1. Introduction
Bothremydidae is an extinct group of morphologically diverse pleurodiran turtles found in Lower Cretaceous to Eocene sediments of South and North America, Africa, Madagascar, India and Europe. Bothremydids (contrary to extant side-necked turtles) were widespread in the northern hemisphere, inhabited both freshwater and nearshore marine environments and developed considerably different feeding strategies (Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006 and references therein). An extensive review of their morphology, diversity, taxonomy and phylogeny has been recently given by Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006) who subdivided the family into four tribes (Kurmademydini, Cearachelyini, Bothremydini and Taphrosphyini); the tribe Bothremydini is further divided into two subtribes, Foxemydina and Bothremydina. Currently, three genera and five species belong to the Foxemydina, including Foxemys mechimorum Tong, Gaffney & Buffetaut, Reference Tong, Gaffney and Buffetaut1998, Polysternon provinciale Matheron, Reference Matheron1869, P. atlanticum Lapparent de Broin & Murelaga, Reference Lapparent de Broin and Murelaga1996, Elochelys perfecta Nopcsa, Reference Nopcsa1931 and E. convenarum Laurent, Tong & Claude, Reference Laurent, Tong and Claude2002, which, perhaps due to their freshwater habitat, were restricted to the Late Cretaceous of Europe. On the other hand the Bothremydina adapted to a near-shore marine lifestyle, which probably allowed them a wider temporal and geographical distribution in the Late Cretaceous to Middle Eocene period of Africa, Europe and North America.
Formerly, the Foxemydina has been recognized as an endemic element of the Campanian–Maastrichtian faunas of France and Spain where their remains are often found abundantly (see Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006 for a review on their distribution). Here we describe a new species of the genus Foxemys from the Santonian of Hungary, which increases the known diversity of the group and proves that they had a wider temporal and stratigraphical distribution than previously thought. The present contribution is intended to provide a brief description of the cranial morphology of F. trabanti sp. nov., solve some taxonomical debates concerning the genus Foxemys (Gaffney, Tong, Meylan, Reference Gaffney, Tong and Meylan2006 contra Lapparent de Broin, Reference Lapparent de Broin2001) and to clarify relationships of the new species within the Bothremydidae via a cladistic analysis. Finally, we also explore the complex biogeographical history of the tribe Bothremydini.
2. Institutional abbreviations
AE – Costa collection, Montpellier, France; MC – Musée de Cruzy, Cruzy, France; MTM – Natural History Museum of Hungary, Budapest; MDE – Musée des Dinosaures, Espéraza, France; PAM – Patrick and Annie Méchin collection, Vitrolles, France.
3. Location and geology
At the Iharkút site, siliciclastic sediments are exposed in an open-pit bauxite mine in the location of the former village of Iharkút in the Bakony Mountains, Veszprém county, western Hungary (Fig. 1). The exposed section of this unit is composed of 50 m thick non-marine deposits and represents the upper part of the Upper Cretaceous Csehbánya Formation. The Csehbánya Formation overlies the bauxite accumulated in dolomitic karst holes and is overlain by an Eocene conglomerate or Oligocene fluvial sediments. Dominantly, the Csehbánya Formation is built up of siltstone and variegated clay, including palaeosoil horizons, and interbedding sandy channel deposits (Jochca-Edelényi, 1997). The vertebrate-bearing beds form 2–3 m thick cyclical units beginning with green clay covered by breccia containing clay clasts, sandstone and siltstone layers (Tuba et al. Reference Tuba, Kiss, Pósfai and Mindszenty2006). Most of the material, including the turtles comes from the matrix-supported breccia consisting of ripped-up variegated clay clasts, dolomite pebbles, carbonized plant remains and sand forming channel-like structures with the isolated bones accumulated in lenses. Fragmentary turtle plates are among the most abundant remains at the site. The brown siltstone layer also yielded some more intact plate fragments and a number of partial shells. Occasional plate fragments were also found in the palaeosoil unit. All of the cranial material was found isolated except one specimen, a fragmentary skull (MTM V2010.216.1.), which was embedded in siltstone next to an anterior lobe of a plastron. In addition to bothremydid turtles, the fauna from Iharkút consists of pycnodontiform and lepisosteid fishes, albanerpetontid and anuran amphibians, dortokid pleurodiran turtles, borioteiid and mosasauroid squamates, ziphosuchian-like, hylaeochampsid and other eusuchian crocodyliforms, nodosaurid, rhabdodontid and theropod dinosaurs, azdarchid pterosaurs and enantiornithine birds (Makádi, Botfalvai & Ősi, Reference Makádi, Botfalvai and Ősi2006; Ősi & Rabi, Reference Ősi and Rabi2006; Szentesi & Venczel, Reference Szentesi and Venczel2009). The palynological study of Knauer & Siegl-Farkas (Reference Knauer and Siegl-Farkas1992) indicates a Santonian age for the Csehbánya Formation, which was recently confirmed by the palaeomagnetic results of Szalai (Reference Szalai2005). The palaeoenvironment is interpreted as an alluvial plain and the deposition of the breccia could be the result of the episodic filling of a channel or pond by debris flows, silt and sand (Ősi & Mindszenty, Reference Ősi, Mindszenty and Babinszky2009).

