Introduction
Seeds of many plant species are dormant at maturity and require special treatment to break dormancy to allow germination. Dormancy is imposed by either coat or embryo, or a combination of the two (Nikolaeva, Reference Nikolaeva and Khan1977). The causes of coat-imposed dormancy include the impermeability of the coat to water and/or gases, the mechanical prevention of radicle extension, and the seed coat preventing inhibitory substances from leaving the embryo or supplying inhibitors to the embryo; whereas embryo dormancy usually depends on hormonal regulation induced by temperature and/or light. When the two types of dormancy are combined, the removal of the seed coat will not result in germination, instead additional treatments directed at the embryo are required (Kelly et al., Reference Kelly, van Staden and Bell1992). Abscisic acid (ABA) is a sesquiterpenoid derived from carotenoids which plays a role in regulating many biological processes, including seed dormancy and germination (Nambara and Marion-Poll, Reference Nambara and Marion-Poll2005). Many studies have shown that ABA accumulates in late maturing seeds and induces dormancy (Finkelstein et al., Reference Finkelstein, Reeves, Ariizumi and Steber2008). Physiological approaches suggest an important role of ABA in the control of apple embryo dormancy (Lewak, Reference Lewak2011). Moreover, exogenous ABA can induce secondary dormancy in non-dormant apple embryos (Rudnicki et al., Reference Rudnicki, Kamińska and Pieniężek1971). Mutants and transgenic lines that overaccumulate ABA show enhanced dormancy or delayed germination (Thompson et al., Reference Thompson, Jackson, Symonds, Mulholland, Dadswell, Blake, Burbidge and Taylor2000; Okamoto et al., Reference Okamoto, Kuwahara, Seo, Kushiro, Asami, Hirai, Kamiya, Koshiba and Nambara2006). ABI (ABSCISIC ACID-INSENSITIVE) mutants resulted in reduced seed dormancy and decreased sensitivity of seed germination and seedling growth to inhibition by ABA (Finkelstein et al., Reference Finkelstein, Reeves, Ariizumi and Steber2008). Gibberellic acid (GA) triggers the production of α-amylase, proteolytic enzymes, nucleases, etc., leading to loss of seed dormancy and promotion of seed germination (Domínguez et al., Reference Domínguez, Moreno and Cejudo2004; Mrva et al., Reference Mrva, Wallwork and Mares2006). The GA signalling genes GAI (GA INSENSITIVE), RGA (REPRESSOR OF GA1-3) and RGL (RGA-LIKE) encode highly homologous DELLA proteins, which are characterized by their N-terminal DELLA domain (Peng et al., Reference Peng, Carol, Richards, King, Cowling, Murphy and Harberd1997; Silverstone et al., Reference Silverstone, Ciampaglio and Sun1998; Sun et al., Reference Sun, Jones, Harvey, Edwards, Pascal, Kirk, Considine, Sheerin, Rakonjac, Oldfield, Xue, Dunker and Uversky2010). The DELLA proteins negatively regulate the GA signalling pathway and are inactivated in response to GA signalling (Silverstone et al., Reference Silverstone, Jung, Dill, Kawaide, Kamiya and Sun2001; Eckardt, Reference Eckardt2007).
GA signalling is suppressed by ABA in dormant seeds. Studies in the aleurone layer of cereal seeds have shown that ABA may suppress the induction of GA-induced Myb-like protein (GAMyb) and α-amylase by GA through an ABA-induced protein kinase (PKABA1) and PKABA1-independent pathway (Gómez-Cadenas et al., Reference Gómez-Cadenas, Zentalla, Walker-Simmons and Ho2001; Zentella et al., Reference Zentella, Yamauchi and Ho2002). Studies using the ABA-deficient mutant aba2-2 suggest that ABA is also involved in the suppression of GA biosynthesis in both imbibed and developing seeds (Seo et al., Reference Seo, Hanada, Kuwahara, Endo, Okamoto, Yamauchi, North, Marion-Poll, Sun, Koshiba, Kamiya, Yamaguchi and Nambara2006). On the contrary, GA can also reverse the inhibition of seed germination by low concentrations of ABA (Liu et al., Reference Liu, Ye, Liu, Chen and Zhang2010). The DELLA proteins that repress GA signalling can stimulate ABA synthesis and activate ABI3 and ABI5 function (Piskurewicz et al., Reference Piskurewicz, Jikumaru, Kinoshita, Nambara, Kamiya and Lopez-Molina2008, Reference Piskurewicz, Turecková, Lacombe and Lopez-Molina2009), but they are inactivated by GA-mediated ubiquitin proteasome-dependent protein degradation (Achard and Genschik, Reference Achard and Genschik2009). ENY (ENHYDROUS) encodes a C2H2 zinc finger protein that plays a role in ABA and GA signal transduction during late seed development (Feurtado et al., Reference Feurtado, Huang, Wicki-Stordeur, Hemstock, Potentier, Tsang and Cutler2011). MFT (MOTHER OF FT AND TFL1) encodes a phosphatidylethanolamine-binding protein (PEBP) and promotes the growth of the germinating seed embryo by constituting a negative feedback loop in the ABA signalling pathway (Xi et al., Reference Xi, Liu, Hou and Yu2010). Although the regulatory framework of ABA and GA signalling factors in seed dormancy and germination is increasingly clear in model plants, knowledge of these signalling genes in woody plants and their function in germinating seeds of trees is limited.
