Introduction
Pectinids of tribe Chlamydini Teppner, Reference Teppner and Diener1922 were among the most abundant bivalves in the latest Oligocene–early Pliocene marine rocks exposed along 1,500 km of the southwestern Atlantic littoral, although they are scarcely represented in the western sector of Patagonia (Meseta Belgrano, Santa Cruz Province) (Figs. 1, 2).
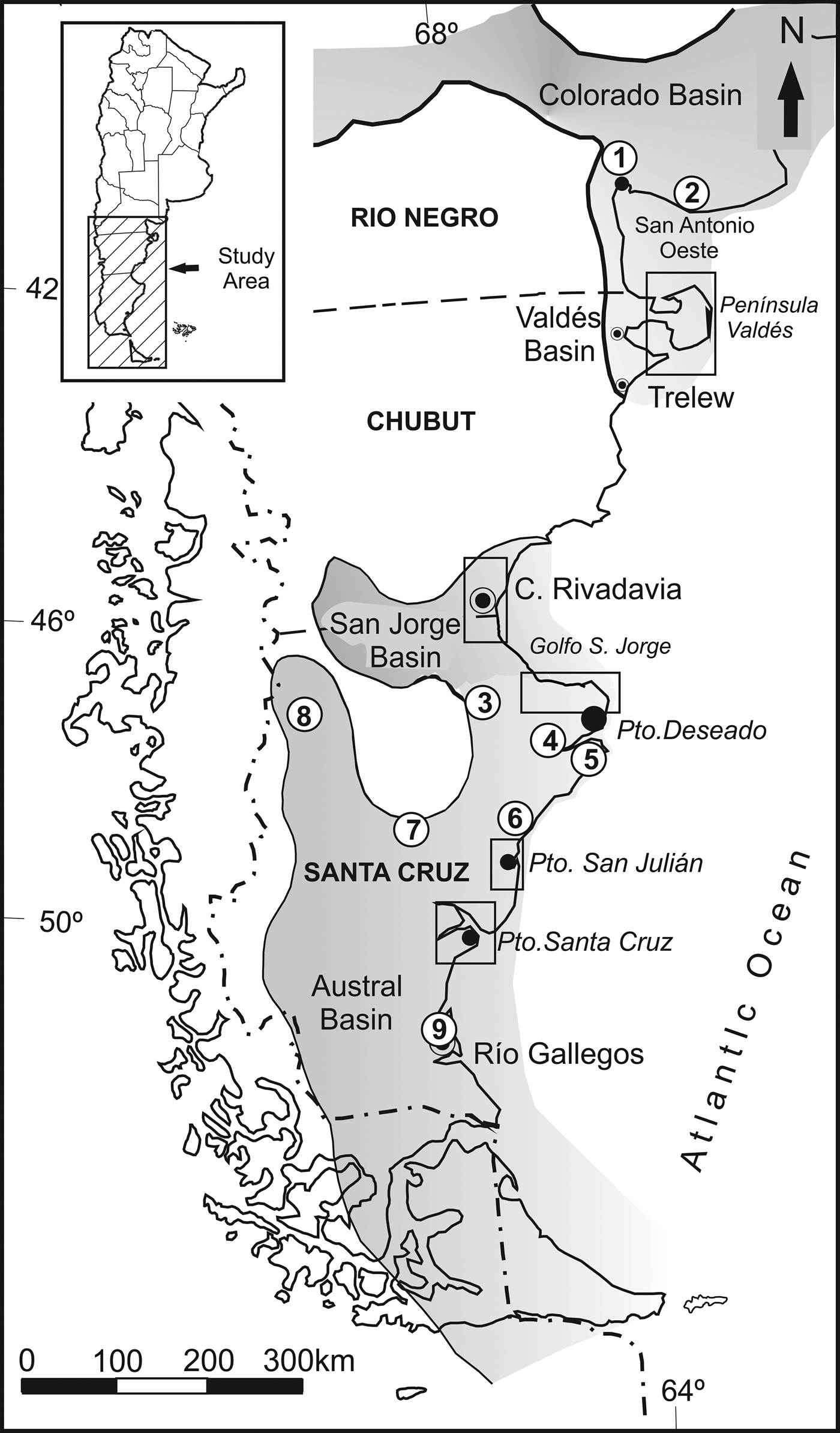
Figure 1. Geographic location of Neogene sedimentary basins and fossiliferous sites: 1 = Salinas del Gualicho; 2 = Barranca Final; 3 = Puesto El Museo; 4 = Bajo La Pava-Cerro Laciar; 5 = Punta Guanacos; 6 = Cerro Espejo; 7 = Estancia La Celestina; 8 = Meseta Belgrano; 9 = Cabo Buentiempo. Squares indicate study areas enlarged in Figure 2.

Figure 2. Enlargements of the study areas. (1) Puerto San Julián: 1 = Playa La Mina; 2 = Punta Cuevas; 3 = Oven Point; 4 = Cerro Pan de Azúcar; 5 = Meseta Chica; 6 = Cañadón Darwin; (2) Southern littoral of the Golfo San Jorge: 1 = Punta Casamayor; 2 = Cañadón El Lobo; 3 = Oficina Telégrafo Mazarredo; 4 = Estancia Pasuco; 5 = Punta Nava; 6 = Cabo Tres Puntas; (3) Mouth of Río Santa Cruz: 1 = Cañadón de los Misioneros; 2 = Monte Entrada; 3 = Estancia Santa Rosa; 4 = Las Cuevas; 5 = Yegua Quemada; (4) Comodoro Rivadavia area: 1 = Bahía del Fondo; 2 = Curva de la Calera; 3 = Playa Las Cuevas; 4 = Baliza Villegas; 5 = Punta Delgada; 6 = Rada Tilly; 7 = Punta Borjas; 8 = Cerro Chenque; 9 = Astra; 10 = Manantiales Behr; 11 = Cerro Cabeza de Papagayo; 12 = Bahía Solano; 13 = Pico Salamanca; (5) Península Valdés: 1 = Cerro Avanzado, 2 = El Doradillo, 3 = Cerro Prismático, 4 = Punta Quiroga, 5 = Eje Tentativo, 6 = Punta Logaritmo, 7 = Punta Tehuelche, 8 = Lote 39; 9 = Puerto San José; 10 = Punta San Román; 11 = Puerto Pirámides-Lobería Punta Pirámide; 12 = Punta Pardela.
Because of its biostratigraphic importance, this group received much attention during recent decades, when some genera were the focus of paleontological and stratigraphic studies. According to modern contributions, the Argentinean Chlamydini are constituted by the genera Swiftopecten Hertlein, Reference Hertlein1936, Reticulochlamys del Río, Reference del Río2004a, Jorgechlamys del Río, Reference del Río2004a, and a group of species that has been highly misinterpreted and assigned to the genus Zygochlamys Ihering, Reference Ihering von1907 (Morra, Reference Morra1985; Jonkers, Reference Jonkers2003) and doubtfully to ‘Chlamys’ Roding, Reference Röding1798 (del Río, Reference del Río1992).
Since the very moment of its creation by von Ihering (Reference Ihering von1907), the validity, specific composition, and origin of the genus Zygochlamys have been debated greatly by paleontologists and neontologists. Great confusion was generated by the lack of identification of its diagnostic characters and because several unrelated groups of circumpolar living and fossil species were included in Zygochlamys uncritically by some authors, while others strongly rejected any relationships among them. Some of these contradictions arose from the frequent homoplasy and high intraspecific variation of those species or due to the incorrect identification of its type species, Z. geminata (Zinsmeister, Reference Zinsmeister1981; Beu, Reference Beu1985; Waller, Reference Waller and Shumway1991).
In light of discrepant ideas about the identity of Zygochlamys, one of the goals of the present contribution is an exhaustive revision of its diagnostic characters, to improve its systematics and taxonomy and those of the species traditionally placed within it. As will be demonstrated herein, these largely belong in other genera. This analysis will lead to a better characterization of the taxonomic composition of the molluscan assemblages defined by del Río (Reference del Río2004b) for the Cenozoic rocks of Patagonia and will contribute to knowledge of the origin of the genus Zygochlamys and its relationships to the remaining species of the circumpolar regions, which during recent decades were thought to be closely related to Zygochlamys.
Geologic setting
Stratigraphic information
The Chlamydini included in this revision have been collected from the fossiliferous horizons of San Julián, Monte León, Chenque, Chacay, Puerto Madryn, and Gran Bajo del Gualicho formations and in the rocks informally known as the ‘Cabo Buentiempo Beds’ and ‘Araucanense Beds.’ These units have been described previously by the authors of the present paper (del Río, Reference del Río1992, Reference del Río2004a, Reference del Ríob; del Río and Camacho, Reference del Río and Camacho1998; del Río et al., Reference del Río, Griffin, McArthur, Martínez and Thirlwall2013), and are illustrated herein to provide accurate stratigraphic occurrences of the studied species (Fig. 3).

Figure 3. Lithologic sections of the San Julián, Monte León, Chenque, and Puerto Madryn formations and the ‘Araucanense Beds’ where analyzed material comes from.
The tribe Chlamydini was poorly represented during late Oligocene times by Zygochlamys geminata (Sowerby, Reference Sowerby and Darwin1846), a species herein restricted to the southern sector of the Austral Basin. It is an abundant species in the San Julián Formation, where it constitutes monospecific horizons exposed in the Bajo de San Julián, but is scarcely represented in the exposures of that unit in the surroundings of Puerto San Julián (Fig. 2.4).
The group became slightly more diverse during the early Miocene, when it was represented by Zygochlamys geminata, Z. sebastiani Morra, Reference Morra1985, and Pixiechlamys quemadensis n. comb. (Ihering, Reference Ihering von1897), which occur in the Monte León Formation, also exposed in the southern sector of the Austral Basin (Fig. 2.5). These species are infrequent taxa associated with other, more common representatives of the tribe, such as Reticulochlamys proximus (Ihering, Reference Ihering von1897), R. zinsmeisteri del Río, Reference del Río2004a, Jorgechlamys juliana (Ihering, Reference Ihering von1907), and Swiftopecten iheringii del Río, Reference del Río1995. In turn, the highly fossiliferous beds of early Miocene age exposed in the northern region of the Austral Basin (San Julián and Monte León formations) and in the Golfo San Jorge Basin (Chenque Formation) record a remarkable increase in the diversification and abundance of Chlamydini. The tribe reached its highest peak then, being represented by eight species of Zygochlamys, Chokekenia n. gen., Pixiechlamys n. gen., Swiftopecten, Reticulochlamys, and Jorgechlamys. Fossiliferous horizons are mostly characterized by pectinids and oysters found in either isolated exposures of the Monte León Formation on the Deseado Massif, in the San Julián Formation in the cliffs of Bahía Mazarredo (Fig. 2.3), or in the base of the Chenque Formation exposed in the Golfo San Jorge Basin (Fig. 2.2). The dominant species is Zygochlamys jorgensis Ihering, Reference Ihering von1907, which locally makes up monospecific horizons or may be associated with Pixiechlamys quemadensis n. comb. (Ihering, Reference Ihering von1907), Chokekenia nicolasi n. comb. (Morra, Reference Morra1985), Jorgechlamys juliana (Ihering, Reference Ihering von1907), J. centralis (Sowerby, Reference Sowerby and Darwin1846), and Swiftopecten iheringii in Bahía Mazarredo. The basal beds of the Chenque Formation, in the surroundings of Comodoro Rivadavia, are characterized by the abundance of all of these species (except for Chokekenia nicolasi n. comb.) and by Reticulochlamys del Río, Reference del Río2004a.
