Introduction
Parasitic nematodes in small ruminants and other livestock can have major economic impacts on animal production worldwide. Host parasitism results in lower levels of weight gain, interference with food intake, delayed reproductive age and even death in severely affected animals (Hansen and Perry, Reference Hansen and Perry1994). Meanwhile, there is a significant economic burden related to the cost of anthelmintic treatments to control parasites (McLeod, Reference McLeod1995). In Brazil, sales in the animal health industry reached nearly US$1.5 billion in 2018, 53% of which was spent on ruminants and 29% on antiparasitic agents (Sindan, Reference Sindan2018).
Commercial anthelmintics are most commonly used to control nematodiasis. Anthelmintic resistance among ruminant nematodes, however, is undoubtedly one of the most serious challenges in the production of small ruminants, and in many countries, the presence of anthelmintic resistant nematodes in small ruminants has become the norm rather than the exception (Leathwick and Besier, Reference Leathwick and Besier2014). A review of studies on anthelmintic resistance of gastrointestinal nematodes of small ruminants in Brazil was conducted by Salgado and Santos (Reference Salgado and Santos2016) and showed research beginning in the 1960s in the South (sheep) and Northeast (goats), where livestock is economically significant, and in the Southeast (sheep), an important region for research and the economy.
Plants are an important resource that can provide alternatives to the conventional anthelmintic control methods of gastrointestinal nematodiasis (Hounzangbe-Adote et al., Reference Hounzangbe-Adote, Paolini, Fouraste, Moutairou and Hoste2005; Eguale and Giday, Reference Eguale and Giday2009; Kamaraj et al., Reference Kamaraj, Rahuman, Elango, Bagavan and Zahir2011; Ahmed et al., Reference Ahmed, Laing and Nsahlai2013, Reference Ahmed, Laing and Nsahlai2014; Santos et al., Reference Santos, Cerqueira, Branco, Batatinha and Botura2019). Plant-based therapies have numerous advantages over commercial anthelmintics, as they are biodegradable, cause no environmental damage, and have fewer residues (Chagas, Reference Chagas2004).
Cymbopogon citratus (Lemon grass), commonly called Capim Limão, Capim Cidreira, or Capim Santo in Brazil, is a herbaceous plant of the Poaceae family that is native to the tropical regions of Asia, especially India (Gupta and Jam, Reference Gupta and Jam1978). Cymbopogon citratus has been identified as a medicinal plant species that can be used with domestic animals, with the highest use value reported at Colares Island, Pará State, eastern Amazon, Brazil. Tea/macerated leaves of C. citratus in water or food have been indicated for the medicinal treatment of helminths in dogs (Ritter et al., Reference Ritter, Monteiro, Monteiro, Rodrigues, Soares, Silva, Md, Biondi, Rahal and Tourinho2012). The nematicidal activity of the plant has been analysed against the root-knot nematode (Oka et al., Reference Oka, Nacar, Putievsky, Ravid, Yaniv and Spiegel2000; Fabiyi et al., Reference Fabiyi, Olatunji, Adebayo and Atolani2018), pinwood nematode (Barbosa et al., Reference Barbosa, Lima, Vieira, Dias, Tinoco, Barroso, Pedro, Figueiredo and Mota2010) and gastrointestinal nematodes of small ruminants (Almeida et al., Reference Almeida, Botura, Santos, Domingues, Costa and Batatinha2003; Silva et al., Reference Silva, Brito, Marinho, Rodrigues and Athayde2005; Macedo et al., Reference Macedo, Oliveira, Ribeiro, Santos, Silva, AraújoFilho, CamurçaVasconcelos and Bevilaqua2015, Reference Macedo, Oliveira, André, Araújo Filho, Santos, Rondon, Ribeiro, Camurça-Vasconcelos, Oliveira, de Paula and Bevilaqua2019). However, to date, no study has examined the morphological effects of C. citratus on nematodes. This study analysed the chemical composition of the plant and assessed its nematicidal activity and morphological and ultrastructural alterations of C. citratus extract and fractions on eggs and third-stage, infective larvae (L 3) of Haemonchus spp. and Trichostrongylus spp. nematodes in sheep.
Materials and methods
Collection and identification of botanical material
Cymbopogon citratus (Lemon grass) was collected at the Research Support Unit of the Centro de Ciências Tecnológicas e Agropecuárias, Universidade Estadual do Norte Fluminense – CCTA/UENF (21°S, 41°W). A voucher specimen (H8225) was deposited in the UENF herbarium, Campos dos Goytacazes City, Rio de Janeiro State, Brazil. Plant names were verified with http://www.theplantlist.org (accessed April 2018).
Collection of nematode eggs and third-stage, infective larvae (L 3)
Feces were collected directly from the rectum of 15 naturally infected sheep and refrigerated until analysis. The animals belonged to a small sheep breeding farm in the municipality of Campos dos Goytacazes. Eggs per gram (EPG) of feces were determined from 4 g of feces per animal as described by Gordon and Whitlock (Reference Gordon and Whitlock1939). Feces were also cultivated to obtain L 3 as described by Bonadiman et al. (Reference Bonadiman, Ederli, Soares, Moraes Neto, Santos and DaMatta2006) and this larval stage was morphometrically identified at genera level as described by Van Wyk et al. (Reference Van Wyk, Cabaret and Michael2004) and Van Wyk and Mayhew (Reference Van Wyk and Mayhew2013).