Sand pear (Pyrus pyrifolia) is a pear species belonging to the Rosaceae pear subfamily. It is cultivated throughout East Asia, and in other countries such as Australia, New Zealand and the USA (e.g. California). The Rosaceae pear subfamily also includes the important fruit apple. Pear seeds usually show dormancy at maturity and require 2–3 months at 0–7°C in a moist, well-aerated medium to break dormancy (Hartmann et al., Reference Hartmann, Kester and Davies1990), which is similar to the requirements for apple seeds (Lewak, Reference Lewak2011). Two types of dormancy, i.e. embryonic and testa-imposed, have been identified in apple seeds, and the dormancy of isolated apple embryos can be eliminated at room temperature by an anaerobic treatment lasting several days (Tissaoui and Côme, Reference Tissaoui and Côme1973; Lewak, Reference Lewak2011). Our previous studies have also demonstrated the important role of the endocarp and seed coat of sand pear in maintaining seed dormancy (Wang et al., Reference Wang, Dai, Zhang and Shi2012). However, the type of sand pear seed dormancy and its regulation remain unclear. In this study we determined the ABA content in endocarp, seed coat and embryo of sand pear, and analysed the influence of ABA on the germination of its embryos. We have cloned a number of key ABA signalling genes and analysed their expression in imbibed embryos or embryos of imbibed nutlets (full seeds with endocarp) and true seeds (endocarp removed) after treatment with ABA or GA. We also analysed the influence of exogenous GA on seed germination and cloned the key GA signalling genes and monitored their expression in sand pear embryos after the different treatments mentioned above. The role of testa and embryo and the hormonal and molecular regulation of sand pear seed dormancy are discussed.
Materials and methods
Fruit harvest and storage and collection of nutlets
Mature fruits of sand pear cv. ‘Cuiguan’ were harvested from trees growing in Yangdu orchard (30°29′ N, 120°15′ E), Haining County, Zhejiang Province, China in August 2011. Harvested fruits were stored at 4°C for 60 d to complete seed maturation. Mature nutlets were removed, and air-dried at room conditions for 1 month to 14% relative water content and stored in a sealed container at 4°C.
Analysing the effect of exogenous ABA and GA on germination
Nutlets, true seeds and isolated embryos from harvested nutlets were either placed directly on filter paper soaked with demineralized water or with a certain treatment in culture plates for germination at 25°C and continuous light (TL57 bulbs, Philips). The ABA treatment included four concentrations: 0.1 mM, 0.5 mM, 1 mM and 2 mM. The GA3 treatment included concentrations of 0.29 mM, 1.15 mM and 4.62 mM. Each treatment and the untreated control consisted of three replicates of ~50 nutlets, true seeds or embryos. Germination, with visible elongation of the embryonic axis, was recorded three times a week, and pictures were taken.