Figure 1. Geographic location of the Iharkút site situated in an open-pit bauxite mine in the Bakony Mountains, Veszprém county, western Hungary, which yielded the Foxemys trabanti n. sp. material. The crossed hammers indicate the location of the mine.
4. Systematic palaeontology
Order TESTUDINES Linnaeus, Reference Linnaeus1758
Infraorder PLEURODIRA Cope, Reference Cope1864
Hyperfamily Pelomedusoides Cope, Reference Cope1868
Family Bothremydidae Baur, Reference Baur1891
Tribe Bothremydini Baur, Reference Baur1891
Subtribe Foxemydina Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006
Genus Foxemys Tong, Gaffney & Buffetaut, Reference Tong, Gaffney and Buffetaut1998
Type species. Foxemys mechinorum Tong, Gaffney & Buffetaut, Reference Tong, Gaffney and Buffetaut1998.
Included species. F. mechinorum, F. trabanti.
Distribution. Late Cretaceous Santonian of Hungary and Late Campanian–Early Maastrichtian of France.
Revised diagnosis. A bothremydid pleurodire of the Foxemydina with the following characters differentiating it from Polysternon: prefrontals do not taper anteriorly; deeper fossa pterygoidei; deeper labial ridge of triturating surface; condylus mandibularis closer to the level of condylus occipitalis; basioccipital shorter than basisphenoid; shell similar to Polysternon but lacking the parallel striations, straight lateral border of posterior lobe of the plastron and a wide anal notch, in contrast to Polysternon.
Foxemys trabanti sp. nov. Figures 2–5
Type specimen. MTM V2010.86.1., incomplete skull.

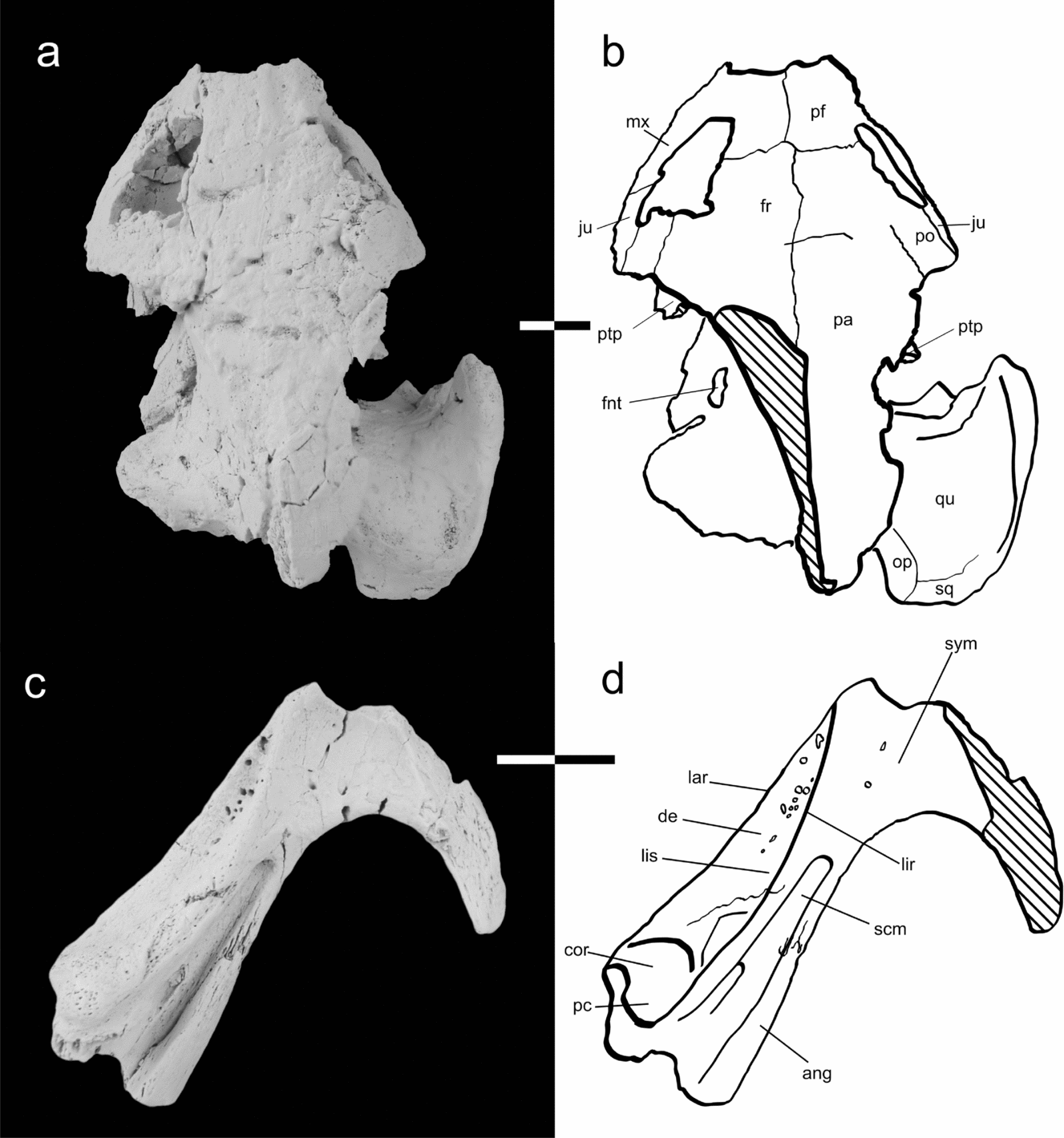
Figure 2. Holotype skull (MTM V2010.86.1.) and referred incomplete lower jaw (MTM V2010.89.1.) of Foxemys trabanti n. sp. in dorsal view. (a) Composite photo of the skull in dorsal view consisting of several layers shot in different planes. (b) Line interpretation of sutures and structures. (c) Composite photo of the lower jaw in dorsal view consisting of several layers shot in different planes. This process required coating with ammonium chloride to make the surface homogenous. (d) Line interpretation of sutures and structures. Anatomical abbreviations: ang – angular, ap – antrum postothicum, bo – basioccipital, bs – basisphenoid, co – condylus occipitalis, cor – coronoid, de – dentary, ex – exoccipital, fjp – foramen jugulare posterius, fm – foramen magnum, fnt – foramen nervi trigemini, fnv – foramen nervi viadini, fpcci – foramen posterius canalis carotici interni, fpp – foramen posterius palatinus, fpt – fossa pterygoidei, fr – frontal, fst – foramen stapedio temporale, ica – incisura columella auris, ju – jugal, lar – labial ridge, lhv – lateral head vein, lir – lingual ridge, lis – lingual shelf, mx – maxilla, op – opisthotic, pa – parietal, pal – palatine, pc – processus coronoideus, pf – prefrontal, po – postorbital, pr – prootic, pt – pterygoid, ptp – processus trochlearis pterygoidei, qu – quadrate, sa – stapedial artery, scm – sulcus cartilaginis meckelii, so – supraoccipital, spp – sulcus palatinopterygoideus, sq – squamosal, sym – symphysis, XII – foramen nervi hypoglossi. Scale bar equals 1 cm.

Figure 3. Holotype skull of Foxemys trabanti n. sp. (MTM V2010.86.1.) and line interpretation of sutures. (a, b) Ventral view; (c, d) right lateral view; (e, f) occipital view. Grey filling indicates pyritized matrix. For anatomical abbreviations see Figure 2. Scale bar equals 1 cm.