Eggs were recovered from faecal samples with an EPG above 2000, as described by Bizimenyera et al. (Reference Bizimenyera, Githiori, Eloff and Swan2006). Briefly, the faecal samples were homogenized in distilled water and filtered through a series of sieves (24-, 48-, 80-, 100-, 200-, 270-, and 400-mesh), the last of which trapped the eggs in the 38 μm pores (400-mesh). This sieve was washed, and the eggs and other contents were transferred to 15 mL Falcon tubes, which were then centrifuged at 1600g for 5 min. This process was repeated with the addition of saline, and the supernatant was removed and filtered through a 400-mesh sieve to recover the eggs. The supernatant was discarded, and the eggs were washed liberally with distilled water to remove all traces of brine and then transferred to a conical sedimentation glass to settle for 2 h. Using a Pasteur pipette, the eggs were then retrieved and transferred to a Falcon tube.
Preparation of C. citratus crude extracts
Leaves of C. citratus were collected, chopped and dried in a greenhouse for 72 h at 30°C. Seventy grams of dried leaves were added to 2.5 L of methanol (MeOH) which was used for extraction. The solution was kept at room temperature for ten days, with the use of a rotary evaporator every 72 h, yielding 9.2 g of crude methanol extract. The crude MeOH extract was stored at 4°C until use. An aqueous extract was prepared from 63.3 g of dried leaves in 6 L of distilled water. The leaves were macerated for 24 h and then filtered, and the extract was frozen at −20°C and lyophilized. Two grams of lyophilized aqueous extract were obtained; the extracts were diluted in 3% Dimethyl sulfoxide (DMSO) to produce stock solutions.
In vitro tests of anthelmintic activity with C. citratus crude extracts
Stock solutions of 1.0 g mL−1 were used to test anthelmintic activity. Crude extracts at concentrations of 25.0, 12.5, 6.2, 3.1 and 1.6 mg mL−1 were used to assess larvicidal activity. Approximately 80 L 3 0.02 mL−1 were included in these tests. Crude extracts at concentrations of 50.0, 25.0, 12.2, 6.2 and 3.1 mg mL−1 and solutions of approximately 110 eggs 0.05 mL−1 were prepared for the hatching test. Negative controls were prepared using 3% DMSO. In vitro assays were performed in 24-well culture plates incubated at 27°C (L 3) and 30°C (eggs) for 48 h in a biochemical oxygen demand (BOD) incubator. To prevent further hatching, a drop of Lugol's solution was added to each well after 48 h (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992; Bizimenyera et al., Reference Bizimenyera, Githiori, Eloff and Swan2006). The number of hatched larvae and dead L 3 were counted using an inverted microscope. All assays were performed in triplicate.
Phytochemical analysis
The MeOH extract was submitted to liquid-liquid partition (MeOH/Hexane, 1:1, v/v). The crude MeOH extract was chromatographed over a silica gel column with a gradient of ethyl acetate/hexane, affording ten fractions. The crude MeOH extract and fractions were analysed by thin-layer chromatography and the first was also analysed by Gas Chromatography-Mass Spectrometry (GC-MS) with a GCMS-QP2010 Plus (Shimadzu, Kyoto, Japan), using a Factor Four/VF-5 ms column (30 m × 0.25 mm × 0.25 μm). The sample injection temperature was 250°C, with a split mode of injection. The sample was injected into the column at 1 mL min−1. Chloroform was used as the eluent in the column dilution of the MeOH extract. This analysis identified the compounds and per cent mass present in each sample using the GCMS-QP2010 Series Software.
The fractions were tested on eggs and larvae at concentrations of 31, 62, 125, 250, 500 and 1000 μg mL−1, as described above. Lethal concentration 50% (LC50) values were performed for the most active fractions of the MeOH extract.
Light and electron microscopy
For electron microscopy, samples were processed as described by Souza (Reference Souza2007). Briefly, the eggs and larvae were fixed for 24 h in 2.5% glutaraldehyde, 4% paraformaldehyde, 5 mm calcium chloride in 0.1 M cacodylate buffer, pH 7.2, post-fixed in 1% osmium tetroxide, 5 mm calcium chloride and 0.8 potassium ferrocyanide in 0.1 M cacodylate buffer. For s.e.m., the fixed eggs and L 3 were bonded on cover slips with the aid of poly-L-lysine to facilitate processing and visualization. The samples were dehydrated in an ethanol series, critical point dried with CO2, mounted in stubs, sputter-coated with gold and examined in a Zeiss DMS962 scanning electron microscope operating at 15 KV. For TEM, the samples were dehydrated in an acetone series, infiltrated in resin Spurr, polymerised in an oven at 60°C, slices of 0.5 μm or 70 nm thickness were obtained and stained with 1% toluidine blue or contrasted in uranyl acetate and lead citrate, respectively. Samples were then examined using a light microscope or a Zeiss 900 transmission electron microscope operating at 80 kV. To produce the control micrographs (untreated eggs), fresh eggs were collected and processed for electron microscopy within 3 hours after collection.