Extraction and measurement of ABA in endocarp, seed coat and embryo by ELISA
Extraction and purification of ABA in plant tissues prior to immunoassay have been described previously (Yang et al., Reference Yang, Xu, Wang and Jia2001). Extraction of 2 g of different homogenized parts of fully imbibed seeds was conducted in cold 80% (v/v) aqueous methanol at a rate of 5 ml g− 1 fresh weight (FW) overnight at 4°C with butylated hydroxytoluene (10 mg l− 1) to prevent oxidation. The supernatant was collected after centrifugation at 10,000 g (4°C) for 20 min. The crude extract was passed through a C18 Sep-Pak cartridge (Waters, Milford, Massachusetts, USA). The efflux was collected, adjusted to pH 2.5, and extracted three times with an equal volume of ethyl acetate. The extracts (ethyl acetate phase) were pooled and dried in a gentle stream of N2, after which the residue was dissolved in 200 μl of 100% methanol for methylation with ethereal diazomethane and taken up with 200 μl of phosphate-buffered saline (PBS) for ABA enzyme-linked immunosorbent assay (ELISA).
The ELISA procedures, based on a monoclonal antibody that showed highly specific immunoreactivity with ABA, have been described by Yang et al. (Reference Yang, Xu, Wang and Jia2001). The ELISA was performed with microtitre plates (Nunc, Roskilde, Denmark). Standard ABA was added in the immune reaction system for normalization of the hormone measurements. All samples were assayed six times.
RNA extraction and cDNA synthesis
Total RNA was extracted from sand pear embryos using the EasyPure™ Plant RNA Kit (TransGen, Beijing, China). DNase I (TaKaRa, Japan) was added to remove genomic DNA and RNase-free columns (TransGen) were used for purifying total RNA. The concentration of total RNA was measured by absorbance at 260 nm using BioPhotometer Plus (Eppendorf, Germany), and the integrity and quality of the RNA was checked using agarose gel electrophoresis and absorbance 260/280 ratio. Subsequently, first-strand cDNA was synthesized from total RNA (2 μg) using oligo(dT).
Cloning of signalling factors involved in ABA and GA pathways
Our previous studies revealed high sequence conservation of apple and pear homologous genes, and pear genes could be amplified directly using the primers designed for apple homologous sequences (Shi et al., Reference Shi, Wang, Dai and Zhang2011). In the present study, apple homologues of Arabidopsis ABI3, ABI4, ABI5, ENY, GAI, RGA, RGL2 and MFT were searched for in the apple genome database (http://www.rosaceae.org/projects/apple_genome) using BLAST. Gene-specific primers were designed according to the sequences of these apple homologues (Table 1). The sand pear cDNAs encoding the putative signal factors were amplified by reverse transcription polymerase chain reaction (RT-PCR) using the gene-specific primers (Table 1). cDNAs synthesized from certain embryos of sand pear cv. ‘Cuiguan’ were used as PCR templates. Amplified PCR products of appropriate length were cloned into T/A cloning vector pEASY-T1 (TransGen) and then transformed into Escherichia coli Trans5a chemically competent cells (TransGen). Positive E. coli cells were identified with the PCR method, using gene-specific primers, and sent to Sangon Biotech (Shanghai) Co. Ltd for sequencing.
Table 1 Primers for fragment cloninga and real-time polymerase chain reaction (PCR) analysisb of ABA and GA signalling genes

Sequence analysis and quantitative real-time PCR
Comparing ABI3, ABI4, ABI5, GAI, RGA, RGL2, ENY and MFT sequences with known apple sequences was carried out using GDR Blast server (Jung et al., Reference Jung, Staton, Lee, Blenda, Svancara, Abbott and Main2008). Multiple sequence alignment was performed using ClustalW (Thompson et al., Reference Thompson, Higgins and Gibson1994).
Isolation of total RNA from the embryos of sand pear and synthesis of first-strand cDNA were performed as described above. Six types of sand pear embryos, i.e. embryos of air-dried nutlets, embryos of imbibed nutlets, imbibed embryos, embryos treated with 0.1 mM ABA, embryos treated with 1 mM ABA and embryos of true seeds treated with 0.29 mM GA3 were used in the expression analysis. All types of embryos except the embryos of air-dried seeds were imbibed at 25°C in light for 5 d. The transcript levels of ABI3, ABI4, ABI5, GAI, RGA, RGL2, ENY and MFT were analysed using quantitative real-time PCR (qRT-PCR) with ABI 7500 Real-Time PCR Systems (Applied Biosystems, California, USA), according to the manufacturer's instructions. Each reaction (final volume 20 μl) contained 10 μl 2 × TransStart™ Top Green qPCR SuperMix (TransGen), 0.5 μl Passive Reference Dye II (50 × ), 0.4 μl of each the forward and reverse primers (10 mM), 2 μl of the cDNA template (corresponding to 50 ng of total RNA) and 7 μl of RNase-free water. The reaction mixtures were heated to 94°C for 30 s, followed by 40 cycles at 94°C for 5 s, 55°C for 15 s and 72°C for 34 s. A melting curve was generated for each sample at the end of each run to ensure the purity of the amplified products.
