Introduction
Maternal depression during pregnancy, including major depressive disorder (MDD), dysthymia, and depressive symptoms, is a major pregnancy complication carrying prevalence rates of 7–20% (Lahti et al., Reference Lahti, Savolainen, Tuovinen, Pesonen, Lahti, Heinonen, Hamalainen, Laivuori, Villa, Reynolds, Kajantie and Raikkonen2017; Woody et al., Reference Woody, Ferrari, Siskind, Whiteford and Harris2017). Maternal depression not only hinders the maternal quality of life, but is often accompanied by overweight/obesity (Kumpulainen et al., Reference Kumpulainen, Girchenko, Lahti-Pulkkinen, Reynolds, Tuovinen, Pesonen, Heinonen, Kajantie, Villa, Hamalainen, Laivuori and Raikkonen2018), diabetes and hypertensive pregnancy disorders (Fenton and Stover, Reference Fenton and Stover2006), and shows high continuity postpartum (Kumpulainen et al., Reference Kumpulainen, Girchenko, Lahti-Pulkkinen, Reynolds, Tuovinen, Pesonen, Heinonen, Kajantie, Villa, Hamalainen, Laivuori and Raikkonen2018). Maternal depression during pregnancy also associates with poorer fetal growth and preterm birth (Jarde et al., Reference Jarde, Morais, Kingston, Giallo, MacQueen, Giglia, Beyene, Wang and McDonald2016) and increases child risk for inflammation, allergies, asthma, poorer neurodevelopment, and psychopathology (Plant et al., Reference Plant, Pawlby, Sharp, Zunszain and Pariante2016; Lahti et al., Reference Lahti, Savolainen, Tuovinen, Pesonen, Lahti, Heinonen, Hamalainen, Laivuori, Villa, Reynolds, Kajantie and Raikkonen2017; Van den Bergh et al., Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017; Flanigan et al., Reference Flanigan, Sheikh, DunnGalvin, Brew, Almqvist and Nwaru2018; Tuovinen et al., Reference Tuovinen, Lahti-Pulkkinen, Girchenko, Lipsanen, Lahti, Heinonen, Reynolds, Hamalainen, Kajantie, Laivuori, Pesonen, Villa and Raikkonen2018).
However, the biological mechanisms underlying the transmission of these effects from the mother to her child remain vague. In addition to depression-related changes in placental structure and function (Raikkonen et al., Reference Raikkonen, Pesonen, O'Reilly, Tuovinen, Lahti, Kajantie, Villa, Laivuori, Hamalainen, Seckl and Reynolds2015; Reynolds et al., Reference Reynolds, Pesonen, O'Reilly, Tuovinen, Lahti, Kajantie, Villa, Laivuori, Hamalainen, Seckl and Raikkonen2015; Lahti-Pulkkinen et al., Reference Lahti-Pulkkinen, Cudmore, Haeussner, Schmitz, Pesonen, Hamalainen, Villa, Mehtala, Kajantie, Laivuori, Reynolds, Frank and Raikkonen2018), stress axes, oxidative stress, and nutrition (Glover, Reference Glover2015; Van den Bergh et al., Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017), it has been suggested that depression may aggravate maternal proinflammatory state set forth in pregnancy (Leff-Gelman et al., Reference Leff-Gelman, Mancilla-Herrera, Flores-Ramos, Cruz-Fuentes, Reyes-Grajeda, Garcia-Cuetara Mdel, Bugnot-Perez and Pulido-Ascencio2016) and link maternal depression with child development (Glover, Reference Glover2015; Van den Bergh et al., Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017).