In the earliest middle Miocene beds of the Chenque Formation, an abrupt decrease is recorded in the abundance of pectinids, and Chlamydini are represented by uncommon representatives of Z. jorgensis and Z. sebastiani. This low diversity trend continued through time, and during the late Miocene, Moirechlamys actinodes n. comb. (Sowerby, Reference Sowerby and Darwin1846) is the only known Chlamydini of Patagonia. However, it is one of the most abundant bivalves of the entire Puerto Madryn Formation exposed at Peninsula Valdés and the surroundings of the city of Puerto Madryn (Fig. 2.1), where it is associated with Aequipecten paranensis (d’ Orbigny, Reference d'Orbigny1842) (tribe Aequipectinini), Amusium paris del Río, Reference del Río1992, and Leopecten piramidesensis (Ihering, Reference Ihering von1907) (tribe Amusiini). Moirechlamys actinodes n. comb. is also present in the uppermost horizons of the Gran Bajo del Gualicho Formation at Salinas del Gualicho and in the southernmost late Miocene exposures in the ‘Cabo Buentiempo Beds’ (on the coast north of Río Gallegos). The early Pliocene species Moirechlamys aurorae n. comb. (Feruglio, Reference Feruglio1954) is the youngest representative of the tribe in Patagonia. It is a very rare species recorded from isolated exposures at Cerro Laciar, Bajo de la Pava, Cañadón Darwin, Estancia Santa Rosa, and Cerro Espejo (Santa Cruz Province) (Figs. 1, 2.5).
Materials and methods
Image treatment
Preradial stages of the early discs of left valves were looked for in all studied material, and 41 valves were treated with a metalizer (Quorum Technologies SC7620) and coated with a gold/palladium alloy (40:60) to obtain scanning electron microscope (SEM) images with a Philips XL 30 scanning electron microscope. However, that early stage has not been preserved on the examined shells, as a result of abrasion or dissolution, except for one valve where it is partially preserved. Consequently, such a very useful character for systematics (Waller, Reference Waller and Shumway1991) is not available for Patagonian fossil Chlamydini. Several images are included here of the earliest stages observed.
Repositories and institutional abbreviations
The fossil material considered in the present paper has been collected in numerous field trips carried out during recent decades by different research teams conducted by H. Camacho and by Claudia del Río. It also includes the type material of historical collections made in Patagonia by C. Darwin in 1834, J.B. Hatcher between 1896 and 1899, and C. Ameghino, later studied by Sowerby (Reference Sowerby and Darwin1846), Ortmann (Reference Ortmann and Scott1902), and Ihering (Reference Ihering von1907), respectively. The repositories and their acronyms are as follows: MACN-Pi, Colección Paleoinvertebrados, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, CABA, Argentina; ex -CIRGEO-PI, Centro de Investigaciones en Recursos Geológicos, currently housed at MACN-Pi, CABA, Argentina; CPBA, Cátedra de Paleontología de la Universidad de Buenos Aires, CABA, Argentina; MLP, Facultad de Ciencias Naturales y Museo de la Universidad Nacional de La Plata, Buenos Aires, Argentina; NHM-L, Palaeontology collections, The Natural History Museum, London, England; PRI, Cenozoic Marine Mollusks, Paleontological Research Institution, Ithaca, United States; MGGC, Giovanni Capellini Geological Museum, Bologna, Italy; SGO.Pi, Colección Paleoinvertebrados, Museo de Historia Natural, Santiago de Chile, Chile.
Systematic paleontology
The Patagonian fossil taxa described in the following are referred to tribe Chlamydini because their shape is chlamydoid, they have asymmetrical auricles with a deep byssal notch and a functional ctenolium in adults, they have dorsal and resilial hinge teeth of similar size, they have no internal rib carinae, and they are sculptured with shagreen microsculpture and prominent antimarginal striae developed early in ontogeny. Shells are highlighted by a diagnostic complex pattern of appearance of ribs and riblets during ontogeny, with intercalations on both valves and bifurcations or ramifications on the right ones, resulting in discs densely ornamented with ribs and riblets. Of utmost importance is the presence of much shorter preradial stages, which allows distinction of the endemic Patagonian taxa from the remaining austral Chlamydini (Equichlamys Iredale, Reference Iredale1929, Notochlamys Cotton, Reference Cotton1930, Semipallium Jousseaume in Lamy, Reference Lamy1928, Talochlamys Iredale, Reference Iredale1929, Veprichlamys Iredale, Reference Iredale1929, and Psychrochlamys Jonkers, Reference Jonkers2003).
Order Pectinida Gray, Reference Gray1854
Superfamily Pectinoidea Rafinesque, Reference Rafinesque1815
Family Pectinidae Rafinesque, Reference Rafinesque1815
Subfamily Chlamydinae Teppner, Reference Teppner and Diener1922
Tribe Chlamydini Teppner, Reference Teppner and Diener1922
Genus Zygochlamys Ihering, Reference Iredale1907
Type species
Zygochlamys geminata (Sowerby, Reference Sowerby and Darwin1846). Late Oligocene–early Miocene, San Julián and Monte León formations, by original designation.
Included species
Zygochlamys geminata; Z. jorgensis Ihering, Reference Ihering von1907 (early–middle Miocene, San Julián, Monte León, El Chacay, and Chenque formations), Z. sebastiani Morra, Reference Morra1985 (early–middle Miocene, Monte León and Chenque Formations).
Diagnosis
Adult shell subtriangular, prosocline or acline. Umbonal angle narrow throughout ontogeny. Byssal notch rectangular. Left anterior auricle with 11–19 radial ribs; posterior auricle with 5–11 radial ribs. Disc sculptured with 8–14 paired plicae on right valve and 7–23 simple plicae on left valve, with numerous ribs and riblets bearing prominent scales.
Occurrence
Late Oligocene–middle Miocene of Austral and Golfo San Jorge basins.
Remarks
As already discussed by Morra (Reference Morra1985) and del Río (Reference del Río2004a), when erecting the genus Zygochlamys, Ihering (Reference Ihering von1907) questioned the validity of the name Chlamys Röding, Reference Röding1798 and replaced it with Myochlamys. He considered Zygochlamys to be a subgenus of Myochlamys, designated Pecten geminatus Sowerby, Reference Sowerby and Darwin1846 as its type species, and referred to Zygochlamys a large group of fossil and recent Patagonian species characterized by the development of simple plicae on left valves and paired plicae on the right valves: Z. fissocostalis (Ihering, Reference Ihering von1899), Z. jorgensis Ihering, Reference Ihering von1907, Z. quemadensis (Ihering, Reference Ihering von1897) (late Oligocene–middle Miocene), and Pecten actinodes Sowerby, Reference Sowerby and Darwin1846 (late Miocene). Ihering (Reference Ihering von1907) also referred with doubts to Zygochlamys the Recent species Chlamys patagonica (King, Reference King1832) and Chlamys natans (Philippi, Reference Philippi1845), both distributed along the southern South American coasts.
Cossmann (Reference Cossmann1909) stated that Myochlamys is a homonym because it had been used previously for a group of coleopterans (Family Curculionidae; Fairmaire, Reference Fairmaire1876), and proposed its replacement by Chlamydina. Therefore, Chlamydina immediately became an objective synonym of Chlamys Röding, Reference Röding1798 (Hertlein, Reference Hertlein and Moore1969). However, Hennig (Reference Hennig1911) continued to consider Myochlamys to be a valid name. Subsequently, Zygochlamys was widely accepted among paleontologists and neontologists who referred at least 15 species from the Cenozoic of the Southern Hemisphere to it. Fleming (Reference Fleming1944) was the first to suggest that Z. geminata could be an ancestor of Chlamys patagonica and of the Pleistocene–Recent New Zealand species Chlamys delicatula (Hutton, Reference Hutton1873). Powell (Reference Powell1950) for the first time used Zygochlamys to locate C. delicatula. Later, Fleming (Reference Fleming1957) proposed the inclusion in that genus of the Patagonian fossil species Pecten actinodes, as well as the Recent species C. patagonica and C. patriae Doello-Jurado, Reference Doello–Jurado1918 and the Neogene circum-Antarctic species C. anderssoni (Hennig, Reference Hennig1911), C. mawsoni Fletcher, Reference Fletcher1938, C. (Zygochlamys) heardensis Fleming, Reference Fleming1957, C. seymouri (Marwick, Reference Marwick1928), and C. delicatula? subantarctica (Hedley, Reference Hedley1916).
Grau (Reference Grau1959) was the pioneer author in claiming that Zygochlamys should not be considered a valid genus because its diagnostic characters had never been properly defined. He synonymized it with Chlamys Röding, Reference Röding1798.
Beu (Reference Beu1985) considered that all taxa placed in Zygochlamys by Fleming (Reference Fleming1957) were related to each other but not to Z. geminata, rejected the use of Zygochlamys, included them in Chlamys sensu lato, and added the Neogene Chilean species C. tenuicostata (Hupé, Reference Hupé and Gay1854), C. vidali (Philippi, Reference Philippi1887), C. hupeana (Philippi, Reference Philippi1887), and a species assigned to C. geminata by Tavera Jerez (Reference Tavera Jerez1979).
At the same time, Morra (Reference Morra1985) included in Zygochlamys the Patagonian Miocene species Z. nicolasi Morra, Reference Morra1985 and Z. sebastiani Morra, Reference Morra1985 and carried out the first detailed description of the genus. He stated that Zygochlamys is a valid taxon with almost equiconvex (Z. geminata) or right-convex shells (Z. jorgensis), whereas in Chlamys s. str. the left valve is substantially more convex than the right valve, the byssal sinus is larger, and the cardinal teeth are less pronounced. Although Morra (Reference Morra1985) failed to provide a clear diagnosis, he mentioned for the first time the presence of shagreen and antimarginal (= ‘camptonectes’) microsculpture to characterize Zygochlamys.
In disagreement with Beu (Reference Beu1985), Waller (Reference Waller and Shumway1991) accepted the use of Zygochlamys for C. patagonica, C. delicatula, C. dichroa (Suter, Reference Suter1909), and C. taiaroa (Powell, Reference Powell1952) (the last considered to be a synonym of C. dichroa by Dijkstra and Marshall [Reference Dijkstra and Marshall2008]) and, like Fleming (Reference Fleming1944, Reference Fleming1957), accepted a probable monophyletic origin for Zygochlamys.
Del Río (Reference del Río1992) restricted the use of Zygochlamys to species older than the late Miocene in Patagonia, and for that reason, specifically excluded Pecten actinodes from it.
Beu (Reference Beu1995) finally accepted the use of Zygochlamys for all the Recent and fossil circumpolar species, continued relating C. actinodes to Zygochlamys, and excluded C. dichroa, which he placed in Talochlamys Iredale, Reference Iredale1929.
More recently, in his monograph of the high southern latitude pectinids, Jonkers (Reference Jonkers2003) placed the Neogene Antarctic species in the new genus Austrochlamys and assigned the circumpolar species Z. patagonica, Z. delicatula, and Z. seymouri to the new genus Psychrochlamys Jonkers (Reference Jonkers2003). Regarding the composition of Zygochlamys, he included the Patagonian fossil species (Z. geminata, Z. jorgensis, and P. actinodes) and the Chilean Neogene species Pecten vidali Philippi, Reference Philippi1887, P. coquimbensis Möricke, Reference Möricke1896, and P. hupeanus Philippi, Reference Philippi1887. Jonkers (Reference Jonkers2003) provided a long list of characters diagnostic of Zygochlamys that actually characterize the Chlamydini, such as the presence of shells with chlamydoid acline, prosocline, or opisthocline outlines, with nearly circular or asymmetrical discs, a high auricular asymmetry, a large and arcuate byssal notch with a functional ctenolium, an oblique resilifer, the presence of scaly plicae, smooth or sculptured interspaces, and the development of “shagreen microsculpture restricted to the umbonal region in ancient species, which would be replaced by antimarginal ridgelets in adults, whereas, in modern species, shagreen microsculpture would completely cover the disc” (Jonkers, Reference Jonkers2003, p. 36).