All gene-specific primers from the identified genes for qRT-PCR were designed using a Primer 6.0 program (PREMIER Biosoft International, Canada) (Table 1). Each assay using the gene-specific primers amplified a single product of correct size with high PCR efficiency (90–110%) (Lefever et al., Reference Lefever, Hellemans, Pattyn, Przybylski, Tayor, Geurts, Untergasser and Vandesompele2009). Elongation factor 1 (EF1-α) gene (GenBank accession number: AY338250) is stably expressed in pear tissues and was used as the reference gene (Malnoy et al., Reference Malnoy, Faize, Venisse, Geider and Chevreau2005; S.J. Zhang et al., Reference Zhang, Wu, Chen, Gu, Tao, Wu and Zhang2011). All qRT-PCR reactions were normalized using Ct value corresponding to the EF1-α. The relative expression levels of target genes were calculated with the 2-ΔΔCT method (Livak and Schmittgen, Reference Livak and Schmittgen2001). Values reported represent the average of three biological replicates.
Results
Effect of ABA on sand pear seed dormancy
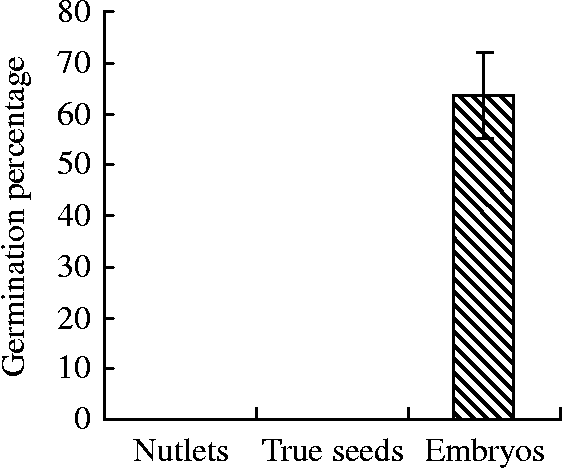
Our previous studies have shown that both mature nutlets and true seeds from mature nutlets of sand pear remained dormant during water incubation for 10 d or longer (Fig. 1). Although the imbibed nutlets displayed signs of embryo imbibition and weak radicle elongation, most of the radicles did not effectively break through the seed coat. However, more than 60% of the isolated embryos showed germination with elongating radicles. These results suggest that the endocarp and seed coat play an important role in maintaining sand pear seed dormancy.
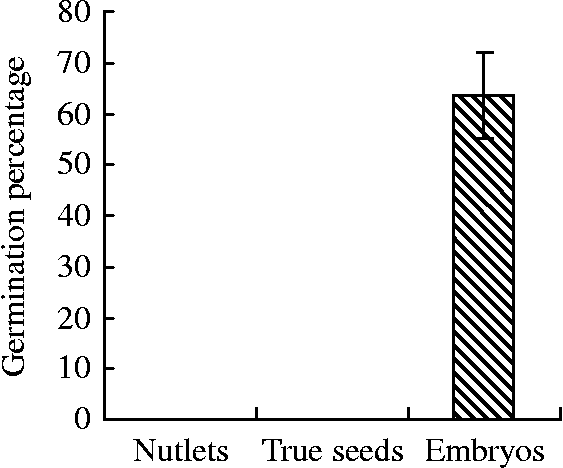
Figure 1 Germination rates of sand pear cv. ‘Cuiguan’ nutlets, true seeds and embryos soaked with demineralized water at 25°C in light for 10 d (from Wang et al., Reference Wang, Dai, Zhang and Shi2012).
ABA content of endocarp, seed coat and embryo
The ABA content was measured in endocarp, seed coat and embryo. The results show that the ABA content of the endocarp was as high as 766.92 ng (g FW)− 1, which was about two times that of the seed coat and embryo (Fig. 2).

Figure 2 ABA content of different seed parts of sand pear cv. ‘Cuiguan’; a–c represent endocarp, seed coat and embryos from fully imbibed seeds, respectively; d–f represent embryos of true seeds, embryos of 1.15 mM GA3-treated true seeds, and embryos, respectively, imbibed in light at 25°C for 5 d. Results are presented as means ± SE.
