod survival, mating success and behaviour that were observed (as one expects to find for organisms designated as ‘parasites’), interactions between parasite species with respect to amphipod photophilia were also significant. Specifically, the presence of Microphallus sp. infection removed the host behavioural changes (increased photophilia) associated with infection by A. galaxii and (non-significantly) C. parvum. This indicates that host/parasite studies which do not explicitly look for interactions among species in the parasite community, in terms of the combined effects of parasites on their hosts, may miss subtle interactive effects such as these. This also reinforces that the effects of different parasite species on their hosts are not always additive.
By conducting a holistic assessment of the results from the different trials conducted here, we can hypothesize potential mechanisms by which the different parasites involved affect their hosts, with this being the first step to understanding when and how the different parasite species can interact in their host effects. Microphallus sp. had a clear negative influence on host survival, most likely as a direct consequence of the high levels of infection intensity reached by this parasite (up to 14 parasites in 1 amphipod observed here). Microphallus sp. infection was also related to decreased probabilities of both male amphipods being paired with females, and female amphipods being gravid. In amphipod reproduction, males compete for access to females, with both pre-copulatory mate guarding (Plaistow et al. Reference Plaistow, Bollache and Cézilly2003; Cothran, Reference Cothran2004; Sutherland et al. Reference Sutherland, Hogg and Waas2007) and actual mating being energetically expensive. After mate selection, females have to expend a great quantity of energy through vitellogenesis and embryonic development (Bollache et al. Reference Bollache, Rigaud and Cézilly2002), processes in which reduction of stored carotenoids probably related to parasitism appears to be one mechanism reducing female fecundity (Rauque and Semenas, Reference Rauque and Semenas2009).
Impacts on host survival were not evident for A. galaxii, C. parvum or Cyclophyllidea sp. However, as for Microphallus sp., infection by all 3 species was related (significantly for A. galaxii) to decreased probabilities of both male amphipods being paired with females, and female amphipods being gravid, suggesting some energetic cost of infection. In addition to these considerations, however, a significant positive relationship between relative photophilia (higher scores) and infection by A. galaxii was observed. Photophilia scores of a similar high magnitude were also observed for amphipods infected by C. parvum, although they were not significantly higher due to the small sample size of C. parvum-infected amphipods. Although such patterns could be generated by more photophilic amphipods being at higher risk of infection, this is unlikely since they generally coincide with the parasite stage that is infectious to the final host, and acanthocephalans at least are proven manipulators of intermediate host behaviour (Moore, Reference Moore2002).
Considering our assessment of the parasite-host interactions of the 4 helminth species detailed above, the most parsimonious explanation for the interactions between parasite species observed in this study is that the potentially manipulative effects of both A. galaxii and C. parvum on host photophilia are impaired by co-infection with Microphallus sp. Specifically, any subtle effects of A. galaxii and C. parvum on their hosts are likely overwhelmed by the greater general effect Microphallus sp. has on the shared hosts, including impacts on survival, and reduced probabilities of infected hosts either being paired or gravid. Such interactions have great implications for the conditions under which putative parasite manipulations of host behaviour evolved, and the persistence of such parasites in the face of other common parasite species whose effects may hinder their attempts at manipulation to increase trophic transmission to the next host. However, to assess whether their influence on host effects is potentially as important, one must also take frequency of co-infection into account. In the current study, only 6% of amphipods had the co-infections documented to interact in their host effects (e.g. either A. galaxii and Microphallus sp., or C. parvum and Microphallus sp.). In contrast to the implications for parasite fitness, this low prevalence of double infections indicates that such interactions likely do not matter above the host individual scale in the current study. Meta-analyses are now needed to investigate whether this is a general pattern, and hence whether our models and interpretations of population and higher scale host effects based on single parasite species studies are generally valid.
Regardless of their importance at greater scales, the form of the interactions documented here is in contrast to the few previous studies also documenting such effects of co-infecting parasite species on their hosts. Here the presence of a parasite species with strong effects on host fitness appeared to disrupt more subtle, potentially manipulative, influences of others. In contrast, Cézilly et al. (Reference Cézilly, Gregoire and Bertin2000) observed that Gammarus pulex amphipods hosting mixed infections of the parasites Pomphorhynchus laevis and Polymorphus minutus showed values of vertical distribution in the water column intermediate to those of amphipods with single infections of each parasite species (Cézilly et al. Reference Cézilly, Gregoire and Bertin2000). Again in contrast, Graham et al. (Reference Graham, Lamb, Read and Allen2005) showed that mice co-infected with malaria and the helminth Litomosoides sigmodontis suffer greater reductions in body mass and red blood cell density than mice only infected with malaria, while data collected by Jolles et al. (Reference Jolles, Ezenwa, Etienne, Turner and Olff2008) strongly suggest that infection with gastrointestinal worms is associated with poor body condition in buffalo Syncerus caffer infected with bovine tuberculosis (i.e. an interaction between parasites that is deleterious to host fitness) due to trade-offs between immune responses. It may be that generic mechanistic rules could be constructed to successfully predict the directions of interactions among parasites of different groups. For example, one potentially constructive hypothesis to explore is that parasites with different final hosts would be more likely to have opposite ‘interests’ than parasites sharing a common final host (Lafferty, Reference Lafferty1999). However, the number and diversity of host/parasite systems in which such mechanisms have been explored is currently insufficient to attempt to do so.
ACKNOWLEDGMENTS
Thanks are given to the Evolutionary and Ecological Parasitology Research Group of the University of Otago, to Bronwen Presswell for taxonomic identification of cestodes, and to Isabel Blasco-Costa and Cynthia Winkworth for assistance with amphipod collection. The comments of two anonymous referees greatly improved the manuscript.
FINANCIAL SUPPORT
This work was supported by The Royal Society of New Zealand ISAT Linkage Fund.