Figure 4. Partial skull of Foxemys trabanti n. sp. (MTM V2010.87.1.). (a, b) Photos in dorsal (a) and ventral (b) view; (c, d) line interpretation of sutures and structures in dorsal (c) and ventral (d) view. Grey filling indicates pyritized matrix. For anatomical abbreviations see Figure 2. Scale bar equals 1 cm.

Figure 5. Left otic chamber of Foxemys trabanti n. sp. (MTM V2010.88.1.). (a, c, e) Composite photos consisting of several layers shot in different planes. This process required coating with ammonium chloride to make the surface homogeneous; (a) anterior; (c) ventral; (e) lateral view. (b, d, f) Line interpretations of sutures and structures; (b) anterior; (d) ventral; (f) lateral view. Note open incisura columella auris. For anatomical abbreviations see Figure 2. Scale bar equals 1 cm.
Type locality. Németbánya II bauxite lens, Iharkút, Bakony Mountains, Hungary.
Horizon. Csehbánya Formation, Santonian, Upper Cretaceous.
Diagnosis. Member of the Foxemys genus differing from the type species in the absence of an accessory ridge on the triturating surface of the maxilla; a narrower incisura columella auris as a result of a triangular, dorsally projecting ventral process of the quadrate; the parietal contacting the pterygoid through the base of the processus trochlearis pterygoidei as in Bothremys arabicus and Chedighaii hutchisoni; the basioccipital concavity hardly extending onto the basisphenoid as in Polysternon; having a dentary with a considerably wider U-shaped depression on the symphysis and more rounded anterior tip.
Etymology. In honour of the cultic East German (former German Democratic Republic) automobile Trabant 601, which is intensely used by the Iharkút research group and serves as an indispensable field car and transporter vehicle during the excavations.
Referred material. MTM V2010.215.1., skull; MTM V2010.216.1., partial skull; MTM V2010.87.1., partial skull; MTM uncatalogued partial skull; MTM V2010.88.1., left otic chamber; MTM V2010.89.1., fragmentary left lower jaw; MTM V2010.219.1., right and left lower jaw; MTM V2010.220.1., left lower jaw.
5. Description
The following brief description is based on the holotype (MTM V2010.86.1., Figs 2a, b, 3), MTM V2010.87.1. a partial skull, which lacks the prefrontal and orbital region of the cranium (Fig. 4) and MTM V2010.88.1., a left otic chamber (Fig. 5). The overlapping sections of both crania are very similar in morphology but MTM V2010.87.1. and MTM V2010.88.1. provide many details of the otic region that are not so clear in the type specimen. We also refer to two other skulls, MTM V2010.215.1. and MTM V2010.216.1., to reflect some variation within F. trabanti, but these specimens will be described elsewhere in more detail. Our comparisons with Foxemys mechinorum are based on personal observations on specimens, including PAM 511A, MDEt 10, MC M1734, MC M2119 skulls and PAM 511B, MC M2114–MC M2118 lower jaws. Comparative material of Polysternon provinciale consisted of skull AE28. For descriptions of most of these materials see Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006), Tong, Gaffney & Buffetaut (Reference Tong, Gaffney and Buffetaut1998) and Tong & Gaffney (Reference Tong and Gaffney2000). Comparative observations with other taxa in the text are based on Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006). Morphological features that correspond to phylogenetic character definitions used in Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006) are indicated by the appropriate numbers in subscript.