Morra (Reference Morra1985) proposed that Pecten fissicostalis Ihering, Reference Ihering von1899 could have been based on specimens of Z. geminata, but P. fissicostalis is known only from fragmented casts where the number, disposition, and size of plicae just allow it to be considered a member of Zygochlamys.
Of the remaining Patagonian species placed in Zygochlamys by Morra (Reference Morra1985), P. nodosoplicata belongs in Swiftopecten Hertlein, Reference Hertlein1936 (del Río, Reference del Río1995) and Pecten quemadensis, Z. nicolasi, and the highly debated Pecten actinodes are herein excluded from Zygochlamys because they belong in new endemic genera, as will be discussed.
According to current knowledge, the presence of the genera Zygochlamys, Austrochlamys, and Psychrochlamys in the Neogene–Recent southern circumpolar regions is accepted here, and in this paper we restrict the use of Zygochlamys to the fossil species Z. geminata, Z. jorgensis, and Z. sebastiani of Patagonia.
The presence of Zygochlamys in Chile is accepted here. It is represented by the early Miocene casts (SGO.PI 4329, SGO.PI 4459, SGO.PI 5178) assigned by Tavera Jerez (Reference Tavera Jerez1979) and Frassinetti and Covacevich (Reference Frassinetti and Covacevich1999) to Zygochlamys geminata. The poor preservation of the material precludes any specific assignment, but the arrangement of the paired plicae on right valves and the number of plicae on left valves allow their placement in the genus Zygochlamys.
Zygochlamys is characterized by the presence of either right- or left-convex shells with umbonal angles between 80° and 98°. In contrast to what was proposed by Jonkers (Reference Jonkers2003), there is a remarkably strong ontogenetic variation from juvenile specimens with higher than long triangular discs to adults with more circular but not orbicular outlines. Valves are sculptured with plicae covered with scaly ribs, shagreen microsculpture is restricted on most specimens to a commarginal band between 3 mm and 30 mm from the beaks on both valves, and antimarginal microsculpture is well developed from at least the beginning of the radial stage down to the ventral margin. The commarginal lirae are well developed on the entire umbonal area and as scattered patches on the remaining surface of the disc.
Zygochlamys differs from Chlamys Röding, Reference Röding1798 (type species: Pecten islandicus Müller, Reference Müller1776, North Atlantic and North Pacific Oceans, early Pleistocene–Recent; MacNeil, Reference MacNeil1967, pl. 2, figs. a–d, pl. 18, fig. 8, pl. 19, figs. 2–5, pl. 20, figs. 7–9, pl. 22, figs. 7–8, pl. 24, figs.12, 13) in having larger and thicker shells, with the antero- and posterodorsal margins of the adult disc less inclined and a shallower byssal sinus. In Zygochlamys shells are sculptured with up to 14 paired plicae on right valves (bifurcated in early ontogeny) and up to 23 simple plicae on left valves, and interspaces on both valves are covered with two or three orders of scaly ribs, whereas discs of Chlamys lack plicae but have 30 ribs on left valves and 36 on right valves that commonly bifurcate in late ontogeny, and interspaces have one or two riblets on left valves but only one on right valves.
Zygochlamys differs from Psychrochlamys (type species: Pecten patagonicus King, Reference King1832, Magellanic and Argentine Malacological provinces, Holocene–Recent; Jonkers, Reference Jonkers2003, pl. 1, fig. 1, pl. 8, figs. c–f, pl. 9, figs. e, f) in having larger, thicker, and less prosocline shells that are higher than long or as high as long in adult stages, a narrower umbonal angle, a longer hinge margin with more asymmetrical auricles, the free margin of left anterior auricles inclined anteriorly, a deeper byssal notch, and a byssal sinus. Sculpture also separates the two genera because Zygochlamys species have higher plicae, interspaces are covered with scaly riblets of two or three orders, and the commarginal microsculpture is almost restricted to the umbonal area or, as happens in Z. sebastiani Morra, Reference Morra1985, to certain narrow bands of the shells, while in Psychrochlamys coarse commarginal lamellae extend over the entire surface and it lacks the shagreen microsculpture that characterizes Zygochlamys.
Zygochlamys differs from Austrochlamys (type species: Pecten natans Philippi, Reference Philippi1845; Antarctica and the Southern Ocean, Miocene–Recent; Jonkers, Reference Jonkers2003, pl. 12, fig. a–i, pl. 13, fig. a–g, pl. 14, fig. a–e, pl. 15, fig. a–h; Pirrie et al., Reference Pirrie, Jonkers, Smellie, Crame and McArthur2011: fig. 3; Beu and Taviani, Reference Beu and Taviani2014, fig. 3d–f) in having subtriangular shells, more elongated posteriorly; a narrower umbonal angle; more asymmetrical auricles covered with strong riblets; a deeper, rectangular byssal notch; and a byssal sinus. Moreover, in Zygochlamys the plicae are covered with up to five riblets, the interspaces have a higher number of ribs, and shells are sculptured with shagreen microsculpture, while in Austrochlamys, when plicae are present, they are covered with only one or two fine ribs and coarse commarginal lamellae.
Zygochlamys is distinguished from Talochlamys Iredale, Reference Iredale1929 (type species: Talochlamys pulleineana (Tate, Reference Tate1887) (= Chlamys famigerator Iredale, Reference Iredale1925) (Recent, Australia; Beu and Darragh, Reference Beu and Darragh2001, fig. 29A–I; Dijkstra and Beu, Reference Dijkstra and Beu2018) (genus: Australia and New Zealand, Eocene–Recent; Beu and Darragh, Reference Beu and Darragh2001) by the presence of a much shorter preradial stage, larger and subtriangular discs with paired plicae on right valves that are covered with up to five riblets, and interspaces with more secondary riblets, which arise in early ontogeny. In Talochlamys, shells are ornamented with ribs or simple plicae on both valves, which are covered with only one or three ribs, and the interspaces are covered with fewer secondary riblets, which appear in late ontogeny. In addition, in Talochlamys shagreen microsculpture is infrequent and the entire surface of the discs is covered with thick commarginal lamellae.
Zygochlamys geminata (Sowerby, Reference Sowerby and Darwin1846)
Figures 4.1–4.9, 5.1, 5.2
- 1846
Pecten geminatus Sowerby, p. 609, pl. 2, fig. 24.
- 1897
Pecten geminata; Ihering, p. 227.
- 1902
Pecten geminatus; Ortmann, pl. 23, fig. 2a–c, e, p. 117.
- 1907
Myochlamys geminata; Ihering, p. 254.
- 1911
Myochlamys geminata; Hennig, p. 13, 15.
- 1914
Myochlamys geminata; Ihering, p. 31.
- 1957
Chlamys (Zygochlamys); Fleming, p. 15.
- 1985
Zygochlamys dominator Morra, p. 303, pl. 2, figs. 1a, b.
- 1985
Chlamys geminata; Beu, p. 2, 7.
- 1991
Pecten geminatus; Waller, p. 29.
- 1995
Zygochlamys geminata; Beu, p. 15.
- 2003
Zygochlamys geminata; Jonkers, p. 36, fig. 4a–f.
- 2008
Zygochlamys geminata; Griffin and Nielsen, p. 268, fig. 5.1.

Figure 4. Zygochlamys geminata (Sowerby, Reference Sowerby and Darwin1846) from the San Julián Formation: (1) external view of cast of right valve, holotype NHM-L 27695, from ‘San Julián, Patagonia’; (2) external view of right valve, MACN-Pi 4458a, from Oven Point; (3) external view of left valve, an articulated specimen, CPBA 21584, from Oven Point; (4) external view of left valve, MACN-Pi 4458b, from Oven Point; (5) external view of right valve, holotype of Z. dominator Morra, Reference Morra1985 CPBA 12446, form Meseta Chica; (6) anterior view, MACN-Pi 5812, from Meseta Chica; (7–9) MACN-Pi 5813, from Playa La Mina, (7) hinge, (8) external view, (9) detail of shagreen microsculpture. (1–8) Scale bar = 10 mm; (9) scale bar = 5 mm.

Figure 5. (1, 2) Zygochlamys geminata (Sowerby, Reference Sowerby and Darwin1846) from the San Julián Formation: SEMs of left valve, MACN-Pi 4460a, from Meseta Chica: (1) an image compiled from four obtained with SEM showing shagreen microsculpture, (2) showing commarginal and antimarginal microsculpture in radial initial stage; (3–5) Zygochlamys jorgensis Ihering, Reference Ihering von1897: (3, 4) SEMs of left valve, MACN-Pi 250, from Punta Nava, San Julián Formation, showing antimarginal and shagreen microsculpture, (5) SEMs of left valve, CPBA 6422, from Bahía Solano, Chenque Formation showing antimarginal microsculpture. (1) Scale bar = 400 µm; (2, 4, 5) scale bars = 500 µm; (3) scale bar = 200 µm.
Type specimens
Holotype of Pecten geminatus Sowerby, Reference Sowerby and Darwin1846, NHM-L27695, a right valve from ‘San Julián, Patagonia,’ San Julián Formation (late Oligocene); holotype of Zygochlamys dominator Morra, Reference Morra1985, CPBA 12446, a right valve, and paratype of Z. dominator, CPBA 12447, a left valve, both from Meseta Chica, San Julián Formation (Santa Cruz Province).
Diagnosis
Shell large, slightly left-convex. Left valve ornamented with 13–23 simple plicae; plicae and interspaces on both valves bearing 3–5 ribs and riblets. Shagreen microsculpture extending beyond umbonal area, reaching a greater distance from beaks than in Z. jorgensis and Z. sebastiani.
Occurrence
San Julián Formation (late Oligocene) at Oven Point, Punta Cuevas, Playa La Mina, Meseta Chica, and Cerro Pan de Azúcar and Monte León Formation (early Miocene) at Canadón Darwin (Santa Cruz Province).
Description
Shell of large size, attaining up to 120 mm, triangular, higher than long, left-convex, acline, rarely prosocline, subequidimensional or posteriorly elongate, posterodorsal margin less concave and longer than the anterodorsal margin. Hinge dorsal margin straight, 46%–55% of total disc length. Umbonal angle 87°–98°. Auricles large, asymmetrical, with free margins inclined anteriorly; right anterior auricle with dorsal margin slightly projected upward, free margin rounded or straight; byssal notch rectangular, wide, deep, with ctenolium functional throughout ontogeny, with 3–6 strong teeth; byssal fasciole with corrugations convex toward umbo; posterior auricles with free margin straight on most specimens, slightly concave on a few; free margin of left anterior auricle sinuous, with shallow byssal sinus. Right anterior auricle sculptured with 6–9 radial ribs, left anterior auricle with 17–19 ribs, posterior auricles with 7–11 ribs; ribs on anterior auricles thicker than on posterior auricles; dorsal rib of right anterior auricle thickened. Discs sculptured with plicae of rounded to subtriangular section; interspaces and plicae with one central primary rib, flanked by 2–4 secondary and tertiary riblets, covered with scales directed ventrally, scales higher toward ventral margins. Right valve with 10–14 paired plicae of equal width or wider than their interspaces, bifurcating at beginning of radial stage, defining conspicuous geminate pattern in central sector of disc. Left valve with 13–23 simple plicae, narrower than their interspaces. Entire disc surface covered with antimarginal ridgelets from beginning of initial radial stage; commarginal microsculpture of fine lirae restricted to beginning of initial radial stage, up to 3.5 mm shell height; shagreen microsculpture extending from 2 mm to 40 mm shell height on both valves. Auricles of both valves covered with antimarginal ridgelets; commarginal lamellae extending from beginning of radial stage up to 5 mm height on left anterior auricle, represented by corrugations on anterior right auricle; shagreen microsculpture developed on left auricles and right posterior auricle of some specimens.
Materials
Five articulated specimens, 67 right valves and 61 left valves: CPBA 6599, 6621, 21582–21584, 21586, 21575; MACN-Pi 246–247, 249, 913, 4457–4460, 5137, 5812–5813, 5815, 6298; MLP 5837; PRI 66230, PRI 66231, PRI 66411, PRI 66412, PRI 66415, PRI 66416, PRI 66417, PRI 72678, PRI 72679, and PRI 72680.