Statistical analysis
The analysis was performed based on generalized linear models, using the Poisson distribution and the GLIMMIX procedure in the Statistical Analysis System software (SAS System, Inc., Cary, NC, USA). In the case of significant difference, the Tukey test was applied.
Results
Faecal samples
Faecal samples revealed the presence of the Haemonchus and Trichostrongylus genera. Haemonchus spp. larvae were predominant (>85%).
In vitro anthelmintic action of C. citratus crude extract
The aqueous and MeOH extracts of C. citratus showed significant larvicidal (Fig. 1A and B) and ovicidal (Fig. 1C and D) activity at all evaluated concentrations, except for the aqueous extract at a concentration of 1.6 mg mL−1. Both extracts were active against Haemonchus spp. and Trichostrongylus spp. larvae.

Fig. 1. Larvicidal and ovicidal assay with Cymbopogon citratus crude extract. Mean number of infective larvae (L 3) dead or hatched after a 48 h interaction with different concentrations of aqueous (A, C) and methanolic (B, D) extract. Concentrations in A and B: 25.0 mg mL−1 (1); 12.5 mg mL−1 (2); 6.2 mg mL−1 (3); 3.1 mg mL−1 (4); 1.6 mg mL−1 (5); and negative control with distilled water (6). Concentrations in C and D: 50.0 mg mL−1 (1); 25.0 mg mL−1 (2); 12.5 mg mL−1 (3); 6.2 mg mL−1 (4); 3.1 mg mL−1 (5); and negative control with distilled water (6). *Means are significantly different when compared to 3% DMSO and distilled water (P < 0.001).
Chromatography and C. citratus fractions
Ten fractions (C. citratus methanolic fraction CCMF-01 to CCMF-10) were used in the assays of biological activity to determine the active fraction. The phytochemical analysis identified the presence of compounds such as terpenoids (monoterpenes and steroids), various ketones, esters and fatty acids. From the C. citratus MeOH extract (Table 1), 11 major compounds were identified through GC-MS: four monoterpenes (linalool, cis-citral, trans-citral and trans-geraniol); one diterpene (phytol); four steroids (campesterol, stigmasterol, α-sitosterol and β-sitosterol); one triterpene (cerin acetate); linoleic acid and α-tocopherol (Supplementary Fig. 1). Among these, cerin acetate, trans-geraniol and phytol were particularly abundant.
Table 1. Substances postulated (Supplementary Fig. 1) by GC/MS analysis of the methanol extract of C. citratus, including retention time (Rt), percentage (%) of each component and molecular formulas

Assay of biological action with C. citratus MeOH fractions
The fractions of C. citratus only showed ovicidal activity (Fig. 2), and mainly at concentrations of 1000 μg mL−1: CCMF-03 (73.3%), CCMF-04 (99.3%), CCMF-05 (89.2%), CCMF-07 (86.6%) and CCMF-08 (100%) (Fig. 2C, D, E, G, and H). No larvae hatched after treatment with the fractions CCMF-09 and CCMF-10 at concentrations ranging from 62 to 1000 μg mL−1 indicating 100% ovicidal activity (Fig. 2I and J). Fractions CCMF-08, CCMF-09 and CCMF-10 were the only fractions that differed significantly from the control at all concentrations evaluated, while CCMF-02 was the only fraction that not differ significantly from the control. LC50 values of the fractions CCMF-09 and CCMF-10 were ~12 and 26 μg mL−1, respectively (Supplementary Fig. 2).

Fig. 2. Ovicidal assay with fractions obtained from Cymbopogon citratus methanolic extract. Mean number of hatched larvae after 48 h interaction with fractions: CCMF-01 (A); CCMF-02 (B); CCMF-03 (C); CCMF-04 (D); CCMF-05 (E); CCMF-06 (F); CCMF-07 (G); CCMF08 (H); CCMF09 (I); CCMF10 (J). Concentrations: 1000 μg mL−1 (1); 500 μg mL−1 (2); 250 μg mL−1 (3); 125 μg mL−1 (4); 62 μg mL−1 (5); 31 μg mL−1 (6); 3% DMSO (7); and distilled water (8). *Means are significantly different when compared to 3% DMSO and distilled water (P < 0.001).
Effects of C. citratus extracts and fractions on the ultrastructure of L 3 and eggs
Several changes were observed in the ultrastructure and morphology of eggs and L 3 after treatment with C. citratus. The cuticular surface of L 3 treated with 25.0 mg mL−1 of aqueous and MeOH extracts showed a modified appearance (Fig. 3C–E) that differed from the control (Fig. 2A and B). The cuticle showed lesions in the larvae treated with both aqueous (Fig. 3C and D) and MeOH extracts (Fig. 3E and F). Untreated eggs showed typical surface morphology (Fig. 4A), while eggs treated with MeOH extract presented an irregular surface with several injuries (Fig. 4B and C).

Fig. 3. s.e.m. micrographs of infective larvae (L 3) of Haemonchus spp. treated with Cymbopogon citratus crude extract. Control with 3% DMSO (A, B), aqueous (C, D) and methanolic (E, F) extract at a concentration of 25 mg mL−1. C, D – L 3 cuticle with lesions (arrow); E, F – lesions (arrow) in the cuticle. Bars: 20 μm, 50 μm.