By using the Newcastle Ottawa Scale (NOS) (Herzog et al., Reference Herzog, Álvarez-Pasquin, Díaz, Del Barrio, Estrada and Gil2013; Wells et al., Reference Wells, Shea, O'Connell, Peterson, Welch, Losos and Tugwell2014a, Reference Wells, Shea, O'Connell, Peterson, Welch, Losos and Tugwell2014b; Anthony and Lin, Reference Anthony and Lin2018), we systematically assessed the quality of evidence of the scant previous studies that have tested if depression is associated with inflammation during pregnancy. Online Supplementary Table ST1 provides a summary of the study characteristics, main findings and NOS quality of evidence assessment. Online Supplementary Table ST2 provides further details of the NOS assessment and criteria for cross-sectional (Herzog et al., Reference Herzog, Álvarez-Pasquin, Díaz, Del Barrio, Estrada and Gil2013; Anthony and Lin, Reference Anthony and Lin2018) and online Supplementary Table ST3 for cohort studies (Wells et al., Reference Wells, Shea, O'Connell, Peterson, Welch, Losos and Tugwell2014a, Reference Wells, Shea, O'Connell, Peterson, Welch, Losos and Tugwell2014b). The NOS assessment of the reviewed studies highlights the limited quality of available evidence: of the 10 reviewed studies 40% were defined as ‘poor’ (Scrandis et al., Reference Scrandis, Langenberg, Tonelli, Sheikh, Manogura, Alberico, Hermanstyne, Fuchs, Mighty, Hasday, Boteva and Postolache2008; Azar and Mercer, Reference Azar and Mercer2013; Cheng and Pickler, Reference Cheng and Pickler2014; Gustafsson et al., Reference Gustafsson, Sullivan, Nousen, Sullivan, Huang, Rincon, Nigg and Loftis2018), 50% as ‘fair’ (Christian et al., Reference Christian, Franco, Glaser and Iams2009; Cassidy-Bushrow et al., Reference Cassidy-Bushrow, Peters, Johnson and Templin2012; Haeri et al., Reference Haeri, Baker and Ruano2013; Simpson et al., Reference Simpson, Steiner, Coote and Frey2016; Osborne et al., Reference Osborne, Biaggi, Chua, Du Preez, Hazelgrove, Nikkheslat, Previti, Zunszain, Conroy and Pariante2018), and 10% as ‘good’ (Blackmore et al., Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O'Connor2011) based on the NOS assessment. Online Supplementary Table ST1 also shows that the findings are mixed with some studies showing that maternal depression is associated with higher levels of a number of inflammatory markers studied and some reporting null associations. In the only study providing good quality of evidence, MDD diagnosis and depressive symptoms at 18 and 32 gestational weeks were not significantly associated with interleukin (IL)-6 or tumor necrosis factor α (TNF-α) measured at these same gestational weeks (Blackmore et al., Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O'Connor2011). There were no longitudinal associations across time between depression and inflammation either (Blackmore et al., Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O'Connor2011). Our review, thus, highlights the need for further studies with good quality of evidence to either refute or confirm the hypothesis that depression aggravates the proinflammatory state set forth in pregnancy.
Apart from the limited quality of evidence, there are also critical knowledge gaps in the existing literature. The studies are based on small samples limiting statistical power, and all but two (Blackmore et al., Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O'Connor2011; Azar and Mercer, Reference Azar and Mercer2013) have reported cross-sectional correlations, even if depression and/or inflammation would have been measured at more than one gestational stage. In addition to the above-mentioned good quality study (Blackmore et al., Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O'Connor2011), the other, small-scale study reporting longitudinal associations showed in 27 women that an increase in depressive symptoms from 7–10 to 16–20 gestational weeks was associated with higher IL-6 at 16–20 gestational weeks, but the increase was not associated with C-reactive protein (CRP) or TNF-α (Azar and Mercer, Reference Azar and Mercer2013). A further knowledge gap relates to the limited evidence available on depression diagnoses: all of the previous studies have focused on depressive symptoms and only three (Blackmore et al., Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O'Connor2011; Haeri et al., Reference Haeri, Baker and Ruano2013; Osborne et al., Reference Osborne, Biaggi, Chua, Du Preez, Hazelgrove, Nikkheslat, Previti, Zunszain, Conroy and Pariante2018) have additionally studied depression diagnoses. Moreover, since convincing