Repression by ABA on embryo germination
The embryo dormancy of sand pear can be broken rapidly by imbibition in light at room temperature. However, true seeds with a seed coat maintain their dormancy for a considerable period of time. We hypothesized that removal of the seed coat leads to a rapid loss of ABA from the embryo, resulting in a rapid loss of embryo dormancy. To test this hypothesis, we detected the ABA content in embryos imbibed for 5 d and embryos of 5-d imbibed true seeds. ABA content was not significantly changed in the embryos of imbibed true seeds, but was significantly reduced in imbibed embryos (P< 0.05), as compared with the initial ABA content of embryos of fresh seeds (Fig. 2). The germination of embryos was not affected by the 0.1 mM ABA treatment, but was completely prevented by ABA treatments above 0.5 mM (Fig. 3).

Figure 3 Effect of exogenous ABA on the germination of embryos of sand pear cv. ‘Cuiguan’. All treatments and the control were incubated in light at 25°C for 10 d.
Effect of exogenous GA on sand pear seed germination
During the 10-d light incubation (Fig. 4), both control and GA3-treated embryos showed obvious radicle elongation. The germination rate of sand pear embryos was weakly affected by GA3 treatment at low concentration, but was suppressed by 4.62 mM GA3. Compared with embryos, the true seeds of either control or with GA3 treatment showed less than 2% germination, so the GA3 treatment can not effectively break the dormancy of true seeds during this incubation. The 1.15 mM GA3 treatment had no effect on ABA degradation or loss in the 5-d imbibed true seeds (Fig. 2).

Figure 4 Effect of exogenous GA3 on the germination of embryos and true seeds of sand pear cv. ‘Cuiguan’ incubated at 25°C in the light for 10 d.
Isolation and sequence analysis of ABA and GA signal factors
Eight Arabidopsis ABA and GA signalling genes were used to query the apple genome database, and a total of 11 genes were identified and selected to study in more detail. These genes include homologues of ABI3 (2610 bp), ABI4 (1092 bp), ABI5 (1413 bp), RGA (1920 bp), RGL2 (1756 bp), ENY (1758 bp), three MFT homologues (MFT1, 519 bp; MFT2, 519 bp and MFT3, 525 bp), and two GAI homologues (GAI-1, 1041 bp and GAI-2, 1095 bp). Sequence analysis showed that ‘MFT2’ and ‘MFT3’ had the highest homology with Arabidopsis TFL1 (TERMINAL FLOWER 1, At5g03840) and FT (FLOWERING LOCUS T, At1G65480), respectively. Therefore, the sand pear ‘MFT2’ and ‘MFT3’ were renamed PpTFL1 and PpFT (Table 2). The apple genome database accession codes of the 11 submitted genes are listed in Table 2. These apple genes share 38–90% peptide homology with the corresponding homologues in Arabidopsis. Although some homologues, such as that of ABI4 in apple and Arabidopsis, do not have high peptide homology, they share functionally conserved domains, in agreement with previous reports in maize and yellow-cedar (Lazarova et al., Reference Lazarova, Zeng and Kermode2002; Niu et al., Reference Niu, Helentjaris and Bate2002).
Table 2 Homologies based on peptide sequences for putative ABA and GA signalling genes isolated from sand pear cv. ‘Cuiguan’

a % Similarity between Arabidopsis and apple homologues.
b % Similarity between apple and sand pear homologues.
Sand pear homologous fragments were isolated following the traditional cloning procedures including RT-PCR and TA ligation from sand pear cv. ‘Cuiguan’, using apple gene-specific primers (Table 1). The sequences obtained were 213– 697 bp in length and encoded for peptides of 71–211 amino acids. GenBank accession codes of the 11 isolated gene fragments are listed in Table 2. The homologues of the ABA and GA signalling factors in sand pear and apple showed high peptide homology: from 87.1 to 100% (Table 2).