Dimensions (in mm)
Holotype NHM-L27695, length (L) = 23.6 , height (H) = 28.8; holotype of Z. dominator CPBA 12446, L = 99.0, H = 102.5; MACN-Pi 5813, L = 62.4, H = 69.5; PRI 72678, L = 77.9, H = 81.8; CPBA 21583, L = 126.0, H = 130.3; CPBA 21582, L = 43.1, H = 51.3.
Remarks
This species was originally described by G.B. Sowerby I (Reference Sowerby and Darwin1846) based on a juvenile right valve collected by C. Darwin in 1834 from an unspecified stratigraphic horizon exposed in the area of Puerto San Julián (Santa Cruz Province). Because the exact geographical occurrence is unknown, the surroundings of Puerto San Julián strata of both late Oligocene (San Julián Formation) and early Miocene ages (Monte León Formation) are exposed, and the holotype is a juvenile specimen, which is extremely difficult to distinguish among species of Zygochlamys, Morra (Reference Morra1985) proposed the new name Z. dominator (Fig. 4.5) as the type species of Zygochlamys. However, according to a 1994 personal communication of C. del Río and T. Waller to A. Beu, Z. dominator Morra, Reference Morra1985 is the adult shell of P. geminatus (Beu, Reference Beu1995), an assertion accepted by both Jonkers (Reference Jonkers2003) and Griffin and Nielsen (Reference Griffin and Nielsen2008).
In the current revision, Zygochlamys geminata is considered to be the valid name because it is the type species of the genus, a name widely cited in paleontological literature, and the only recognizable species of Zygochlamys in the area where it was originally described by Sowerby (Reference Sowerby and Darwin1846), despite the fact that he failed to provide its accurate stratigraphical and geographical location. Zygochlamys geminata is restricted to the southern sector of the Austral Basin, where it was an abundant species during the late Oligocene–early Miocene interval. The material mentioned by Ihering (Reference Ihering von1907) for the Golfo San Jorge Basin actually belongs to Z. jorgensis Ihering (see discussion that follows).
Since the pioneer descriptions by Ortmann (Reference Ortmann and Scott1902) and Ihering (Reference Ihering von1907), and through the discussions by Zinsmeister (Reference Zinsmeister1981), Beu (Reference Beu1985), and Waller (Reference Waller and Shumway1991), Zygochlamys geminata repeatedly has been confused with other taxonomic entities. Even Ihering (Reference Ihering von1907) himself curiously synonymized with it a right valve of Aequipecten paranensis (d'Orbigny, Reference d'Orbigny1842) illustrated by Sowerby (Reference Sowerby and Darwin1846, p. 376, pl. 3, fig. 30), which in fact is a guide fossil of the late Miocene (del Río, Reference del Río1988, Reference del Río1992).
Two varieties of Zygochlamys geminata have been proposed. Ortmann (Reference Ortmann and Scott1902) included shells from Darwin Station in Z. geminata var. quemadensis Ihering (Ortmann Reference Ortmann and Scott1902, pl. 23, fig. 2d), but that material belongs in a new genus (see the following). Ihering (Reference Ihering von1907) named as Zygochlamys geminata radana (Chenque Formation, early Miocene, Rada Tilly, Chubut Province) a single valve that is lost and has never been illustrated. However, according to its locality, it seems likely to correspond to Z. jorgensis.
The number of plicae and the position of appearance of riblets in the interspaces of Zygochlamys geminata are highly variable. This is particularly the case of specimens from playa La Mina (Fig. 4.8, 4.9) and Oven Point (PRI 72678; Ortmann, Reference Ortmann and Scott1902, pl. 23, fig. 2c) where secondary riblets appear 40 mm from the umbo, whereas they usually arise between 4 and 6 mm in shells from Meseta Chica, Cerro Pan de Azúcar, Punta Cuevas, Cañadón Darwin, and Oven Point. Moreover, shagreen microsculpture, although present on both valves in some specimens, is more common on right valves.
Zygochlamys jorgensis Ihering, Reference Ihering von1907
Figures 5.3–5.5, 6.1–6.9
- 1907
Myochlamys (Zygochlamys) jorgensis lhering, p. 258, pl. 9, fig. 57b–d.
- 1981
Chlamys geminata (Sowerby, Reference Sowerby and Darwin1846); Zinsmeister, p. 1092, pl. 1, figs. 3, 7–9 (not Pecten geminatus Sowerby, Reference Sowerby and Darwin1846).
- 1985
Zygochlamys jorgensis; Morra, p. 302.
- 1995
Zygochlamys jorgensis; Beu, p. 15.
- 2003
Zygochlamys jorgensis; Jonkers, p. 38.
- 2004b
“Chlamys” jorgensis; del Río, p. 1103, fig. 13.1.
Figure 6. Zygochlamys jorgensis Ihering, Reference Ihering von1897. (1–3) Holotype, an articulated specimen MACN-Pi 274, from Punta Casamayor, San Julián Formation: (1) external view of left valve; (2) anterior view; (3) external view of right valve. (4) External view of left valve, MACN-Pi 5821a, from Punta Delgada, Chenque Formation; (5) detail of shagreen microsculpture, MACN-Pi 6422a, from Bahía Solano, Chenque Formation; (6) external view of left valve, MACN-Pi 6406, from Ea. Pasuco, Monte León Formation; (7) external view of left valve, MACN-Pi 6413, from Ea. La celestina, Monte Léon Formation. (8, 9) MACN-Pi 5821b, from Punta Delgada, Chenque Formation: (8) external view of right valve, (9) hinge. (1–4, 6–9) Scale bar = 10 mm (graphed at the right end of the figure); (5) scale bar = 5 mm (below the figured specimen).
Type specimens
Holotype MACN-Pi 274, an articulated specimen from Punta Casamayor (Santa Cruz Province), San Julián Formation (early Miocene).
Diagnosis
Right valve more convex than left valve. Umbonal angle narrow. Hinge dorsal margin long for genus. Number and width of plicae strongly dissimilar on the two valves; crest and flanks of plicae sculptured with only one wide primary rib with coarse, high scales; secondary riblets arising in advanced ontogeny on sides of plicae; left valve sculptured with 6–11 plicae; interspaces wide for genus, with 5–11 ribs and riblets.
Occurrence
San Julián Formation (early Miocene) at Punta Casamayor, Punta Nava, Cañadón El Lobo, Mazarredo, Oficina Telégrafo Mazarredo, and Cabo Tres Puntas; Monte León Formation (early Miocene) at Ea. La Celestina, Ea. Pasuco, and Puesto Museo; El Chacay Formation (early Miocene) at Meseta Belgrano; and Chenque Formation (early–middle Miocene) at Bahía del Fondo, Curva de la Calera, Playa Las Cuevas, Baliza Villegas, Punta Delgada, Rada Tilly, Punta Borjas, Cerro Chenque, Cerro Cabeza de Papagayo, Astra, manantial Behr, Bahía Solano, and Pico Salamanca.
Description
Shell of moderate size, up to 92 mm in height, triangular, right-convex, acline, rarely prosocline, elongate posteriorly or subequilateral, with anterodorsal margin concave, posterodorsal margin concave to straight. Hinge dorsal margin straight, 54%–60% of total disc length. Umbonal angle 80°–90°. Auricles large, asymmetrical, with free margins sloping anteriorly; right auricle rounded or straight, sloping anteriorly; byssal notch very deep and wide, rectangular in outline, with functional ctenolium with 3–5 strong teeth; byssal fasciole with corrugations convex toward umbo; free margins of right and left posterior auricles straight, concave in a few specimens, that of left anterior auricle concave, sinuous in a few specimens, with shallow byssal sinus. Right anterior auricle sculptured with 4–6 radial ribs, left anterior auricle with 13–19 ribs, posterior auricles with 7–9 ribs; ribs on anterior auricles thicker than those on posterior auricles, dorsal rib of right anterior auricle thickened. Disc folded by rounded plicae, widening ventrally, sculptured with one primary rib covering crests and flanks and with secondary and tertiary riblets that appear at 70 mm and at 75–85 mm height from beaks; interspaces sculptured with up to four orders of ribs ornamented with upright scales, ventrally concave, rarely flat, higher toward ventral margin. Right valve sculptured with 8–11 paired plicae, separated by interspaces each narrower than one plica, with central rib and 3–5 secondary and tertiary riblets. Left valve ornamented with 6–11 single plicae, separated by concave interspaces each twice width of one plica, sculptured with central primary rib appearing 2–5 mm from beaks and 4–6 secondary and tertiary riblets or up to 11 riblets of equal width. Antimarginal microsculpture extending over entire disc from initial radial stage; commarginal lirae restricted to initial radial stage; shagreen developed as a band between 2 and 27 mm height on plicae and interspaces on both valves of some specimens. Auricles of both valves sculptured with antimarginal microsculpture; thick commarginal corrugations on anterior auricles, present from beginning of radial stage up to 5 mm height on left auricles of most specimens; when present on disc, shagreen microsculpture extends up to 10 mm on left anterior auricle and up to 4 mm height from beaks on posterior auricle.
Materials
Six articulated specimens, 124 right valves, and 105 left valves: CPBA 14442–14443,16280, 16371,16385, 8573, 8581– 8582, 8609, 8611–8612, 8620, 8665, 8729, 8731, 14456, 21576, 21578, 21579, 21850, 23615, 23617–23620; MACN-Pi 250, 275, 277–278, 3887, 5816–5818, 5820–5821, 6401–6402, 6406, 6408–6409, 6422, 6413; ex-CIRGEO-PI 2056, 2063, 2107, 2110, 2122, 2181; MLP 2032, 2369, 2378, 2393, 2401, 2413, 2421, 2440, 2455, 2458, 2463, 2465, 5169, 6778, 8388, and 8904.
Dimensions (in mm)
Holotype MACN-Pi 274, L = 67.0 , H = 72.0; MACN-Pi 5821, L = 68.1, H = 75.6; MACN-Pi 5821, L = 81.1, H = 91.4; MACN-Pi 5821, L = 77.1, H = 85.5; CPBA 21580, L = 58.4, H = 65.1; MACN-Pi 277, L = 47.1, H = 56.6; CPBA 21576, L = 57.7, H = 63.4; MACN-Pi 5817, L = 86.6, H = 92.0; MLP 2455, L = 78.0, H = 86.1.
Remarks
Zygochlamys jorgensis is the most common species of Chlamydini in Patagonia, being abundant in the Golfo San Jorge Basin, but a poorly represented species in the western sector of the Austral Basin (El Chacay Formation). It was described by Ihering (Reference Ihering von1907) from material from the San Julián Formation exposed at Bahía Mazarredo; Ihering chose as the holotype of this species an articulated shell from Punta Casamayor, illustrating the left valve (MACN-Pi 274; Fig. 6.1–6.3).
Collections made during recent decades extended the stratigraphic range of Zygochlamys jorgensis to the earliest middle Miocene (middle and upper Chenque Formation) and allowed its recognition in the Monte León Formation (early Miocene) exposed on the Deseado Massif (i.e., Estancia La Celestina and Puesto Museo) and in the Chacay Formation (early Miocene).
Zinsmeister (Reference Zinsmeister1981, pl. 1, figs. 3, 7–9) illustrated specimens of Zygochlamys jorgensis from Punta Casamayor but wrongly attributed them to Chlamys geminata (Sowerby, Reference Sowerby and Darwin1846). The two species can be differentiated because Z. jorgensis has right-convex, smaller, and more-inequiconvex shells, a narrower umbonal angle, the right anterior auricle covered with only four to six ribs with higher scales, and the left valve sculptured with fewer plicae that are much wider than those on right valves and separated by wider interspaces than those on left valves of Z. geminata. In addition, the crests of plicae of Z. jorgensis are ornamented with only one coarse primary rib extending onto the flanks and by riblets that appear at 70 mm from the beaks; in Z. geminata, the crests and flanks of plicae are covered with several narrower riblets that arise earlier in ontogeny. Moreover, the shagreen microsculpture extends further down the disc on Z. geminata.