Preservation. The surface of MTM V2010.86.1. is badly weathered and some elements and sutures are thus barely recognizable compared to MTM V2010.87.1. and MTM V2010.88.1. The type skull suffered considerable dorsomedial deformation on the right anterior side of the orbital region. It completely lacks the premaxillae, the vomer and the quadratojugals, and the edges of the parietals are damaged. On the right side both the quadrate and the squamosal are broken off but the left ones are in good condition. Dorsally, the elements of the otic chamber are not distinguishable in the type specimen owing to weathering. MTM V2010.87.1. excellently preserves the basicranium and the bones of the otic chamber including part of the parietal and the entire supraoccipital process.
Description and comparisons. The prefrontal resembles that of Foxemys mechinorum in both shape and position. It is roughly rectangular and forms the anteromedial edge of the orbits. As in all Pelomedusoides the prefrontals meet in the midline4. The anterior and posterior margins are almost equally wide in MTM V2010.86.1. and F. mechinorum, contrasting with the anteriorly notably narrower prefrontal of Polysternon (Fig. 2a, b).
The visible contacts of the frontal of F. trabanti are with the prefrontal anteriorly and the other frontal medially. Laterally, the frontals border the laterally placed, subcircular orbits11 to an equal extent as the prefrontals do (Figs 2a, b, 3c, d). This is slightly different to F. mechinorum where the contribution of the frontal is less pronounced and not equal to the extension of the prefrontal. F. trabanti has a subcircular orbit with a higher ventral orbital rim than that of F. mechinorum (Fig. 3c, d).
The extent of the temporal emargination, partly depending on the shape of the parietals, shows a considerable variation among bothremydid turtles from extreme emargination seen in Kurmademys to the completely roofed condition of Taphrosphys. In MTM V2010.86.1. most of the lateral margins of the parietals are damaged but on the right side of MTM V2010.87.1. the posterior segment is more or less intact. The rim preserved here implies a slight emargination14 with the prootic covered dorsally, similar to F. mechinorum and Polysternon and unlike Cearachelys or Kurmademys (Figs 2a, b, 4).
The jugal meets the maxilla ventrally, and dorsally there is a contact with the postorbital (Figs 2a, b, 3a–d). The absence of the quadratojugal in MTM V2010.86.1. provides insight into the composition of the septum orbitotemporale. Here the medial process of the jugal is exposed and has a wide contact with the palatine as in F. mechinorum and unlike Bothremys maghrebiana where the medial extension of the maxilla separates these two elements. Like in F. mechinorum and Polysternon the jugal does not contribute in the build-up of the triturating surface23 (Fig. 3a, b).
In the septum orbitotemporale the medial process of the postorbital forms a closed wall28 and has a ventrolateral contact with the jugal, a ventromedial contact with the palatine and a dorsolateral contact with the parietal; all as in F. mechinorum (Fig. 2a). With the exception of the parietal and inclusion of the maxilla, these elements define the fossa orbitalis, which has a posterior enlargement27 as in Foxemys and most other bothremydids (Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006).
The squamosal is cone-shaped and resembles that of F. mechinorum, Polysternon and other Bothremydini. Like in the Bothremydini and some Taphrosphyini the squamosal process is greatly overhanging the posterior termination of the opisthotic24 (Figs 2a, b, 3, 4).
In ventral view, the triturating surface of MTM V2010.86.1. has a posteriorly broadening outline similar to that of F. mechinorum and Polysternon 34. The triturating surface of this skull is relatively narrower with a less curved labial ridge in ventral view compared to F. mechinorum (PAM 511A and MDEt 10), resembling that of Polysternon in this respect. On the other hand, it possesses deeper labial ridges posteriorly more similarly to F. mechinorum 37 (Fig. 3a, b). However, MTM V2010.215.1. and MTM V2010.216.1. have wide and labially curved triturating surfaces in ventral view, which indicates that these structures can vary intraspecifically. An accessory ridge on the triturating surface is absent in all known specimens of F. trabanti 36.
As preserved in MTM V2010.86.1. the horizontal plate of the palatine meets the maxilla anterolaterally and takes part in the formation of the triturating surface to a similar extent as in F. mechinorum 50. The foramen palatinum posterius is placed behind the orbit close to the processus trochlearis pterygoidei48 (Fig. 3a, b).
On the ventral side of MTM V2010.88.1. the foramen posterius canalis carotici interni lies at the pterygoid–basisphenoid suture74,75 (Fig. 5c, d). The processus trochlearis pterygoidei is present in MTM V2010.86.1. (Figs 2a, b, 3a–d, 4b, d) like in all members of the Pleurodira70, and its base narrowly contacts the processus inferior parietalis of the parietal, in contrast to F. mechinorum.