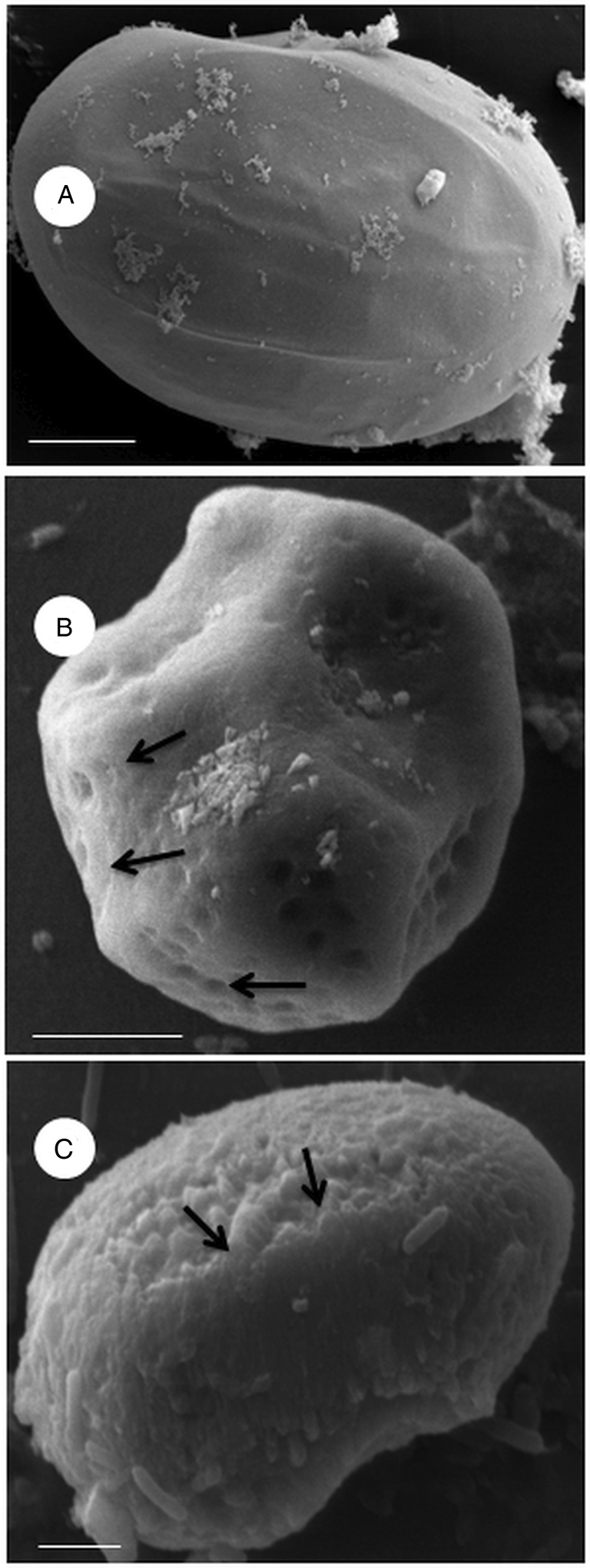
Fig. 4. s.e.m. micrographs of nematode egg treated with Cymbopogon citratus crude extract. Control with distilled water (A); methanolic (B) and aqueous (C) extract at a concentration of 50 mg mL−1. Eggs treated with aqueous extract presented an irregular surface with numerous circular concave injuries (arrow) or with clusters of lumps (arrow) when treated with MeOH extract. Bars: 2 μm, 5 μm and 20 μm.
Ultra-thin or semi-fine sections of untreated L 3 revealed a typical internal morphology (Fig. 5A and C, 6A and B). For the treated L 3, we observed an irregular pattern of cuticle striation in many regions of the body (Fig. 5D). The sheath demonstrated strong interaction with the extract, with the external region being more intensely marked with dye (Fig. 5B) or electron-dense (Figs 5D and 6D). An alteration of the hypodermis was found with a slight decoupling from the cuticle (Figs 5D and 6C). Further, the muscular layer, intestinal cells and the mitochondria profile all showed damage compared to the typical pattern: muscle cells were degraded (Figs 5B, 5D, 6C and 6D); intestinal cells, when present, appeared distorted and numerous vacuoles filled the interior cavity (Figs 5B, 5D and 6C); and the mitochondria profile did not contain the matrix and cristae (Fig. 6D). Electron-dense vesicles were also observed filling the body cavity of the larvae (Fig. 6D).