evidence shows associations between depression and obesity in pregnant populations (Molyneaux et al., Reference Molyneaux, Poston, Ashurst-Williams and Howard2014; Kumpulainen et al., Reference Kumpulainen, Girchenko, Lahti-Pulkkinen, Reynolds, Tuovinen, Pesonen, Heinonen, Kajantie, Villa, Hamalainen, Laivuori and Raikkonen2018); and inflammatory state in pregnancy is aggravated in response to obesity (Choi et al., Reference Choi, Joseph and Pilote2013), most studies on depression and inflammation during pregnancy have accounted for pre-pregnancy overweight/obesity (Christian et al., Reference Christian, Franco, Glaser and Iams2009; Blackmore et al., Reference Blackmore, Moynihan, Rubinow, Pressman, Gilchrist and O'Connor2011; Cassidy-Bushrow et al., Reference Cassidy-Bushrow, Peters, Johnson and Templin2012; Haeri et al., Reference Haeri, Baker and Ruano2013; Simpson et al., Reference Simpson, Steiner, Coote and Frey2016; Osborne et al., Reference Osborne, Biaggi, Chua, Du Preez, Hazelgrove, Nikkheslat, Previti, Zunszain, Conroy and Pariante2018). However, few studies have considered diabetes and hypertensive pregnancy disorders (Azar and Mercer, Reference Azar and Mercer2013; Haeri et al., Reference Haeri, Baker and Ruano2013; Simpson et al., Reference Simpson, Steiner, Coote and Frey2016; Osborne et al., Reference Osborne, Biaggi, Chua, Du Preez, Hazelgrove, Nikkheslat, Previti, Zunszain, Conroy and Pariante2018) even though these conditions are associated with depression (Fenton and Stover, Reference Fenton and Stover2006), often complicate overweight/obese pregnancies (Ovesen et al., Reference Ovesen, Rasmussen and Kesmodel2011) and associate with increased inflammation as well (Rebelo et al., Reference Rebelo, Schlussel, Vaz, Franco-Sena, Pinto, Bastos, Adegboye and Kac2013; Pantham et al., Reference Pantham, Aye and Powell2015). Finally, none of the studies has tested whether depression adds to the inflammatory effects of overweight/obesity, diabetes, and hypertensive pregnancy disorders.
To address these knowledge gaps, we tested the hypotheses that (1) history of depression diagnoses before pregnancy, derived from healthcare registry, and (2) from self-reports, and (3) higher levels of depressive symptoms reported during pregnancy were associated with higher levels of and increases in plasma high-sensitive CRP (hsCRP) and glycoprotein acetyls measured across three consecutive stages during pregnancy. We also tested the hypotheses that early pregnancy body mass index (BMI), diabetes, and hypertensive pregnancy disorders accounted for and, at least partially mediated these associations, and tested if depression added to the inflammation that accompanies these conditions.
We focused on two proinflammatory biomarkers: hsCRP and glycoprotein acetyls, because they both have long half-lives and indicate systemic, low-grade chronic inflammation (Ritchie et al., Reference Ritchie, Würtz, Nath, Abraham, Havulinna, Fearnley, Sarin, Kangas, Soininen, Aalto, Seppälä, Raitoharju, Salmi, Maksimow, Männistö, Kähönen, Juonala, Ripatti, Lehtimäki, Jalkanen, Perola, Raitakari, Salomaa, Ala-Korpela, Kettunen and Inouye2015). HsCRP is among the most commonly used inflammatory biomarkers in research. Vast evidence in the general population supports its longitudinal associations with depression (Copeland et al., Reference Copeland, Shanahan, Worthman, Angold and Costello2012; Valkanova et al., Reference Valkanova, Ebmeier and Allan2013; Huang et al., Reference Huang, Su, Goldberg, Miller, Levantsevych, Shallenberger, Pimple, Pearce, Bremner and Vaccarino2019) and cardiovascular mortality (Li et al., Reference Li, Zhong, Cheng, Zhao, Zhang, Hong, Wan, He and Wang2017). Glycoprotein acetyls are, in turn, a novel inflammatory biomarker. It is a composite signal of changes in multiple circulating glycoproteins. Glycoprotein acetyls predict the risk of infectious illnesses (Ritchie et al., Reference Ritchie, Würtz, Nath, Abraham, Havulinna, Fearnley, Sarin, Kangas, Soininen, Aalto, Seppälä, Raitoharju, Salmi, Maksimow, Männistö, Kähönen, Juonala, Ripatti, Lehtimäki, Jalkanen, Perola, Raitakari, Salomaa, Ala-Korpela, Kettunen and Inouye2015). Importantly, both hsCRP and glycoprotein acetyl levels rise markedly during pregnancy (Wang et al., Reference Wang, Würtz, Auro, Mäkinen, Kangas, Soininen, Tiainen, Tynkkynen, Jokelainen, Santalahti, Salmi, Blankenberg, Zeller, Viikari, Kähönen, Lehtimäki, Salomaa, Perola, Jalkanen, Järvelin, Raitakari, Kettunen, Lawlor and Ala-Korpela2016), making them suitable candidate biomarkers for our study.