The incisura columella auris is damaged in MTM V2010.86.1. but excellently preserved in MTM V2010.88.1. The incisura is nearly closed and narrows posteriorly by the near meeting of the dorsal and ventral processes of the quadrate, forming a marked slit-like structure, typical of the Foxemydina52. However, MTM V2010.88.1. is unique in the morphology of the incisura, as a dorsally projecting triangular ventral process almost completely separated the Eustachian tube from the stapes (Fig. 5a, b, e, f). In dorsal view the foramen stapedio-temporale is not visible, the result of an anterior shift92 (Figs 2a, b, 4a–c, 5a, b). Like other members of the Foxemydina, the Hungarian taxon has deep fossa pterygoidei68 which are similar to those of F. mechinorum, but deeper than in Polysternon. Unlike in Polysternon the position of the condylus mandibularis is close to the level of the condylus occipitalis60 although not as close as in F. mechinorum (Figs 3a, b, 5c, d).
The supraoccipital is in contact with the quadrate79 and the processus supraoccipitalis appears to be complete in MTM V2010.87.1. It appears that F. trabanti probably has one of the longest processes among bothremydids, extending much farther to the posterior than the posterior tip of the squamosals. However, this region is often damaged and incompletely known in other taxa (Fig. 4).
Ventrally, the exoccipital widely contacts the quadrate85 as evident from the right otic chamber (MTM V2010.88.1.). This character is considered to be a synapomorphy of the Bothremydidae by Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006). The foramen jugulare posterius is exposed solely on the posterior surface of the exoccipital, and as in other Foxemydina it is laterally open82 (Figs 3e, f, 5c, d).
As in the French bothremydids, the basisphenoid has a pentagonal shape in ventral view106 but the basioccipital concavity in F. trabanti barely extends onto the basisphenoid, which is more reminiscent of Polysternon (Figs 3a, b, 4b, 5c). In F. mechinorum this concavity is larger, extending onto the posterior half of the basisphenoid.
As in F. mechinorum, the ventral exposure of the basioccipital is half as short as that of the basisphenoid in all specimens of F. trabanti. In Polysternon, the basioccipital and the basisphenoid are similar in length in ventral view. The occipital condyle is preserved in MTM V2010.87.1. and is formed by the exoccipitals only84. Its position relative to the mandibular condyle of the quadrate is comparable to F. mechinorum (Figs 3a, b, 4b, c, 5c, d).
A fragmentary left ramus of a lower jaw (MTM V2010.89.1.; Fig. 2c, d) is also referred to F. trabanti on the basis of the typical posteriorly broadening triangular triturating surface118 shared with many other bothremydids and particularly because of its close resemblance to F. mechinorum. MTM V2010.89.1. is composed of the entire dentary with the anterior half of the angular attached to the coronoid. The anterior tip of the symphysis is missing and a small fragment of the right dentary is fused to the left ramus. As in other Bothremydini, the triturating surface is built up mainly by the dentary, and the wide coronoid contributes to the posteromedial part. Similarly to F. mechinorum, the labial ridge of the triturating surface is acute anteriorly and it becomes swollen and lower posteriorly. The lingual ridge forms a wide shelf and a shallow trough runs along it with the medial sidewall being the labial ridge. The lingual ridges of the two triturating surfaces form a U-shaped depression on the symphysis116 similarly to F. mechinorum. A particular feature of all known lower jaws from Iharkút is that the depression on the symphysis is considerably wider than in any specimens of F. mechinorum (PAM 511B, MC M2114–M2118; Fig. 2c, d).
6. Discussion
The specimens described above are well suited to reveal and accurately clarify the relationships of the Iharkút turtle material. The new taxon was a pleurodire as shown by the presence of the processus trochlearis pterygoidei, the position of the foramen palatinum posterius behind the orbit and the medially extending ventral process of the quadrate below the cranioquadrate space. It shares diagnostic features with Pelomedusoides, like the midline contact of the prefrontals and the absence of nasals. Based on the following characters, the side-neck turtle from Iharkút is attributed to the Bothremydidae: wide exoccipital–quadrate contact present; fossa precolumellaris absent; fossa orbitalis posterior enlargement present; and foramen stapedio-temporale faces anteriorly. Within the Bothremydidae the new species belongs to the Bothremydini as indicated by the broad preorbital part of the skull, the moderate temporal emargination (deep in the Cearachelyini and Kurmademydini and slight in the Taphrosphyini), a large palatine contribution to the triturating surface and the presence of a supraoccipital–quadrate contact. Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006) divided the tribe Bothremydini into two subtribes, the Foxemydina and Bothremydina, and we include the Iharkút form in the former because the triturating surface of both the maxilla and dentary lack pits, the jugal is not exposed in the triturating surface, the Eustachian tube and the stapes were separated by a narrow fissure (not by bone as in all other Bothremydidae except Cearachelys), the fossa pterygoidei is deep and narrow, the foramen jugulare posterius is only partially closed and the shape of the basisphenoid is pentagonal in ventral view (all listed characters are after Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006).