Zygochlamys sebastiani Morra, Reference Morra1985
Figure 7.1–7.6
- 1985
Zygochlamys sebastiani Morra, p. 304, pl. 1, fig. 2a, b.

Figure 7. Zygochlamys sebastiani Morra, Reference Morra1985. (1) External view of left valve, holotype CPBA 12457, from Monte Entrada, Monte León Formation; (2, 3) paratype CPBA 12458, from Monte Entrada, Monte León Formation: (2) external view of right valve; (3) detail of microsculpture (3×). (4) External view of left valve, CPBA 11010, from Pico Salamanca, Chenque Formation; (5) external view of right valve, MACN-Pi 5819, from Punta Guanacos, Monte León Formation; (6) external view of left valve, CPBA 21577, from Cerro Chenque, Chenque Formation. (1, 2, 4–6) Scale bar = 10 mm (graphed at the right end of the figure); (3) scale bar = 5 mm (below the figured specimen).
Type specimens
Holotype CPBA 12457, a left valve, and paratype CPBA 12458, a right valve, both from Monte Entrada (Santa Cruz Province), Monte León Formation (early Miocene).
Diagnosis
Shell equiconvex. Spaces between plicae sculptured with up to three riblets. Antimarginal microsculpture thickened on top of plicae; commarginal lamellae present on entire disc, but dominant from 40 or 50 mm height from beaks; developing a reticulate pattern when crossing antimarginal ridgelets.
Occurrence
Monte León Formation (early Miocene) at Monte Entrada, Cañadón de los Misioneros, mouth of Santa Cruz River, and Punta Guanacos; Chenque Formation (earliest–middle Miocene) at Pico Salamanca and Cerro Chenque.
Description
Shell of moderate size, up to 95 mm in height, triangular, higher than long, equiconvex, acline, subequilateral, with anterodorsal margin concave and posterodorsal margin straight. Hinge dorsal margin straight, 45%–50% of total shell length. Umbonal angle 90°–95°. Auricles large, asymmetrical, and with free margins sloping anteriorly, those on posterior auricles concave, dorsal margin of anterior auricle slightly projected upward, free margin rounded; byssal notch very deep, wide, and rounded, with functional ctenolium with 5 or 6 strong teeth; byssal fasciole with corrugations convex toward umbo; byssal sinus shallow. Right auricles sculptured with 5 or 6 radial ribs, left anterior auricle with 11–13 ribs, left posterior auricle with 5–7 ribs; ribs on anterior auricles thicker than on posterior auricles, dorsal rib of right anterior auricle thickened. Exterior surface with rounded plicae sculptured with one primary rib that appears between 9 and 11 mm height from beaks and two narrower secondary riblets that originate between 25 and 60 mm height; interspaces with secondary riblets; ribs and riblets covered with ventrally concave scales. Right valve with 10 or 11 paired plicae with a well-defined geminate pattern at beginning of radial stage but vanishing toward ventral margin, where they are separated by narrower interspaces; left valve with 15–20 single plicae of two intercalated sizes and interspaces of equal width. Antimarginal microsculpture extending from 18 mm on plicae and interspaces, thickening on primary ribs over crest of plicae; commarginal sculpture of very narrow lamellae that arise at 40–50 mm height from beaks; when crossing, the two microsculptures form a reticulate pattern. Shagreen microsculpture uncommon, restricted to umbonal area of both valves up to 10–12 mm height; auricles of both valves covered with antimarginal microsculpture.
Materials
One articulated specimen, two right valves, one left valve, three fragments of right valves, and four fragments of left valves: CPBA 11010, 15961–15963, 21577; MACN-Pi 5819; PRI 72684 and PRI 72685.
Dimensions (in mm)
Holotype CPBA 12457, L = 78.9, H = 90.9; CPBA 12458, L = 50.4 , H = 60.2; MACN-Pi 5819, L = 56.2, H = 61.4.
Remarks
Morra (Reference Morra1985) described Z. sebastiani based on a left (holotype) valve and a right (paratype) valve collected at Monte Entrada, and new collections expand its presence northward to the Monte León Formation exposed at Punta Guanacos (northern sector of the Austral Basin, Santa Cruz Province) and to the upper beds of the Chenque Formation in the Chubut Province (Golfo San Jorge Basin).
Zygochlamys sebastiani differs from Z. geminata in having smaller shells, and its auricles are covered with fewer ribs. It differs from both Z. geminata and Z. jorgensis in having equiconvex shells characterized by the dominance of very fine commarginal lirae still present in the advanced stages of ontogeny, where they give rise to a reticulate pattern when crossing the thick antimarginal striae on the tops of plicae. Moreover, Z. sebastiani can be distinguished from Z. jorgensis by having shells sculptured with more numerous plicae with a primary rib restricted to their crests and interspaces on the left valve with fewer riblets.
Chokekenia new genus
Type species
Zygochlamys nicolasi Morra, Reference Morra1985. Early Miocene, San Julián and Monte León Formations, by monotypy.
Diagnosis
As for type species.
Occurrence
Early Miocene of southern littoral of the Golfo de San Jorge and of northern and southern sectors of the Austral Basin.
Etymology
From the word chokeken, which in the language of the Tehuelche native tribe means “plateau,” in reference to the growth ledges on the shells.
Remarks
Chokekenia n. gen. is here defined to include the Miocene species C. nicolasi n. comb. (Morra, Reference Morra1985), characterized by having few plicae that are paired on right valve and being a monospecific genus clearly differentiated from the genera described so far.
Chokekenia n. gen. is distinguished from Zygochlamys by its shells with orbicular outlines, one ledge, a wider umbonal angle, more symmetrical auricles (Fig. 8.3, 8.4, 8.6, 8.7), and narrower plicae covered with finer ribs of equal width that bifurcate twice on right plicae. Moreover, the ribs are sculptured with scales that appear at 20–40 mm shell height, and left interspaces are narrower than plicae, whereas in Zygochlamys scales appear earlier in ontogeny and interspaces are wider than the plicae.

Figure 8. (1–7) Chokekenia nicolasi (Morra, Reference Morra1985) n. comb. (1, 2) An articulated specimen, holotype CPBA 12465, from Cañadón El Lobo, San Julián Formation: (1) external view of right valve; (2) external view of left valve. (3–7) A specimen with matching valves, MACN-Pi 6407, from Punta Guanacos, Monte León Formation: (3) external view of right valve; (4) external view of left valve; (5) detail of microsculpture of left valve; (6) anterior view; (7) hinge. (8–13) Pixiechlamys quemadensis (Ihering, Reference Ihering von1897) n. comb. (8, 9) Holotype MACN-Pi 272, from Las Cuevas, Monte León Formation: (8) external view; (9) internal view of right valve. (10) external view of right valve, CPBA 23622a, from Curva de la Calera, Chenque Formation; (11) external view of left valve of an articulated specimen, MACN-Pi 6403, from Rada Tilly, Chenque Formation; (12) external view of right valve, CPBA 23622b, from Curva de la Calera, Chenque Formation; (13) external view of left valve, MACN-Pi 6400, from Punta Delgada, Chenque Formation. (1–4, 6–13) Scale bar = 10 mm (graphed at the right end of the figure); (5) scale bar = 5 mm (below the figured specimen).
Chokekenia n. gen. can be separated from Swiftopecten because it has a wider umbonal angle and a shorter hinge margin, and shells are sculptured with more plicae without nodes and with fewer ledges, covered with fewer scaly ribs.
Chokekenia n. gen. is differentiated from Semipallium Jousseaume in Lamy, Reference Lamy1928 (type species: Pecten tigris Lamarck, Reference Lamarck1819 [= Semipallium flavicans {Linnaeus, Reference Linnaeus1758}], Recent, Indo-West Pacific; Dijkstra, Reference Dijkstra2013, fig. 22, 1a–d) in having plicae entirely subdivided and bifurcated and arranged in conspicuous pairs, larger posterior auricles, and a deeper byssal notch. The Patagonian genus also has shagreen microsculpture always restricted to the umbonal area, whereas in Semipallium it extends over the entire disc. Chokekenia n. gen. is particularly similar to Semipallium hallae (Cotton, Reference Cotton1960) (Recent, between Adelaide and Glenelg, South Australia; Dijkstra and Beu, [2018] figs. 84 g–h, 86a–c) in having shells sculptured with ribs of homogenous size. In addition, the Australian species has an incipient bifurcation on some right valve plicae.
Chokekenia nicolasi (Morra, Reference Morra1985) new combination
Figure 8.1–8.7
- 1902
Pecten geminatus quemadensis Ihering; Ortmann, pl. 23, fig. 2d.
- 1985
Zygochlamys nicolasi Morra, p. 304, pl. 2, fíg. 2 a, b.
Type specimens
Holotype, an articulated shell, CPBA 12465 and CPBA 12466, right and left valves, respectively. Morra (Reference Morra1985) designated a right valve, CPBA 12465, as the holotype and the left valve of the same specimen, CPBA 12466, as a paratype. As they constitute a single articulated specimen, they are both part of the holotype; from Cañadón El Lobo (Santa Cruz Province), San Julián Formation (early Miocene).
Diagnosis
Shell medium sized with one growth ledge. Left valve with nine simple plicae of two widths and right valve with eight paired plicae. Plicae covered with numerous ribs of equal width that bifurcate twice on right valves, covered with scales that appear at 20–40 mm height from beaks.
Occurrence
Monte León Formation (early Miocene) at Punta Guanacos and Estancia Darwin (= Canadón Darwin) and San Julián Formation (early Miocene) at Estancia El Lobo (Punta Casamayor).
Description
Shell medium sized, up to 75 mm high, thin, subcircular in outline, higher than long or almost as long as high, right valve slightly more convex, opisthocline and posteriorly elongate; dorsal margins of disc concave, strongly inclined ventrally. Hinge dorsal margin straight, 45%–53% of total disc length. Both valves with one growth ledge at 20–40 mm from beaks. Umbonal angle 86°–93°. Auricles asymmetrical, moderately large, and with free margins concave, sloping anteriorly; right anterior auricle with dorsal margin straight, not projected upward, free margin rounded; byssal notch rectangular, moderately deep; ctenolium functional throughout ontogeny, with four or five strong teeth; byssal fasciole wide with corrugations convex toward umbo; byssal sinus very shallow. Right auricles sculptured with 7–10 radial ribs, left auricles with 10–12 ribs; ribs on right anterior auricle thicker than those on remaining auricles. Disc sculptured with wide plicae of subrectangular section that flatten toward ventral margin, interspaces each narrower than one plica, both covered with ribs ornamented with low, ventrally concave scales that appear at 25–40 mm height from beaks, along with development of ledges; right interspaces with one central rib and two secondary riblets, left interspaces with one secondary and one or two tertiary riblets. Right valve sculptured with eight paired plicae, partially bifurcated in some specimens, ornamented with five to six ribs that bifurcate on tops of plicae at 4–5 mm and at 15–20 mm height from beaks, and two weakly defined single plicae on each side of each plica. Left valve with nine single plicae of two widths; wider plicae covered with six ribs, narrower plicae with three or four ribs. Entire surface of disc with antimarginal ridgelets that appear in radial stage; commarginal microsculpture absent; shagreen microsculpture restricted to a band situated between 2 and 40 mm from beaks on most specimens; commarginal lamellae restricted to initial radial stage to 2.5 mm height on left anterior auricle and on right posterior auricle of some specimens; coarse commarginal corrugations covering right anterior auricle; left auricles with shagreen on proximal area.
Materials
Four articulated specimens, six right valves and four left valves MACN-Pi 6404; 6407; CPBA 16373–16374; 21585, PRI 66414.