Effect of endocarp and seed coat on the expression of ABA and GA signalling genes
After 5 d of germination in the light, the expression of the ABA signalling genes ABI3–5 was very weak in the imbibed embryos, but remained at higher levels in imbibed nutlets (Fig. 5B, graphs a–c), which indicates that there was still a relatively strong ABA signal in the imbibed nutlets. Except for PpGAI-2, the expression of the other three GA signal repressors PpGAI-1, PpRGA and PpRGL2 was significantly decreased in the germinating embryos. The original expression of PpRGL2 was highest among the four GA signal repressors, but a minimum in the germinating embryos. Whereas the expression of PpRGL2 was reduced, the expression of PpGAI-1 and PpGAI-2 was increased in imbibed nutlets, and all the GA signal repressors had a similar relatively high level of expression in the imbibed nutlets (Fig. 5B, graphs d–g). The expression of PpENY was relatively higher than other genes at the initial phase and significantly decreased in both nutlets and embryos imbibed for 5 d, but its transcriptional level in imbibed nutlets remained several times higher than that in the germinating embryos (Fig. 5B, graph h). These results suggest that there is still strong signal suppression of GA in the imbibed nutlets. Comparing with the nearly null expression of PpMFT, the expression of PpFT was significantly up-regulated in the germinating embryos. All three MFT homologues maintained their initial transcriptional level in the imbibed nutlets (Fig. 5B, graphs i–k). The expression of PpTFL1 remained at the initial level in both imbibed nutlets and germinating embryos.

Figure 5 (A) Sand pear germination. 1–6 represent different types of embryos or seeds of sand pear: 1, embryos of air-dried seeds; 2, imbibed nutlets; 3, imbibed embryos; 4, embryos treated with 0.1 mM ABA; 5, embryos treated with 1 mM ABA; 6, true seeds treated with 0.29 mM GA3. All types of embryos, except 1, were imbibed at 25°C in light for 5 d. (B) ABA, GA signalling gene expression analysis. The P values for expression data were calculated between embryos of air-dried seeds (type 1) and embryos with certain treatment (type 2–6) by Student's t-test (⋆, P< 0.05; ★, P< 0.01). (A colour version of this figure can be found online at http://www.journals.cambridge.org/ssr)
Effect of exogenous ABA and GA on the expression of ABA and GA signalling genes
The expression of ABA and GA signalling genes in embryos treated with a low concentration of ABA was similar to that of the imbibed embryos, while the gene expression pattern in embryos treated with a high concentration of ABA was similar to that in nutlets (Fig. 5). Except that of PpTFL1, the gene expression level in ABA-treated embryos showed a significant ABA-dose dependency. The expression of ABA and GA signalling factors in true seeds under GA3 treatment was similar to that of imbibed nutlets or embryos treated with 1 mM ABA, which is consistent with the result that the corresponding GA3 treatment had no significant effect on the germination of true seeds imbibed for 10 d (Fig. 4). The expression of PpTFL1 was repressed similarly in both types of ABA-treated embryos.
Discussion
Pear is well known as an important fruit, but few studies have been carried out on pear seed dormancy, and its regulation mechanism is thus still unclear. Our previous studies make clear that the endocarp and seed coat play an important role in maintaining pear seed dormancy (Wang et al., Reference Wang, Dai, Zhang and Shi2012). It has been shown that the seed coat of apple seeds contains over two times the ABA concentration found in the embryos (Subbaiah and Powell, Reference Subbaiah and Powell1987). The ABA content of fresh seeds of red bayberry (Myrica rubra) was distributed in the order: endocarp > seed coat > embryo, with the ABA content of the endocarp about 132-fold higher than in the seed coat and embryo (Chen et al., Reference Chen, Kuo and Chien2008). Therefore, we hypothesized that the presence of high concentrations of endogenous ABA in the endocarp and seed coat suppress the germination of the embryo. Our research shows that the ABA concentration in sand pear embryo and seed coat is similar, and much higher than in red bayberry and apple embryos. Apparently, this is not entirely consistent with the results in apple and red bayberry.