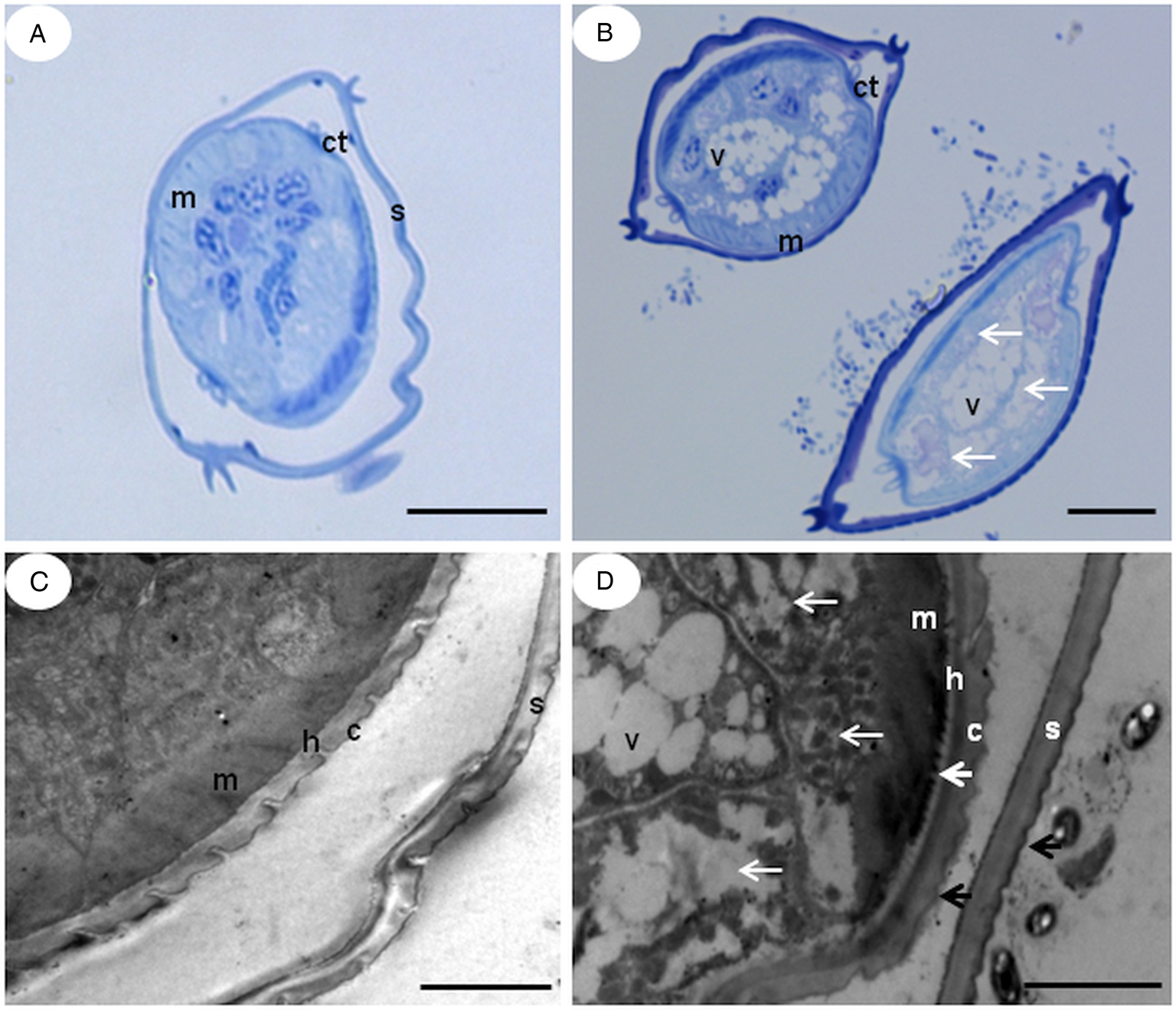
Fig. 5. TEM and semi-fine section micrographs of infective larvae (L 3) untreated (A, C) and treated (B, D) with Cymbopogon citratus aqueous extract 25.0 mg mL−1. S – sheath, C – cuticle, H – hypodermis, M – Somatic muscle cells, V – Vacuoles, Ct – Longitudinal Cuticular thickening. Thin white arrows show the internal disorganization of the cells that make up the tissue. Large white arrow shows slight decoupling of the hypodermis-cuticle. Black arrows show the affected outer surface of the sheath and cuticle. Bars: a, b- 2 μ; c, d – 50 μ.

Fig. 6. TEM micrographs of infective larvae (L 3) untreated (A, B) and treated (C, D) with Cymbopogon citratus methanolic extract. Larvae were incubated with 25.0 mg mL−1 of the extract for 48 h at 27°C in BOD. Cuticle (C); hypodermis (h); somatic muscle cells (m); intestinal cells (ic); vacuoles (v); vesicles (white arrow); mitochondrial profiles (black arrow). Ultrastructural organization was not preserved in the treated larvae and showed vacuoles and vesicles. Mitochondria detail (inset). Bar: 1 μm, 2 μm and 5 μm.
Ultra-thin sections of untreated eggs revealed an internal morphology of the embryo in formation with organelles preserved (Fig. 7A), while eggs treated with methanolic fractions of C. citratus presented modifications (Fig. 7B–D). Eggs treated with fractions CCMF-08 and CCMF-10 at concentrations of 500 and 1000 μg mL−1, respectively, showed a deformed embryo at an early stage of the embryogenesis process, while eggs treated with the CCMF-09 fraction at concentrations of 1000 μg mL−1 also showed deformed embryos but at a later stage of the embryogenesis process. Electron-dense vesicles were observed traversing the lipid and chitinous layers of the eggshell (inset Fig. 7D) and dispersed in the space between the eggshell and plasma membrane (arrow Fig. 7D).