Dimensions (in mm)
Holotype CPBA 12465, L = 59.8, H = 60.9; CPBA16373, L = 52.2, H = 56.0; CPBA16374 L = 49.3, H = 51.3.
Remarks
This species was originally described from an articulated specimen from Estancia El Lobo and included in the genus Zygochlamys by Morra (Reference Morra1985), who designated only the right valve as the holotype.
Analysis of the right valve illustrated by Ortmann (Reference Ortmann and Scott1902, pl. 23, fig. 2d) from Estancia Darwin identified as Pecten geminatus quemadensis showed that it is a specimen of Chokekenia nicolasi and allows us to expand the range of C. nicolasi to the early Miocene Monte León Formation exposed in the Austral Basin.
Pixiechlamys new genus
Type species
Pecten quemadensis Ihering, Reference Ihering von1897. Early Miocene, Monte Léon, San Julián, and Chenque formations, by monotype.
Diagnosis
As for type species.
Occurrence
Early Miocene of Austral and Golfo San Jorge Basins
Etymology
From pixie, a word of probable Celtic origin, meaning dwarf, mythical creatures, in reference to the small shells of the genus.
Remarks
Pixiechlamys n. gen. is here proposed to include the small Miocene species P. quemadensis (Ihering, Reference Ihering von1897) n. comb., well differentiated from other Patagonian taxa in having nonfolded shell, which is sculptured with ribs.
Pixiechlamys n. gen. is characterized by having shells with one or two growth ledges (Fig. 8.8, 8.11–8.13) sculptured with very fine ribs covered with low, closely packed scales instead of the ribbed plicae ornamented with prominent scaly ribs as in Zygochlamys. In addition, Pixiechlamys n. gen. has a smaller, more circular disc, more asymmetrical auricles, a smaller posterior auricle, a strongly sinuous free margin of the left anterior auricle, and a deeper byssal sinus than Zygochlamys.
Pixiechlamys n. gen. differs from Chokekenia n. gen. in having smaller shells sculptured with primary and secondary ribs covered with scales that arise earlier in ontogeny, up to two growth ledges, a narrower umbonal angle, more asymmetrical auricles, and a markedly deeper byssal notch and byssal sinus.
Pixiechlamys n. gen. is distinguished from Swiftopecten Hertlein, Reference Hertlein1936 (type species Swiftopecten swiftii [Bernardi, Reference Bernardi1858], Recent, Japan) in having more-circular shells, a deeper byssal sinus, valves ornamented with ribs of different orders that lack nodes, and weaker and less frequent growth ledges.
This new genus could be confused with small Australian and New Zealand species of Talochlamys Iredale, Reference Iredale1929, which have more circular shells, sculptured with primary, secondary, and tertiary ribs in some species, and a deep byssal sinus. Beu and Darragh (Reference Beu and Darragh2001, p. 97) also indicated the presence of shagreen microsculpture in some specimens of T. eyrei (Tate, Reference Tate1886) as also happens in Pixiechlamys n. gen. However, remarkable differences separate the two genera, such as the presence in Pixiechlamys n. gen. of more-triangular, ledged shells and microsculpture represented by fine commarginal lirae at the beginning of the radial stage rather than by coarse commarginal lamellae as in Talochlamys. Pixiechlamys n. gen. also has a much shorter preradial stage, more-obvious and frequent bifurcations of ribs on the right valve, and many rib intercalations on both valves, and the interspaces are ornamented with secondary ribs from the initial radial stage; in Talochlamys, when present, secondary ribs appear in late ontogeny. Some specimens of T. eyrei have superimposed secondary ribs on the sides of the primary ribs, but they arise as intercalations on both valves and later in ontogeny than in Pixiechlamys n. gen.
Pixiechlamys quemadensis (Ihering, Reference Ihering von1897) new combination
Figures 8.8–8.13, 9.1
- 1897
Pecten quemadensis Ihering, p. 228, pl. 6, fig. 38.
- 1907
Myochlamys quemadensis; Ihering, p. 256.
- 1985
Zygochlamys quemadensis; Morra, p. 303.

Figure 9. (1) Pixiechlamys quemadensis (Ihering, Reference Ihering von1897) n. comb., SEMs of an entire right valve, MACN-Pi 271 from Yegua Quemada, Monte León Formation showing shagreen, commarginal, and antimarginal microsculpture; (2–4) Moirechlamys actinodes (Sowerby, Reference Sowerby and Darwin1846) n. comb, from Puerto Madryn Formation, SEMs of left valve: (2) CPBA 15112, from Eje Tentativo, showing antimarginal and commarginal microsculpture in radial initial stage; (3, 4) CPBA 15118, from Eje Tentativo, showing antimarginal microsculpture, poorly preserved in the preradial stage. (1, 4) Scale bars = 200 µm; (2, 3) scale bars = 500 µm.
Type specimens
Holotype MACN-Pi 272, a right valve from Las Cuevas (Santa Cruz Province), Monte León Formation (early Miocene).
Diagnosis
Shell small and equiconvex. Posterior auricles tiny. Byssal sinus very deep. Valves sculptured with bunches of up to three fine ribs.
Occurrence
Monte León Formation (early Miocene) at Las Cuevas and Yegua Quemada; Chenque Formation (early Miocene) at Curva la Calera, Punta Maqueda, Punta Delgada, Rada Tilly, Baliza Villegas, Punta Borjas, and Cerro Chenque; and San Julián Formation (early Miocene) at Punta Nava.
Description
Shell small, up to 52 mm high, thin, subcircular in outline, higher than long, equiconvex, acline or slightly prosocline, and elongate posteriorly; dorsal margins of disc concave and steeply inclined ventrally. Hinge dorsal margin straight, 51%–61% of total disc length. Umbonal angle 84°–94°. Auricles strongly asymmetrical, anterior auricles up to four times as long as posterior auricles, which are tiny; auricles with free margins concave and sloping anteriorly, except for that of left anterior auricle, which is strongly sinuous; right anterior auricle with dorsal margin straight, not projected upward, free margin rounded; byssal notch rectangular, very deep, moderately wide, with functional ctenolium throughout ontogeny, with five or six strong teeth; byssal fasciole narrow with corrugations convex toward umbo; byssal sinus very deep. Right auricles sculptured with 5–7 radial ribs, left anterior auricles with 8–14 ribs, left posterior auricles with 7–9 ribs; anterior auricles with thicker ribs than on posterior auricles. Disc sculptured with low, narrow, rounded radial ribs covered with closely packed ventrally concave scales, with interspaces each narrower than one rib; ribs covered with three orders of riblets appearing at 3–10 mm, 16–25 mm, and 40–50 mm height from beaks. Both valves of most specimens with one or two growth ledges. Right valve sculptured with 12–16 primary ribs that bifurcate at 1–2 mm and at 4–19 mm height from beaks, most of them clustered into groups of 2 or 3 ribs, reaching a total of 60 ribs near ventral margin of adult specimens. Left valve ornamented with up to 70 ribs of two orders that become equal in width in adults. Antimarginal, shagreen, and commarginal microsculptures developed on both valves; antimarginal ridgelets appear at beginning of radial stage, expanding over entire surface; shagreen microsculpture present in one or two bands between 4–10 mm and 30–40 mm height from beaks; commarginal sculpture developed from initial radial stage to 3 mm height from beaks; antimarginal sculpture present on posterior auricles, commarginal sculpture on right anterior auricle, in proximal area of left anterior auricle, and in some specimens, on right posterior auricle.
Materials
Two articulated specimens, 16 right valves, and 13 left valves: MACN-Pi 271, MACN-Pi 6159, MACN-Pi 6400, MACN-Pi 6403, MACN-Pi 6017; MLP 2421; MLP 2998, MLP 8448; CPBA 8516, CPBA 21581, and CPBA 23621–23623.
Dimensions (in mm)
Holotype MACN-Pi 272, L = 31.0, H = 36.0; MLP 8448, L = 37.6 , H = 41.2; MLP 2998, L = 19.0, H = 22.7; MLP 8448, L = 37.6, H = 41.2; MLP 2998, L = 27.5, H = 28.0.
Remarks
Ihering (Reference Ihering von1897) described this species based on a right valve from Las Cuevas (Santa Cruz Province, Monte Léon Formation, early Miocene) and suggested that Pixiechlamys quemadensis n. comb. and Pecten nodosoplicatus Ihering, Reference Ihering von1897, today placed in Swiftopecten iheringii del Río, Reference del Río1995, could be one variable species. However, in 1907 he considered them to be separate taxa.
Ortmann (Reference Ortmann and Scott1902) considered Pixiechlamys quemadensis n. comb. to be a variety of Pecten geminatus and illustrated one specimen from Cañadón Darwin (Ortmann, Reference Ortmann and Scott1902, pl. 23, fig. 2d), but that specimen is herein considered to be Chokekenia nicolasi n. comb. (Morra, Reference Morra1985).
Moirechlamys new genus
Type species
Pecten actinodes Sowerby, Reference Sowerby and Darwin1846. Late Miocene, Puerto Madryn, Gran Bajo del Gualicho and Barranca Final formations and ‘Cabo Buentiempo Beds.’
Included species
Moirechlamys aurorae (Feruglio, Reference Feruglio1954). Early Pliocene ‘Araucanense Beds.’
Diagnosis
Shell large, left-convex, opisthocline, elongate anteriorly. Umbonal angle 90°–115°. Byssal notch deeper than in other Patagonian Chlamydini. Left anterior auricles with 21–30 radial ribs and posterior auricles with 13–25 radial ribs. Both valves sculptured with 24–44 simple plicae separated by interspaces narrower than those of Zygochlamys.
Occurrence
Late Miocene from the Peninsula Valdés area (Chubut Province), Barranca Final and Salinas del Gualicho (Río Negro Province), and Río Gallegos area (Santa Cruz Province); early Pliocene from ‘Araucanense Beds’ (Santa Cruz Province).
Etymology
From the Moiré optical effect in reference to the pattern of ornamentation.
Remarks
Moirechlamys n. gen. develops very large and convex shells for the tribe Chlamydini, with subcircular or flabelliform, opisthocline adult discs, with a very narrow right anterior auricle and valves sculptured with a large number of single plicae, of variable height, covered with small, low scales that extend over the entire surface except for the umbonal area and rather frequent shagreen microsculpture on both valves. There is a trend toward the elongation of the anterior auricles from the late Miocene M. actinodes to the early Pliocene M. aurorae, which leads to the development of a deeper byssal notch and sinus in the Pliocene species.
The type species of this new genus, Pecten actinodes, has been assigned to Chlamys (Fleming, Reference Fleming1957; Beu, Reference Beu1985) and to Zygochlamys (Ihering Reference Ihering von1907; Waller, Reference Waller and Shumway1991; Beu, Reference Beu1995; Jonkers; Reference Jonkers2003; Griffin and Nielsen, Reference Griffin and Nielsen2008), but its distinctive characters show that it deserves to be included in a new genus.
Moirechlamys n. gen. is separated from Chlamys s. str. (type species: Chlamys islandica [Müller, Reference Müller1776], Arctic Sea and North Atlantic and North Pacific Oceans, early Pleistocene–Recent) in having nonfolded, larger, and left-convex shells, opisthocline discs that are elongated anteriorly, a longer hinge margin, and narrower and longer anterior auricles. In Chlamys, shagreen microsculpture is always present, at least on right valves; in Moirechlamys n. gen. it is present less frequently.