Based on our direct comparisons with skulls of bothremydids from France (F. mechinorum: PAM 511A, MDEt 10, MC M1734, MC M2119; P. provinciale: AE 28) the Iharkút turtle seems to be more closely related to F. mechinorum than to P. provinciale as the prefrontals do not taper anteriorly, the ventral orbital rim is a low, curved surface obscuring the borders of the fossa orbitalis and the lateral surface of the maxilla, the labial ridge of the triturating surface is deeper, the fossa pterygoidea is deeper and the basioccipital is shorter than the basisphenoid. Related to the short length of the basioccipital, the plane of the occipital condyle appears to be shifted anteriorly and lies closer to the level of the condylus mandibularis of the quadrate in P. provinciale than in F. trabanti and F. mechinorum. Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006) and Lapparent de Broin (Reference Lapparent de Broin2001) noted that an additional difference between P. provinciale and F. mechinorum is the wider and more rounded triturating surface in the latter, but the series of specimens from Iharkút show that this morphology is variable and there are narrower, triangular morphotypes (MTM V2010.86.1. and MTM uncatalogued) which are very similar to Polysternon. Lapparent de Broin (Reference Lapparent de Broin2001) considered Foxemys a junior synonym of Polysternon, but accepted the presence of a separate species as P. mechinorum. We consider Foxemys to be a valid genus and as mentioned above, the Iharkút taxon shares more characters with F. mechinorum than with Polysternon provinciale. We recognize the Iharkút taxon to be a new species because the labial ridge of the maxillary triturating surface is deeper; the incisura columella auris is narrower, owing to the near contact of the triangular ventral quadrate process and the vertical dorsal process, not seen in any other Bothremydidae; the parietal contacts the pterygoid at the base of the processus trochlearis pterygoidei; the median concavity of the basioccipital hardly extends onto the basisphenoid (the anterior half of this depression lies on the basisphenoid in F. mechinorum) and unlike F. mechinorum the symphyseal basin of the dentary is considerably wider, resulting in a more rounded anterior tip of the lower jaw. In addition, Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006) reported an accessory ridge on the upper triturating surface of one F. mechinorum skull (MC M1734). The re-examination of PAM 511A and MDEt 10 skulls reveals that these specimens also bear a worn accessory ridge on the triturating surface. Neither of the skulls of F. trabanti show an accessory ridge on the upper triturating surface.
7. Phylogenetic analysis
Cranial and postcranial character states from F. trabanti were coded into a modified version of the comprehensive published matrix of Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006). All 175 characters were treated as unordered and unweighted. Analyses were conducted in PAUP 4.0 (Swofford, Reference Swofford2001) using a heuristic search algorithm. Our runs of the data excluded shell only taxa and employed two modified characters for F. mechinorum (no. 36 to 1 and no. 120 to 1) based on new observations (see Appendix for character scorings). One additional character was changed for E. convenarum (no. 142 to 2) following the description of Laurent, Tong & Claude (Reference Laurent, Tong and Claude2002). Nodal support was evaluated via non-parametric bootstrapping with 500 replicates and a Bremer decay analysis conducted with TreeRotv3. The single resulting tree (tree length = 522, CI = 0.7050, HI = 0.5421, RI = 0.8353) was identical in topology to the cladogram 1 of Gaffney, Tong & Meylan (Reference Gaffney, Tong and Meylan2006, fig. 288) and placed F. trabanti with F. mechinorum in a monophyletic sister clade to Polysternon provinciale (Fig. 6a). Bootstrap and Bremer values indicate reasonable support for both the Foxemydina (bootstrap percentage/Bremer decay index = 78/2) and Foxemys spp. (73/1) but lower (less than 70% bootstrap) for the majority of the nodes within the Bothremydina. Consequently, additional phylogenetic data might alter the palaeobiogeographic hypothesis concerning the latter group (see Section 8).

Figure 6. Phylogeny and palaeobiogeography of the Bothremydini. (a) Temporally calibrated cladogram showing the relationships, geographic distribution and habitat of the tribe Bothremydini with indications of the main events in its biogeographic history and changes in lifestyle. Barr. – Barremian; Cen. – Cenomanian; Tur. – Turonian; Con. – Coniacian; San. – Santonian; Cam. – Campanian; Maa. – Maastrichtian; Dan. – Danian; Sel. – Selandian; Tha. – Thanetian; Ypr. – Ypresian. (b, c) Palaeogeographic reconstruction of the continents during the Santonian (b) and the Campanian (c) with the Bothremydini localities added from this period. Circles correspond to tribe Foxemydina; squares correspond to tribe Bothremydina. Numbers correspond to more than one taxon in a few cases and only taxa identified at least at tribe level are indicated. 1 – Foxemys trabanti (Hungary, present work); 2 – Bothremys arabicus (Jordan, Zalmout et al. Reference Zalmout, Mustafa and Wilson2005; Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006); 3 – Chedighaii or Bothremys barberi (Kansas, USA, Gaffney & Zangerl, Reference Gaffney and Zangerl1968; Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006); 4 – Rosasia soutoi (Portugal, Antunes & Broin, Reference Antunes and Broin1988); 5 – Foxemys mechinorum (Tong, Gaffney & Buffetaut, Reference Tong, Gaffney and Buffetaut1998), Polysternon provinciale (Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006) and Elochelys perfecta (France, Nopcsa, Reference Nopcsa1931); 6 – Polysternon atlanticum and ?Elochelys sp. (Spain, Lapparent de Broin & Murelaga, Reference Lapparent de Broin and Murelaga1996, Reference Lapparent de Broin and Murelaga1999); 7 – Chedighaii hutchisoni (New Mexico, USA, Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006); 8 – Chedighaii or Bothremys barberi (Arkansas, USA, Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006); 9 – Bothremys sp. and Chedighaii sp. (Alabama, USA, Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006; Gaffney, Hooks & Schneider, Reference Gaffney, Hooks and Schneider2009); 10 – Chedighaii or Bothremys barberi (Georgia, USA, Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006); 11 – Bothremys sp, Chedighaii sp. and Bothremydina indet. (North Carolina, USA, Gaffney, Hooks & Schneider, Reference Gaffney, Hooks and Schneider2009); 12 – Chedighaii or Bothremys barberi (New Jersey, USA, Gaffney, Hooks & Schneider, Reference Gaffney, Hooks and Schneider2009).