Testa- and embryo-imposed seed dormancy
The ABA concentration in sand pear endocarp was more than two times higher than that in seed coat and embryo. This is in accordance with the observation that the dormancy of sand pear nutlets was deeper than that of true seeds and embryos (Fig. 1; Wang et al., Reference Wang, Dai, Zhang and Shi2012). These concentration differences of ABA could help to prevent embryo ABA from leaching out to the medium, and to maintain the suppression of seed germination by ABA. It has been shown that light is involved in the metabolic regulation of ABA and GA during seed germination (Oh et al., Reference Oh, Kang, Yamaguchi, Park, Lee, Kamiya and Choi2009; Seo et al., Reference Seo, Nambara, Choi and Yamaguchi2009). Our analysis showed that the radicle elongation of imbibed embryos was clearly suppressed in the dark. The average length of the radicles of 10-d imbibed embryos in the dark was ~3 mm, which is less than half the length of those in 10-d imbibed embryos in the light. Light can also strongly stimulate the slow germination of embryos isolated from imbibed dormant apple seeds (Lewak, Reference Lewak2011). Therefore, shading of the imbibed embryos by the dark-brown endocarp could be another way of regulating sand pear seed dormancy. It has also been reported in other species that dark seeds had deeper dormancy and germinated more slowly than light-coloured seeds (Wyatt, Reference Wyatt1977; Khan et al., Reference Khan, Cavers, Kane and Thompson1997). The seed coat may have semi-permeable membrane properties, and can in this way reduce leakage from the embryo during imbibition (Duke and Kakefuda, Reference Duke and Kakefuda1981; Kelly et al., Reference Kelly, van Staden and Bell1992). We have found that when true seeds were imbibed with deionized water, the seed coat was separated from embryo, obviously by water absorption (Wang et al., Reference Wang, Dai, Zhang and Shi2012). This indicates that the seed coat of sand pear is selectively permeable for water. Thus, the sand pear seed coat may inhibit leaching of endogenous ABA, and maintain its suppression of embryo germination. Oxygen is one basic requirement for seed germination, and the seed coat is implied in the regulation of diffusion of gases to the embryo (Kelly et al., Reference Kelly, van Staden and Bell1992). Hypoxia interferes with ABA metabolism and increases ABA sensitivity of embryos of dormant barley grains. In contrast, increases in both permeability of the hull to oxygen and embryo sensitivity to oxygen contribute to the improvement in germination capacity during afterripening (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008). The apple seed coat has been suggested to limit the entrance of oxygen to the embryo, thereby affecting oxidative processes (Lewak, Reference Lewak2011). Thus, the endocarp and seed coat of sand pear may also be decisive in decreasing ABA metabolism and oxygen supply to the embryo, and increasing sensitivity to ABA to maintain dormancy. Suppression of germination of imbibed embryos in the dark is suggestive of embryo-imposed dormancy in sand pear seeds. This type of dormancy also exists in apple seeds, and can be eliminated at room temperature by an anaerobic treatment for several days (Tissaoui and Côme, Reference Tissaoui and Côme1973; Lewak, Reference Lewak2011).
Role of ABA and GA in sand pear embryo dormancy
Exogenous ABA at 0.5 mM (~132.2 ng g− 1 ABA water solution, 25°C) may suppress the germination of sand pear embryos (Fig. 3). The ABA content in fresh seed embryos is as high as 342.59 ng (g FW)− 1, which should be sufficient to suppress germination. High concentrations of ABA in embryos suggest embryo-imposed seed dormancy in sand pear. The GA3 treatment at low concentration had little effect on the germination of either true seeds or embryos (Fig. 4). We hypothesize that the seed coat prevents the loss of endogenous ABA and thus maintains the suppression of endogenous ABA on true seed germination. GA is not sufficient to significantly accelerate the loss of embryo dormancy in the 10-d light culture. GA3 at high concentration dramatically suppressed sand pear embryo germination. Similar results were reported by Zaman et al. (Reference Zaman, Padmesh and Tawfiq2011) in Convolvulus oxyphyllus. It was suggested that GA3 at high concentrations could be toxic.