Moirechlamys n. gen. can be distinguished from Zygochlamys by having more-inflated, left-convex shells, circular or flabelliform in shape, with opisthocline, anteriorly elongate adult discs, a narrower and longer right anterior auricle, a deeper byssal notch, posterior auricles ornamented with up to 25 riblets, and shagreen microsculpture more frequently extending to the ventral margin on right valves. Moreover, species of Moirechlamys n. gen. are sculptured with almost the same number of unpaired plicae covered with fewer and smaller scales, whereas those of Zygochlamys differ strongly in the number of plicae. Moirechlamys n. gen. differs from Pixiechlamys n. gen. in having larger, opisthocline shells without ledges, a shallower byssal sinus ornamented with lower and much more numerous plicae, covered with fewer ribs, and shagreen microsculpture extending further over the disc surface. Finally, Moirechlamys n. gen. differs from Chokekenia n. gen. in having larger shells without ledges, a larger byssal notch, and byssal sinus, sculptured with numerous plicae covered with narrower and lower ribs of different sizes. In addition, the shagreen microsculpture extends over more of the disc than in Chokekenia n. gen.
Moirechlamys actinodes (G.B. Sowerby I, Reference Sowerby and Darwin1846) new combination
Figures 9.2–9.4, 10.1–10.11
- 1846
Pecten actinodes Sowerby in Darwin, p. 253, 376, pl. 3, fig. 33.
- 1897
Pecten actinodes; Pilsbry, p. 329.
- 1902
Pecten actinodes; Ortmann, p. 119, pl. 24, fig. 1a, b.
- 1921
Chlamys Theresinae Rovereto, p. 27, fig. 12 a.
- 1957
Chlamys (Zygochlamys) actinodes; Fleming, p. 15.
- 1985
Chlamys actinodes; Beu, p. 7.
- 1992
Chlamys actinodes; del Río, p. 27, pl. 5, figs. 1–4, pl. 6, figs. 1, 2, text figs. 5, 6, 7a, b, 8a–e.
- 1995
Zygochlamys actinodes; Beu, p. 15.
- 2000
‘Chlamys’ actinodes; del Río, fig. 10.2.
- 2002
‘Chlamys’ actinodes; Martínez and del Río, fig. 13.1.
- 2003
Zygochlamys actinodes; Jonkers, p. 39, pl. 6, figs. A, b.
- 2008
Zygochlamys actinodes; Griffin and Nielsen, p. 257, pl. 1, fig. 1.
- 2010
Zygochlamys rizzoloi Reichler, p. 201, pl. 2, figs. 1–6.

Figure 10. Moirechlamys actinodes (Sowerby, Reference Sowerby and Darwin1846) n. comb. (1) External view of left valve, holotype MHM-L 27969, from “San José,” Puerto Madryn Formation; (2) external view of right valve, CPBA 7832, from Lobería Punta Pirámides, Puerto Madryn Formation; (3) exterior view of a left valve, CPBA 13745, from Puerto San José, Puerto Madryn Formation; (4–6) articulated specimen, CPBA 14421, from Punta Quiroga, Puerto Madryn Formation: (4) anterior view, (5) external view of right valve, (6) external view of left valve; (7) external view of right valve, holotype of Zygochlamys rizzoloi (Reichler, Reference Reichler2010) MACN-Pi 4794, from Salinas del Gualicho, Gran Bajo del Gualicho Formation; (8) external view of left valve, holotype of Z. rizzoloi (Reichler, Reference Reichler2010) MACN-Pi 4795, from Salinas del Gualicho, Gran Bajo del Gualicho Formation; (9) detail of microsculpture, MACN-Pi 4796a, from Salinas del Gualicho, Gran Bajo del Gualicho Formation; (10) external view of right valve, MACN- Pi 235a, from “Cabo Buentiempo Beds”; (11) external view of left valve, MACN- Pi 235b, from “Cabo Buentiempo Beds.” (1–8, 10, 11) Scale bar = 10 mm (graphed at the right end of the figure); (9) scale bar = 5 mm (below the figured specimen).
Type specimens
Lectotype of Pecten actinodes Sowerby, Reference Sowerby and Darwin1846, NHM-L27960, one left valve from ‘San José Port,’ Puerto Madryn Formation (late Miocene). Holotype of Zygochlamys rizzoloi Reichler, Reference Reichler2010 MACN-Pi 4794, one right valve from Salinas del Gualicho, upper part of the Gran Bajo del Gualicho Formation (late Miocene).
Emended diagnosis
Shell large, flabelliform, longer than high or as high as long in adult stages. Umbonal angle 100°–115°. Hinge margin 49%–55% of total disc length. Free margin of left anterior auricle concave. Byssal sinus shallow. Left posterior auricle with 16 to 25 radial ribs. Shell sculptured with wide, entire plicae.
Occurrence
Puerto Madryn Formation (late Miocene) at Punta Quiroga, Cerro Avanzado, El Doradillo, Cerro Prismático, Eje Tentativo, Punta Logaritmo, Punta Pardela, Punta Tehuelche, Puerto San José, Puerto Pirámides, Lobería Punta Pirámides, Lote 39, and San Román; Gran Bajo del Gualicho Formation (late Miocene) at Salinas del Gualicho (Río Negro Province); Barranca Final Formation (late Miocene) at Barranca Final (Río Negro Province) and ‘Cabo Buentiempo Beds’ (late Miocene) at Cabo Buentiempo, on the coast north of Río Gallegos (Santa Cruz Province).
Description
Shell of very large size, attaining height of 136 mm, flabelliform, as high as long as or longer than high in adult stages, inflated, left valve markedly more convex than right valve, opisthocline, elongate anteriorly and with anterodorsal margin more concave than posterior margin. Hinge dorsal margin straight, 49%–55% of total disc length. Umbonal angle 100°–115°. Auricles asymmetrical, large, with free margins sloping anteriorly; right anterior auricle with dorsal margin projected slightly upward, free margin rounded or straight; byssal notch rectangular or subrounded, deep, high, with ctenolium functional throughout ontogeny, with 4–7 strong teeth; byssal fasciole with corrugations convex toward umbo; free margins of right posterior auricles straight in most specimens, convex in a few, that of left anterior auricle sinuous or concave, with a moderately shallow byssal sinus. Right anterior auricle sculptured with 6–10 radial ribs, left anterior auricle with 22–30 ribs, right posterior auricle with 15–20 ribs, left posterior auricle with 16–25 ribs; dorsal ribs on right auricles thickened. Exterior sculpture of disc of 24–44 simple, rounded or flattened plicae, flattening toward ventral margins, variable in height, separated by equally wide or narrower interspaces, plicae on both valves sculptured with one central primary rib and four secondary and tertiary riblets, all covered with ventrally directed low scales, scales becoming larger and more numerous toward ventral and lateral margins of disc; some plicae on right valve bifurcate at commencement of radial stage. Entire surface of disc covered with coarse antimarginal ridgelets that appear in preradial stage; commarginal microsculpture represented by fine lirae restricted to a band situated 1.5–3 mm from beaks; shagreen microsculpture extended over entire disc from initial radial stage or restricted to umbonal zone. Auricles of both valves covered with antimarginal and shagreen microsculpture; commarginal sculpture of coarse lamellae restricted to initial radial stage and up to 8 mm height on left anterior auricle, and coarse corrugations covering right anterior auricle.
Materials
Sixteen articulated specimens, 236 right valves and 244 left valves: CPBA 7831–7832, CPBA 9330, CPBA 9333, 11417, 11553–11585, 11602–11604,11621, 11623–11625, 13337, 13341, 13381–13422, 13549–13560, 13586–13607, 13701–13745, 13747–14355, 14397–14435, 14768, 15111–15166, 15168 a–i, 15322, 15324, 15326–15332, 15334–15342, 15533–15546, 17398; 17850–17864; MACN-Pi 235, 3927, 4794–4795, 4931 a–ñ, 4796, 4932 a–o, 5390, 6410, 8414, 5737, 5810a–b, 5811 a–c; MLP 2444, 2640, 3029, 3932, 5172, 5333, 7091, 7658, 10337; PRI 66426, and PRI 66450.
Dimensions (in mm)
Lectotype NHM-L 27960, L = 60.5, H = 62.6; CPBA15115, L = 46.4, H = 52.0; CPBA14424, L = 114.2, H = 110.0; CPBA14421, L = 122.9, H = 121.0; CPBA7831b, L = 139.0, H = 136.6; MACN-Pi4931b, L = 89.4, H = 88.3; MACN-Pi4932a, L = 79.0 H = 78.9; CPBA9330a L = 28.9, H = 33.3; MLP 5333, L = 122.7, H = 122.3; MLP 7091, L = 78.0, H = 81.0.
Remarks
Sowerby (Reference Sowerby and Darwin1846, pl. 1, fig.1) illustrated a left valve collected by Charles Darwin from the Puerto Madryn Formation exposed at Puerto San José (Chubut Province). It was identified as the holotype by del Río (Reference del Río1992, p. 29, fig. 7a, b), who provided a diagnosis of the species. However, Griffin and Nielsen (Reference Griffin and Nielsen2008) recognized that the type material consisted of three syntypes, so Sowerby's (Reference Sowerby and Darwin1846) illustrated specimen was designated as the lectotype and is accompanied by two paralectotypes.
Moirechlamys actinodes n. comb. is restricted to late Miocene time, being represented in the Puerto Madryn Formation, the upper part of the Gran Bajo del Gualicho Formation, and the Barranca Final Formation. Ortmann (Reference Ortmann and Scott1902) was the first author to mention its occurrence in the late Miocene at ‘Cabo Buentiempo Beds’ (= Cape Fairweather Beds) (coast north of Río Gallegos, Santa Cruz Province). Although del Río (Reference del Río1992) considered that Ortmann's (Reference Ortmann and Scott1902) material could not be Pecten actinodes, the present analysis confirms that it does belong in M. actinodes n. comb. because shells from Cabo Buentiempo have flabelliform discs with an umbonal angle of 100°–115° as in M. actinodes n. comb., and the shape and size of the auricles with a shallow byssal sinus, the length of the hinge margins, and the sculptural pattern allow us to place the material from Cabo Buentiempo in M. actinodes n. comb. This species was mentioned by Ihering (Reference Ihering von1897, Reference Ihering von1907) as occurring in the early Pliocene ‘Araucanense Beds’ exposed at Bajo La Pava, Estancia Santa Rosa, and Monte Espejo. However, those specimens, as del Río (Reference del Río1992) suggested, do not belong in M. actinodes n. comb. and are included here in Moirechlamys aurorae n. comb. (Feruglio, Reference Feruglio1954) (see the following).
Reichler (Reference Reichler2010) proposed Zygochlamys rizzoloi for specimens from Salinas del Gualicho (Río Negro Province) (upper part of the Gran Bajo del Gualicho Formation, late Miocene) (Fig. 10.7–10.9), but the presence of opisthocline, flabelliform, and left-convex shells with a shallow byssal sinus, ornamented with 26–42 plicae, each covered with up to five riblets, indicates that Z. rizzoloi is a synonym of M. actinodes n. comb.
Del Río (Reference del Río1992) and del Río et al. (Reference del Río, Santelli and Márquez2016) demonstrated the presence of two well-differentiated morphotypes, one with valves strongly ornamented with high plicae (Fig. 10.4–10.6) from the Transgressive Phase of the Puerto Madryn Formation, and the other with low plicae (Fig. 10.1–10.3) recorded from the Highstand and Regressive Phases. This intraspecific variation is associated with changes in hydraulic energy. The material corresponding to Moirechlamys actinodes n. comb. (Sowerby, Reference Sowerby and Darwin1846) from ‘Cabo Buentiempo Beds’ has low plicae.
Moirechlamys aurorae (Feruglio, Reference Feruglio1954) new combination
Figure 11.1–11.5
- 1897
Pecten actinodes Sowerby; Ihering, p. 277
- 1902
Pecten actinodes Sowerby; Ortmann, (in part) p. 119.
- 1907
Myochlamys actinodes (Sowerby); Ihering, p. 408.
- 1933
Myochlamys actinodes Ihering; Feruglio, p. 138, pl. 8, fig. 21.
- 1954
Chlamys aurorae Feruglio, p. 5, 10.
- 1954
Chlamys cf. aurorae Feruglio, p. 9, pl. 1, figs. 7a, b, 8.