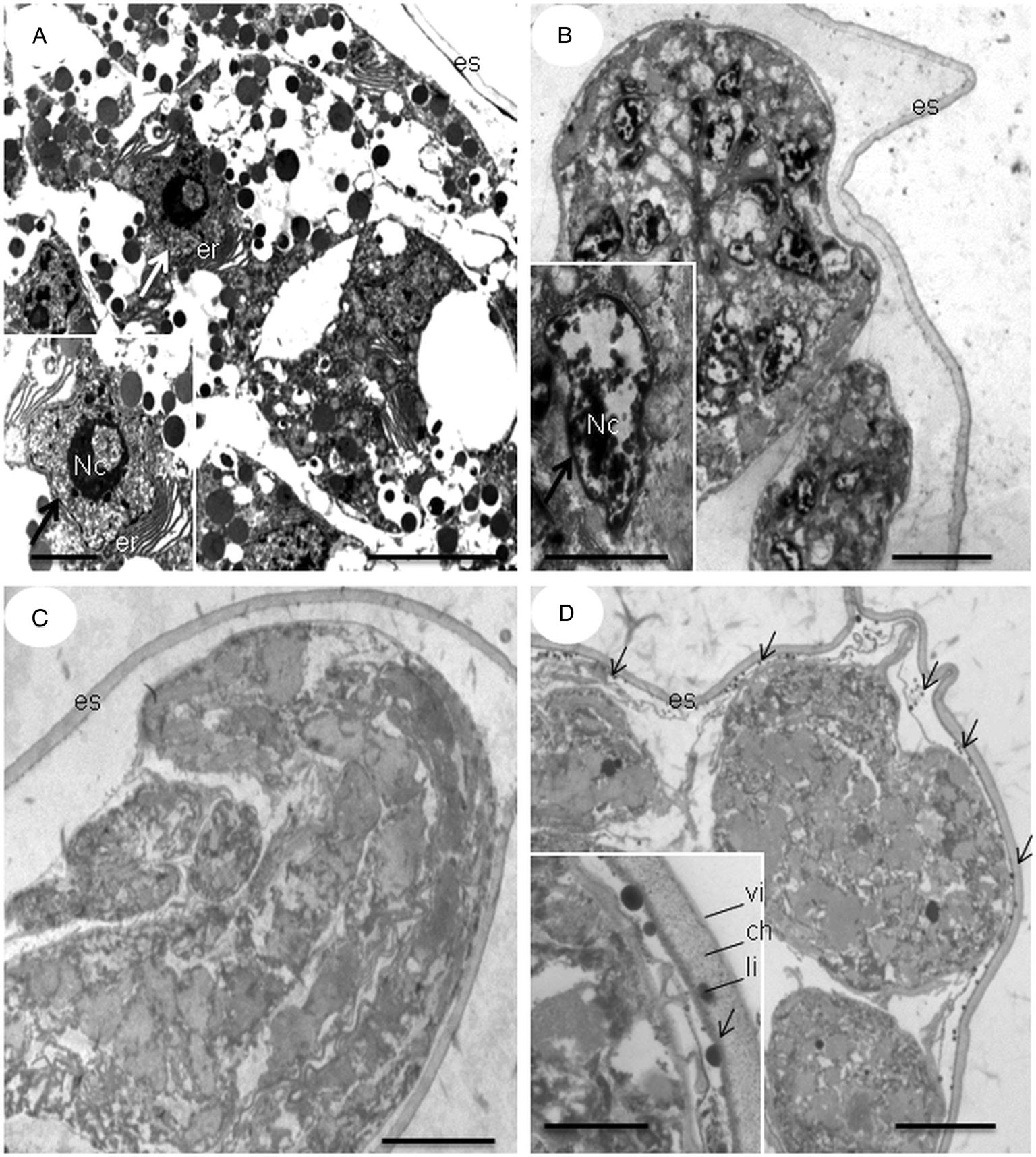
Fig. 7. TEM micrographs of nematode eggs treated with methanolic fractions of Cymbopogon citratus. Incubated egg with distilled water (A), and fractions CCMF-08 (B), CCMF-09 (C) and CCMF-10 (D) at concentrations of 500, 1000 and 1000 μg mL−1, respectively. Nucleolus (Nc); rough endoplasmic reticulum (er); eggshell (es); vitelline layer (vi); chitinous layer (ch); lipid layer (li); nucleus (large arrow); vesicles (narrow arrow). Detail of the vesicles traversing the eggshell (inset). Bars: 2 μm and 5 μm.
Discussion
Research on medicinal plants has been increasing in recent years, and new discoveries are constantly being made. Due to interest in addressing the challenges that arise from the use of anthelmintics, particularly parasite resistance to these compounds, the biological activity of many plants has been tested. As such, interest from the pharmaceutical industry in developing new drugs from natural bioactive compounds has been growing, further promoting research on medicinal plants. In this study, extracts from the leaves of C. citratus demonstrated nematicidal activity against eggs and L 3 of gastrointestinal nematodes in sheep.