Dormancy regulation expressed by ABA and GA signalling factors
The expression of sand pear ABI3–5 in embryos was induced by ABA treatment, and showed high levels in the embryos of air-dried seeds, but was down-regulated in germinating embryos (Fig. 5B, graphs a–c), which is similar to the results from previous studies in other species (Lopez-Molina et al., Reference Lopez-Molina, Mongrand, McLachlin, Chait and Chua2002; L. Zhang et al., Reference Zhang, Qiu, Hu, Yang, Yan, Zhao, Li, He, Huang, Li and Li2011). As the expression of DELLA protein genes was induced by ABA treatment, it can be speculated that the high concentration of endogenous ABA induces the expression of PpGAI-1 and PpGAI-2 in the imbibed nutlets. The high levels of expression of DELLA protein genes could suppress GA signalling and maintain dormancy of the nutlets. Unlike PpGAI, transcript levels of PpRGL2 were reduced in imbibed nutlets, but this reduction was suppressed by ABA treatment. Similar results have been reported for Arabidopsis seeds. Arabidopsis RGL2 also plays a role in ABA biosynthesis and regulation of ABI5 activity (Ko et al., Reference Ko, Yang and Han2006; Zentella et al., Reference Zentella, Zhang, Park, Thomas, Endo, Murase, Fleet, Jikumaru, Nambara, Kamiya and Sun2007; Piskurewicz et al., Reference Piskurewicz, Jikumaru, Kinoshita, Nambara, Kamiya and Lopez-Molina2008). The different responses of transcript levels suggests that the DELLA protein genes could be involved in the co-regulation of seed dormancy release, but detailed functions of the gene family members for dormancy regulation need to be studied further. High expression of PpENY was detected in mature embryos, but the transcript level sharply fell in the germinating embryos. This is consistent with the results obtained for Arabidopsis. Thus, PpENY could have similar functions as its Arabidopsis homologue, interacting with DELLA proteins, triggering accumulation of DELLA transcripts and mediating GA effects to balance ABA-promoted maturation during late seed development (Feurtado et al., Reference Feurtado, Huang, Wicki-Stordeur, Hemstock, Potentier, Tsang and Cutler2011).
PpMFT expression was down-regulated in imbibed embryos. This is consistent with the down-regulated expression of ABI3, ABI5 and DELLA protein genes in germinating embryos of sand pear. In germinating Arabidopsis seeds, MFT expression was directly up-regulated by ABI3 and ABI5, and was also up-regulated by DELLA proteins in the GA-signalling pathway. The up-regulation of MFT by ABI and DELLA proteins, in turn, provided negative feedback regulation of ABA signalling by directly repressing ABI5 (Xi et al., Reference Xi, Liu, Hou and Yu2010). The expression of another PEBP gene, PpFT, was significantly induced in germinating embryos (Fig. 5B, graph j). Chiang et al. (Reference Chiang, Barua, Kramer, Amasino and Donohue2009) have shown that FT was involved in FLC (FLOWERING LOCUS C)-mediated germination, involving the ABA catabolic and GA biosynthetic pathways in seeds. This regulation could be imposed during the late stages of seed development, when FLC expression peaked and corresponding levels of FT, SOC1 and AP1 decreased. So the high level of expression of PpFT could promote seed germination in the putative FLC homologous signalling pathway in sand pear. FT and TFL1 have highly conserved amino acid sequences but opposite flowering-regulation functions (Kardailsky et al., Reference Kardailsky, Shukla, Ahn, Dagenais, Christensen, Nguyen, Chory, Harrison and Weigel1999; Kobayashi et al., Reference Kobayashi, Kaya, Goto, Iwabuchi and Araki1999). However, the expression of PpTFL1 showed no significant change in both imbibed nutlets and imbibed embryos, which suggests that PpTFL1 could not be involved in the regulation of sand pear seed dormancy and germination.
The regulation of seed dormancy is a complex process. In addition to hormonal suppression, the mechanical constraint, reactive oxygen species, anti-oxidants and modifying proteins of endocarp and seed coat can also prevent seed germination. The mobilization and transformation of the seed reserves of lipids, proteins, soluble sugars, pentosanes and a small amount of starch have been considered as unquestionably cardinal processes for seed germination, whereas the control of these processes has been considered an important element in the metabolic regulation of dormancy (Wierszyłłowski, Reference Wierszyłłowski1960; Kawęcki, Reference Kawęcki1970; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Lewak, Reference Lewak2011).
In previous studies, apple seeds have often been used as model material, due to their well-expressed dormancy, easy availability and economic value (Lewak, Reference Lewak2011). Both pear and apple belong to the pear subfamily of Rosaceae. Compared to apple, pear is also an important fruit tree, and has stronger environmental adaptability and a wider cultivation area. However, biological research on many aspects of pear is scarcer than that of apple. This study analysed the hormonal and molecular regulation of sand pear seed dormancy, and the results contribute to the knowledge of Rosaceae nutlet dormancy. With the completion of the pear genome sequencing and the accelerated development of pear molecular biology, pear could be used as another model plant of Rosaceae.
Acknowledgements
This study was supported by the Special Funds for China Agriculture Research System (CARS-29-04) and the Doctoral Start-up Research Funds of Zhejiang Academy of Agricultural Sciences.