Figure 11. Moirechlamys aurorae (Feruglio, Reference Feruglio1933) n. comb., from Cerro Laciar, “Araucanense Beds.” (1, 2) Lectotype MGGC 21980a, a right valve: (1) external view, (2) hinge; (3) external view of right valve, MACN-Pi 239a; (4) external view of right valve, MACN-Pi 239 b; (5) external view of left valve MACN-Pi 239c. Scale bar = 10 mm.
Type specimens
Lectotype designated here, a right valve illustrated by Feruglio (Reference Feruglio1933), MGGC-21980a, paralectotypes, three right valves and two left valves, MGGC-21980b–f from the ‘Araucanense Beds’ exposed at Cerro Laciar (early Pliocene).
Diagnosis
Shell subcircular. Umbonal angle 89°–93°. Hinge margins 58%–68% of total disc length. Right anterior auricle extends to distal anterior end of disc. Byssal notch deep. Free margin of left anterior auricle strongly sinuous. Left posterior auricle with 13–16 radial ribs. Plicae on right valve grooved in early ontogeny, bifurcated twice in adult stages.
Occurrence
'Araucanense Beds’ (early Pliocene) at Cerro Laciar–Bajo de la Pava, Cañadón Darwin, Estancia Santa Rosa, and Cerro Espejo (Santa Cruz Province).
Description
Shell large, reaching 105 mm high, subcircular, higher than long, left-convex, opisthocline, anteriorly elongate, with anterodorsal margin concave, posterodorsal margin straight. Hinge dorsal margin straight, 58%–68% of total length of disc. Umbonal angle 89°–93°. Auricles highly asymmetrical, large, with free margins sloping anteriorly; anterior auricles elongate, anterior extremities extending to anterior extremities of disc; right anterior auricle with dorsal margin projected upward, free margin rounded or straight; byssal notch deep, rectangular or rounded, with ctenolium functional throughout ontogeny, with five or six strong teeth; byssal fasciole with corrugations convex toward umbo; free margins of posterior auricles slightly concave, straight in a few specimens; free margin of left anterior auricle strongly sinuous, with very deep byssal sinus. Right anterior auricle sculptured with 7–9 radial ribs, left anterior auricle with 22–26 ribs, right posterior auricle with 14–20 ribs, left posterior auricle with 13–16 ribs; dorsal ribs on right anterior auricle thickened. Exterior sculpture of disc of 36–44 simple, narrow, rounded plicae and interspaces, rectangular in section in a few specimens, both bearing a central primary rib and riblets of different orders; left valve plicae wider than or the same width as interspaces, both bearing one primary rib and two secondary riblets; plicae on right valve wider than interspaces, grooved in early ontogeny, later bifurcated and bearing one primary rib and secondary and tertiary riblets ornamented with small, low scales directed ventrally. Antimarginal ridgelets over entire disc surface; commarginal microsculpture absent; shagreen microsculpture over plicae and interspaces, extending from 8 mm from beak to ventral margin on right valve, restricted to a band between 5 mm and 35 mm from beaks on left valve. Auricles of both valves covered with antimarginal microsculpture, commarginal microsculpture represented by coarse corrugations over anterior right auricle and on initial radial stage of left anterior auricle up to 3 mm height; shagreen microsculpture developed over left anterior and right posterior auricles of some specimens.
Materials
Thirty right and 23 left valves: MACN-Pi 232–234, 236–239, 6405, 6295–6297; MGGC-21980b–f.
Dimensions (in mm)
Holotype MGGC-21980a L = 65.6, H = 72.9; MACN-Pi 239, L = 53.1, H = 58.5; MACN-Pi 239, L = 63.0, H = 70.5; MACN-Pi 239, L = 55.5, H = 59.0; MACN-Pi 233, L = 98.9, H = 105.2; MACN-Pi 232, L = 97.1, H = 100.0; MACN-Pi 232, L = 92.1, H = 99.6.
Remarks
Material studied herein was considered previously to belong in Pecten actinodes Sowerby, Reference Sowerby and Darwin1846 (Ihering, Reference Ihering von1897, Reference Ihering von1907; Feruglio, Reference Feruglio1933). The right valve illustrated by Feruglio (Reference Feruglio1933, pl. 8, fig. 21, MGGC-21980a) was later placed by that author in a new species, Myochlamys aurorae n. comb., illustrated by two fragments of shells (Feruglio, Reference Feruglio1954, pl. 1, figs. 7a, b, 8). Although Feruglio (Reference Feruglio1954) did not specify it, the type material of M. aurorae n. comb. corresponds to the right valve illustrated in 1933 that is here designated as the lectotype.
Moirechlamys aurorae n. comb. differs from M. actinodes n. comb. in having smaller, subcircular, higher than long shells, a longer hinge margin, a narrower umbonal angle, and more-asymmetrical auricles, with longer anterior ones. The byssal sinus and byssal notch are deeper, the free margin of the left anterior auricle is strongly sinuous, and the left posterior auricle is covered with more ribs than in M. actinodes n. comb. Moirechlamys aurorae n. comb. is also distinguished by the development of narrower plicae; those on right valves bifurcate more than once and later in ontogeny than those of M. actinodes n. comb.
Conclusions and discussion
Representatives of the tribe Chlamydini Teppner, Reference Teppner and Diener1922 were the most abundant components of the late Paleogene–Neogene molluscan faunas of Patagonia, where they are recorded in the Austral, Golfo San Jorge, Valdés, and Colorado basins. The group is represented by Zygochlamys Ihering, Reference Ihering von1907, Pixiechlamys n. gen., Chokekenia n. gen., and Moirechlamys n. gen., constituted by seven species.
According to accurate stratigraphic information based on numerical ages obtained recently (Parras et al., Reference Parras, Dix and Griffin2012; del Río et al., Reference del Río, Griffin, McArthur, Martínez and Thirlwall2013; Cuitiño et al., Reference Cuitiño, Scasso, Ventura Santos and Mancini2015) for Neogene sedimentary rocks, the oldest occurrence of the Patagonian Chlamydini is late Oligocene (Zygochlamys geminata) while the youngest is early Pliocene (Moirechlamys aurorae n. comb.). This tribe strongly increased its species richness during the Miocene, when it reached its maximum peak in the early Miocene of the Golfo San Jorge Basin and was represented by four Chlamydini species alone, those studied here plus representatives of other Chlamydini such as Reticulochlamys del Río, Reference del Río2004a and Jorgechlamys del Río, Reference del Río2004a.
Pectinids have been shown to be excellent biostratigraphic tools in the Neogene horizons of Patagonia (del Río, Reference del Río1988; Reference del Río2004b), and the updated knowledge of the composition of the tribe Chlamydini presented here allows us to improve their usefulness for a better characterization of the Molluscan Assemblages defined by del Río (Reference del Río2004b).
The most abundant species are Zygochlamys geminata, Z. jorgensis, and Moirechlamys actinodes n. comb. As shown in Table 1, while Z. geminata is restricted to the southern sector of the Austral basin where it is a guide fossil of the Panopea sierrana–Parinomya patagonensis (PP; late Oligocene) and Reticulochlamys zinsmeisteri–Struthiolarella paragonensis–Pleuromeris cruzensis (RSP; early Miocene) assemblages, Z. jorgensis constitutes a guide fossil of the Jorgechlamys centralis–Reticulochlamys borjasiensis (JR) and Nodipecten sp.–Venericor abasolensis (NVG) assemblages (early–middle Miocene) restricted to the Golfo San Jorge and northern Austral Basins. The genus Moirechlamys n. gen. appeared in the late Miocene and characterizes the Aequipecten paranensis Biozone, having its last occurrences in the early Pliocene. Zygochlamys sebastiani and Chokekenia nicolasi n. comb. are rather infrequent species, and both occur in the Austral and Golfo San Jorge basins, indicating the base of the RSP Assemblage. Pixiechlamys quemadensis n. comb. is also an uncommon species and is the only pectinid taxon in the Pseudoportlandia glabra–Antimelatoma quemadensis (PA) Assemblage. While the latter species and C. nicolasi n. comb. are restricted to early Miocene sedimentary rocks (RSP and JR assemblages), Z. sebastiani extended its temporal range to the middle Miocene (NVG Assemblage).
Table 1. Biostratigraphy of the tribe Chlamydini in the Patagonian basins. PP = Panopea sierrana–Parinomya patagonensis Assemblage; RSP = Reticulochlamys zinsmeisteri–Struthiolarella patagonensis–Pleuromeris cruzensis Assemblage; PA = Pseudoportlandia glabra–Antimelatoma quemadensis Assemblage; JR = Jorgechlamys centralis–Reticulochlamys borjasiensis Assemblage; NVG = Nodipecten sp.–Venericor abasolensis–Glycymerita camaronesia Assemblage.
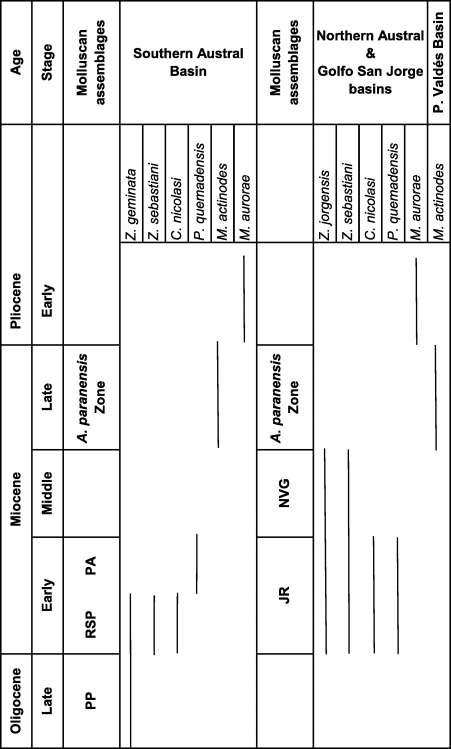
According to the present analysis, Zygochlamys is endemic to the southernmost tip of South America, not being related to other circumpolar genera such as Austrochlamys Jonkers, Reference Jonkers2003 and Psychrochlamys Jonkers, Reference Jonkers2003 (P. patagonica [King, Reference King1832]–P. delicatula [Hutton, Reference Hutton1873] group), precluding its circumpolar dispersal through the Antarctic Circumpolar Current as previously proposed (Beu, Reference Beu1985; Beu et al., Reference Beu, Griffin and Maxwell1997). It appeared in the late Oligocene of southern Patagonia and dispersed during the early Miocene (Navidad and Guadal formations), probably through the Drake Passage or northward, at the latitude of Central Chile, as evidenced by the paleogeographical reconstruction proposed by Malumián and Nañez (Reference Malumián and Náñez2011) for late Oligocene–early Miocene times.
Acknowledgments
The authors are in debt to A. Beu for improving the English language of the paper and for his insightful comments and valuable suggestions.
We acknowledge with thanks S. Martínez and D. Perez for their help in one of the field trips; the curators M. Tanuz (Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina), M. Longobucco (Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia,’ Buenos Aires, Argentina), A. Riccardi (Facultad de Ciencias Naturales y Museo, La Plata, Argentina), D. Rubilar (Museo Nacional de Historia Natural de Chile, Santiago de Chile), and C. Sarti (Universidad de Bolonga, Italy) for permitting access to fossil collections; and F. Tricárico for taking the SEM images.
M. Griffin provided material from Cabo Buentiempo, and G. Parma provided geographic information on some localities in the Golfo de San Jorge.
Ae are also grateful to the editors J. Jin and J. Kastigar and to the anonymous reviewer for their constructive and thoughtful comments.
This paper has been supported by PIP 1649 and PIP 320 (Consejo Nacional de Investigaciones Científicas y Técnicas) and PICT–ANPCyT 57 (Agencia Nacional de Promoción Científica y Técnica) to CDR.