8. Biogeography
The members of the Foxemydina appear to be common elements of the European Late Cretaceous non-marine turtle faunas but their possible absence is noteworthy in the Maastrichtian of Transylvania, where the basal cryptodire Kallokibotion dominates (Gaffney & Meylan, Reference Gaffney and Meylan1992; Vremir & Codrea, Reference Vremir and Codrea2009). The five or six taxa of the Foxemydina (depending on the validity of Polysternon atlanticum) are endemic to the Mediterranean Basin, unlike the Bothremydina, which were present in North America also. Among the freshwater turtles of the Late Cretaceous of Europe, the Foxemydina seem to acquire the greatest diversity compared to the less diverse cryptodires and dortokid pleurodires (Lapparent de Broin & Murelaga, Reference Lapparent de Broin and Murelaga1996, Reference Lapparent de Broin and Murelaga1999; Vremir & Codrea, Reference Vremir and Codrea2009). While the turtle fauna of Gondwanan landmasses was almost exclusively formed by side-necks during the Late Cretaceous period, within Laurasia, Europe was the only area where pleurodires dominated the non-marine aquatic environments (Hirayama, Danilov & Brinkman, Reference Hirayama, Brinkman and Danilov2000; Gaffney, Hooks & Schneider, Reference Gaffney, Hooks and Schneider2009). Thus while in the Early Cretaceous period the faunal connections of Europe were restricted to North America (as indicated by the distribution of solemydids; Hirayama, Danilov & Brinkman, Reference Hirayama, Brinkman and Danilov2000; Larson & Brinkman, Reference Larson, Brinkman and Braman2009; Milner, Reference Milner2004; Lapparent de Broin & Murelaga, Reference Lapparent de Broin and Murelaga1999), in the Late Cretaceous period it was completed with an African influence as indicated by the presence of bothremydids. The distribution of the latter, however, implies that connections with North America were not broken off at least until Santonian time (see next Sections).
8.a. Origin of Bothremydini and dispersion to the Mediterranean
During the Late Cretaceous period the Bothremydini reached remarkable diversity, accompanied by a wide distribution. From the Santonian to the Maastrichtian at least ten separate taxa were present along the northern shores of Africa, in the European archipelago and in North America (Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006; Gaffney, Hooks & Schneider, Reference Gaffney, Hooks and Schneider2009). The outgroups Cearachelyini and Kurmademydini are all from the Gondwana landmass and the Bothremydini thus likely had a Gondwanan origin as well (Broin, Reference Broin1988; Lapparent de Broin, Reference Lapparent de Broin2000; Lapparent de Broin & Murelaga, Reference Lapparent de Broin and Murelaga1999). Based on the results of our phylogenetic analysis the first dispersal event to Europe is represented by the subtribe Foxemydina (Fig. 6a). The oldest representative of this lineage is Foxemys trabanti, which indicates that the clade probably migrated to Europe prior to the Santonian. This occurrence together with the possible affinity of ‘Podocnemis parva’ Haas, Reference Haas1978a and ‘Podocnemis judea’ Haas, Reference Haas1978b from the Cenomanian of Israel with Foxemydina (see Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006) may suggest the migration of the group along the eastern edge of the Mediterranean via Apulia to Iberia. Our phylogenetic analysis indicates a complex biogeographic history of Late Cretaceous European bothremydids: although F. mechinorum lived in the same geographical area as P. provinciale (i.e. southern France, but later in time) it is more closely related to F. trabanti from the East Central European region.
A second dispersal event must have occurred within the Bothremydina, supposedly from North Africa to Iberia, as Rosasia soutoi Carrington da Costa, Reference Carrington da Costa1940 nested with this clade is known from the Campanian to Maastrichtian of Portugal (Antunes & Broin, Reference Antunes and Broin1988). Zolhafah bella Lapparent de Broin & Werner, Reference Lapparent de Broin and Werner1998 from the Maastrichtian of Egypt (Lapparent de Broin & Werner, Reference Lapparent de Broin and Werner1998) is basal to all other Bothremydina, and although it is more derived than Foxemys or Polysternon, it does not necessarily imply a back dispersal to Africa from Europe (Fig. 6a, c). It seems more parsimonious that the Bothremydina had an African common ancestor with Foxemydina well before the Santonian.