Both the aqueous and MeOH extracts of C. citratus showed nematicidal activity against eggs and L 3, but the MeOH extract showed greater effectiveness in terms of ovicidal and larvicidal activity. Previous studies on extracts and essential oil of C. citratus have shown effects on nematodes in animals. C. citratus aqueous extract reduced the number of Haemonchus contortus L 3 by 97% at a concentration of 224 mg mL−1 (Almeida et al., Reference Almeida, Botura, Santos, Domingues, Costa and Batatinha2003). Sheep treated with an oral dose of C. citratus alcoholic extract at a concentration of 20 mg kg−1 for 1, 3 or 4 consecutive days presented a significant reduction in EPG (Silva et al., Reference Silva, Brito, Marinho, Rodrigues and Athayde2005). Meanwhile, C. citratus essential oil or decoction inhibited H. contortus egg hatching and larval development by 90–99% at concentrations of 0.62–10 mg mL−1 (Macedo et al., Reference Macedo, Oliveira, Ribeiro, Santos, Silva, AraújoFilho, CamurçaVasconcelos and Bevilaqua2015). Cymbopogon citratus essential oil nanoemulsion was also shown to inhibit 97.1% of H. contortus larvae hatching; however, it was not effective when used in live sheep infected with gastrointestinal nematodes (Macedo et al., Reference Macedo, Oliveira, André, Araújo Filho, Santos, Rondon, Ribeiro, Camurça-Vasconcelos, Oliveira, de Paula and Bevilaqua2019). Moreover, C. citratus essential oil reduced the H. contortus burden by 38.5% in Meriones unguiculatus (gerbils) treated with 800 mg kg−1 (Macedo et al., Reference Macedo, Oliveira, André, Araújo Filho, Santos, Rondon, Ribeiro, Camurça-Vasconcelos, Oliveira, de Paula and Bevilaqua2019). Thus, compounds in C. citratus have a lethal effect on nematodes.
Most of the studies on the chemical composition of C. citratus relate to its essential oil. Chemical compounds found in the essential oil vary according to the maturity of the plant (Tajidin et al., Reference Tajidin, Ahmad, Rosenani, Azimah and Munirah2012), environmental and geographical conditions (Abegaz et al., Reference Abegaz, Yohannes and Dieter1983; Torres and Ragadio, Reference Torres and Ragadio1996; Chisowa et al., Reference Chisowa, Hall and Farman1998; Bassolé et al., Reference Bassolé, Lamien-Meda, Bayala, Obame, Ilboudo, Franz, Novak, Nebié and Dicko2011) and extraction method (Barbosa et al., Reference Barbosa, Pereira, Martinazzo, Maltha, Teixeira and Melo2008; Mohamed Hanna et al., Reference Mohamed Hanna, Sallaam, El-Leity and Safaa2012). Nevertheless, citral (a combination of geranial and neral) has been identified as the most abundant compound (Saddiq and Khayyat, Reference Saddiq and Khayyat2010). However, an exception was found for Ethiopian C. citratus essential oil that contained geraniol as its main component (Abegaz et al., Reference Abegaz, Yohannes and Dieter1983). Similarly, analysis of the MeOH extract of C. citratus herein did not identify citral as the major compound, but rather a triterpenoid identified as cerin acetate (2α-acetoxyfriedelin).
Unlike the extracts, fractions of C. citratus only had an effect on nematode eggs. The lack of activity of C. citratus fractions against L 3 indicates that the various individual compounds of the fractions do not interfere with the survival of the larvae or a synergistic action of more fractions is necessary. In contrast, the extracts and fractions from Combretum molle and Vernonia amygdalina have shown similar effects on both nematode eggs and larvae (Ademola and Eloff, Reference Ademola and Eloff2011).
Eggs and L 3 have different structural characteristics. The egg has a shell consisting of three layers, the inner lipid, medial chitin and outer vitelline layers (Bird and Bird, Reference Bird and Bird1991), while L 3 has a double cuticle. The cuticle consists of four parts: a triple-layered epicuticle at the external surface, a cortical zone, a median zone and a basal zone. Biochemically, the cuticle consists of structural components, mostly collagen (insoluble in detergent), soluble proteins and low molecular weight components such as lipids (Decraemer et al., Reference Decraemer, Karanastasi, Brown and Backeljau2003). The L 3 cuticle is covered with an additional sheath retained from the L 2. The nematicidal activity of the evaluated extracts was more intense in eggs than in L 3, which is likely due to the ability of the extracts to pass more easily through the eggshell than through the double cuticle of the L 3. As such, the extracts are able to reach the L 1 forming inside the egg, which is at a more fragile stage of life. This limited protective ability of the eggshell may vary with the stage of embryogenesis because the vitelline layer becomes more brittle and water-soluble toward the end of embryo formation (Bird and Bird, Reference Bird and Bird1991). The L 2 cuticle retained by the L 3 functions as an extra protective sheath against environmental conditions (Hansen and Perry, Reference Hansen and Perry1994) and extracts have more difficulty crossing this barrier.