8.b. Dispersion to North America
The Bothremydini is currently also represented in North America by three taxa: Bothremys cooki Leidy, Reference Leidy1865; Chedighaii hutchisoni Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006 and a species with uncertain generic assignment (Chedighaii or Bothremys barberi, see Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006 and Gaffney, Hooks & Schneider, Reference Gaffney, Hooks and Schneider2009; Fig. 6b, c). The earliest occurrence of the Bothremydini in North America is based on shell material from the Coniacian–Santonian Niobrara Formation of Kansas; thus, the colonization must have occurred by the Coniacian or before. The cladogram in Figure 6a predicts two dispersal events: one for Chedighaii and one for Bothremys. Bothremys is also present with a separate species, Bothremys arabicus, in the Santonian of Jordan, then linked to Africa (Fig. 6c). Thus, based on our current knowledge, the most simple explanation is to originate both North American lineages from North Africa.
Although originally inhabiting a freshwater habitat, most bothremydids (including Bothremys) are considered to have led a nearshore marine lifestyle; thus, the salinity could probably not have been a constraining factor in the case of a trans-oceanic migration (Fig. 6a). However, the dispersion of the group from Africa to North America could have also happened along the high-latitude Thulean route (via Europe), which was a variably terrestrial and shallow marine connection between Europe, the British Isles, Greenland and eastern North America during the Late Cretaceous period (Sanmartin, Enghoff & Ronquist, Reference Sanmartin, Enghoff and Ronquist2001). For such a near-shore environment, they were better adapted considering their unreduced shell and the absence of paddle-like digits. Moreover, as indicated by their enlarged triturating surface, often with palatal pits, members of the Bothremydini were durophagous (Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006) and were likely bottom dwelling. As shown by Martin et al. (Reference Martin, Case, Jagt, Schulp and Mulder2005), the high-latitude Thulean corridor played an important role in terrestrial faunal exchanges at least in the Maastrichtian period, and determined the intercontinental spread of dromeosaurid and hadrosaurid dinosaurs, boid snakes and herpetotheriid marsupials from North America to Europe. However, the migration could only happen after the Bothremydina physiologically adapted to a marine habitat and their osmoregulatory systems were developed enough to constantly live in saltwater. This adaptation already appeared by the Santonian as both Bothremys arabicus and Chedighaii or Bothremys barberi probably had a near-shore marine lifestyle. However, some taxa were able to live in freshwater environments as Chedighaii hutchisoni from the Late Campanian of New Mexico, USA is known from fluvial and deltaic sediments, while material referred to Chedighaii sp. and Bothremys sp. have been reported from the deltaic sequence of the Tar Heel Formation of North Carolina (Fig. 6a; Gaffney, Tong & Meylan, Reference Gaffney, Tong and Meylan2006; Gaffney, Hooks & Schneider, Reference Gaffney, Hooks and Schneider2009).
Another constraint for a high-latitude migration for turtles is their temperature tolerance. Extreme climatic warmth during the early Late Cretaceous period has been attested to (Tarduno et al. Reference Tarduno, Brinkman, Renne, Cottrell, Scher and Castillo1998), which allowed turtles to migrate from Asia to North America via the High Canadian Arctic (Brinkman & Tarduno, Reference Brinkman and Tarduno2005; Vandermark et al. Reference Vandermark, Tarduno, Brinkman, Cottrell and Mason2009) and Alaska (Parrish et al. Reference Parrish, Parrish, Hutchison and Spicer1987).
Acknowledgements
The authors wish to say thanks to the numerous participants who attended the fieldworks at Iharkút between 2000 and 2006. The Bakony Bauxite Mining Company and the Geovolán Zrt. significantly contributed to the success of the excavations. RM is grateful to Eugene S. Gaffney for sharing literature and his data set of character codings and Walter G. Joyce for useful discussions on cladistic palaeobiogeography. Walter G. Joyce is further acknowledged for reviewing and carefully correcting the manuscript. Benjamin Kear made useful comments on the manuscript and together with László Makádi gave assistance to the phylogenetical analysis. The suggestions of an anonymous reviewer also improved the quality of the paper. Fieldwork was supported by the Hungarian Natural History Museum, the National Geographic Society (Grant No. 7228–02, 7508–03), the Hungarian Research Fund (OTKA NF 84193, PD 73021), the Jurassic Foundation, the Hantken Foundation and the Pro Renovanda Cultura Hungariae Foundation. This project was also funded by the Synthesys Program, the ELTE-MTA ‘Lendulet’ Dinosaur Research Group, Eötvös Loránd University and the SECyT-NKTH.
Appendix 1. Character codings
Foxemys mechinorum
1111100010 0100101010 0001001110 1102000100 0100110101 3100001110 1111111201 10041?0111 111110110? 111200??11 010112?11? 2?11010111 1101112??1 1?11111122 2(12)10222121 210012?211 111111(01)110 10111
Foxemys trabanti
1111100?10 01?0?01010 0001001110 ?1?2010?00 010????101 3100001110 1111111201 1004110111 111110110? 111200??11 010112?110 2?11010111 110??????1 1?11111122 2(12)102221?1 2101?2?211 1111110110 ?0?11