Few studies have evaluated the effects of plant extracts on nematode ultrastructure. In a previous study analysing the ultrastructural changes to L 3 of ruminant nematodes treated with Onobrychis viciifolia extract, Brunet et al. (Reference Brunet, Fourquaux and Hoste2011) observed that in the ensheathed L 3, the lesions were mainly located in the hypodermis layer and the muscle cells. However, in the exsheathed L 3, the main damage seemed to occur in the intestinal cells, while changes in the hypodermis and muscular layers were less pronounced than in the ensheathed L 3. Furthermore, no alteration was observed in the sheath or the cuticle. Changes to the body surface of H. contortus were observed by s.e.m. after exposure to extracts of Lysiloma latisiliquum and Onobrychis viciifolia (Martínez-Ortíz-de-Montellano et al., Reference Martínez-Ortíz-de-Montellano, Arroyo-López, Fourquaux, Torres-Acosta J, Sandoval-Castro and Hoste2013) and Acacia mearnsii (Yoshihara et al., Reference Yoshihara, Minho, Tabacow, Cardim and Yamamura2015). All of these plants contain condensed tannins that are phenolic compounds, to which anthelmintic effects have been attributed. Here, C. citratus was shown to have an effect on the outermost walls of the body of L 3 of Haemonchus spp. and Trichostrongylus spp., i.e., sheath and cuticle. An irregular pattern striation, lesions and desquamations were observed in the cuticle and the sheath was electron-dense. Regions such as the hypodermis, muscular layer, intestinal cells and the mitochondria profile were also affected.
Previous studies using plants or synthetics compounds have demonstrated that their anthelmintic activity is related to the presence of terpenoids. Citral and lemonene (Macedo et al., Reference Macedo, Bevilaqua, Oliveira, Camurça-Vasconcelos, Vieira, Oliveira, Queiroz-Junior, Tomé and Nascimento2010), linalool and carvacrol (Zhu et al., Reference Zhu, Dai, Yang and Qiu2013), essential oil (Armstrong et al., Reference Armstrong, Klein, Whitney, Scott, Muird, Lambert and Craig2013), carvacrol (Andre et al., Reference Andre, Ribeiro, Cavalcante, Santos, Macedo, de Paula, Freitas, Morais, Melo and Bevilaqua2016), thymol, carvacrol and eugenol (Hernando et al., Reference Hernando, Turani and Bouzat2019), triterpenoids identified as urs-19(29)-en-3-yl acetate, (3β)-Urs-19(29)-en-3-ol and 1-(2’,5’-dimethoxy phenyl)-glycerol (Cavalcante et al., Reference Cavalcante, Morais, Andre, Ribeiro, Rodrigues, De Lira, Viana and Bevilaqua2016), triterpenoid glycosides (saponins) identified as joazeiroside B and lotoside A (Gomes et al., Reference Gomes, Lima, Vaz, Santos, Santos, Dias, Botura, Branco and Batatinha2016) were some of the related compounds. Besides, other saponins revealed the presence of in vitro anthelmintic activity against nematodes eggs of goat (Botura et al., Reference Botura, dos Santos, da Silva, de Lima, de Oliveira, de Almeida, Batatinha and Branco2013; Santos et al., Reference Santos, Santos, Lima, Silva, Uzêda, Dias, Branco, Cardoso, David, Botura, Costa and Batatinha2018), donkey (Maestrini et al., Reference Maestrini, Tava, Mancini, Salari and Perrucci2019), sheep (Maestrini et al., Reference Maestrini, Tava, Mancini, Tedesco and Perrucci2020) and L 3 of goat (Santos et al., Reference Santos, Santos, Lima, Silva, Uzêda, Dias, Branco, Cardoso, David, Botura, Costa and Batatinha2018). In a similar way, β-sitosterol steroid demonstrated anthelmintic activity against Ascaris suum (Villaseñor et al., Reference Villaseñor, Angelada, Canlas and Echegoyen2002) and sheep gastrointestinal nematodes (Giovanelli et al., Reference Giovanelli, Mattellini, Fichi, Flamini and Perrucci2018). Interestingly, it was found that a hydroethanolic extract of the fungus Pleurotus djamor contained a fraction with ovicidal activity against H. contortus, constituted mainly of free fatty acids with less than 1% of β-sitosterol steroid (Pineda-Alegria et al., Reference Pineda-Alegria, Sanchez-Vazquez, Gonzalez-Cortazar, Zamilpa, Lopez-Arellano, Cuevas-Padilla, Mendoza-de-Gives and Aguilar-Marcelino2017). Thus, terpenoids and β-sitosterol steroid found in C. citratus may be responsible for the anthelmintic effects described here.
Concluding remarks
This in vitro study of C. citratus plant extract is quite promising and validates popular knowledge of the medicinal properties of the plant. The extracts and fractions from C. citratus showed nematicidal activity in the assay as well as ultrastructural changes at various nematode life stages. In the continuing search for alternative means of controlling gastrointestinal nematodiasis, medicinal plants represent an important opportunity to pursue.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020001432.
Acknowledgements
The authors would like to thank Marcia Adriana, Beatriz Ferreira, and Giovana Alves for technical assistance and the referees for their helpful suggestions.
Financial support
This study was supported by Brazilian funding agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho do Rio de Janeiro (FAPERJ grant number E-26/110.577/2011), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). IJCV and RBF are CNPq Senior Researchers.
Conflict of interest
None of the authors has any competing interests in the manuscript.
Ethical standards
Not applicable.