Method
Sample
The participants came from the Prediction and Prevention of Pre-eclampsia and Intrauterine Growth Restriction (PREDO) Study, described in detail elsewhere (Girchenko et al., Reference Girchenko, Lahti, Tuovinen, Savolainen, Lahti, Binder, Reynolds, Entringer, Buss, Wadhwa, Hamalainen, Kajantie, Pesonen, Villa, Laivuori and Raikkonen2017). Briefly, in 2005–2009, 1079 pregnant women were enrolled in the clinical subsample of the PREDO when they arrived for their first ultrasound screening at 12–13 weeks of gestation. Of them, 969 had one or more and 110 none of the known risk factors for pre-eclampsia and intrauterine growth restriction (IUGR). The study sites comprised 10 hospitals in Southern and Eastern Finland.
Of the 1079 women, 420 underwent venous blood sampling at one to three consecutive stages during pregnancy; due to economic constraints, blood was sampled only at three study hospitals. Because of large within-individual variation in the levels of hsCRP and glycoprotein acetyls across the three samplings, we did not impute missing data (n = 41 with one or two missing blood samples).
Hence, our sample comprised 379 women providing three blood samples taken at the median (interquartile range) 13.0 (12.6–13.4), 19.3 (19.0–19.7), and 27.0 (26.6–27.6) gestational weeks. Health registry data on the history of depression diagnoses before pregnancy were available for 375 women (two women had no data available and two women who received depression diagnosis during pregnancy were excluded); 347 had data on self-reported history of depression diagnosis before pregnancy (29 did not complete the questionnaire and 3 did not specify when they were diagnosed); and 295 women reported depressive symptoms concurrently to the three blood samplings during pregnancy (84 did not complete the symptom questionnaire). Women with these three analytic samples differed from women of the entire sample only in two respects: they were more often younger than 40 years, and less often reported a history of depression diagnosis before pregnancy (Table 1).
Table 1. Characteristics of the sample

Depressive symptoms during pregnancy in the entire sample are reported as the mean of all available observations, and for the analytic samples as the mean of depressive symptom scores measured at the time of the three blood samplings during pregnancy.
P1 reflects p value from the analyses exploring the difference between the entire sample (N = 1079) and the sample with data on the history of depression diagnosis before pregnancy derived from HILMO (N = 375).
P2 reflects p value from the analyses exploring the difference between the entire sample (N = 1079) and the sample with data on the history of depression diagnosis before pregnancy derived from self-reports (N = 348).
P3 reflects p value from the analyses exploring the difference between the entire sample (N = 1079) and the sample with data on depressive symptoms reported (Center for Epidemiological Studies Depression Scale) at the time of the three blood samplings during pregnancy (N = 295).
HILMO refers to Care Register for Healthcare.
a For glycoprotein acetyls, the analytic samples comprised 344, 317, and 271 women with three blood samples with glycoprotein acetyls and history of depression diagnosis before pregnancy from HILMO and from self-reports and depressive symptoms reported concurrent to the blood samplings during pregnancy, respectively.
All participants signed written informed consents. The PREDO study protocol was approved by ethics committees of the Helsinki and Uusimaa Hospital District. All study procedures were in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Inflammation
The participants came for blood sampling from antecubital vein between 19:00 to 21:00 h, after having fasted for at least 10 h. Plasma was separated immediately. Ethylenediaminetetraacetic acid plasma samples were stored at −80 °C until analyzed. The hsCRP concentration (mg/L) was analyzed with a Beckman-Coulter CRP immunoturbidometric assay and Olympus AU680 analyzer (Beckman Coulter Inc., CA, USA). The intra-assay variation (CV%) of the method in our laboratory was between 2.6% (n = 10, mean 1.20 mg/L) and 0.7% (n = 10, mean 65 mg/L) and inter-assay variations were (CV%) 3.5% (n = 30, mean 1.07 mg/L), 1. 2% (n = 30, mean 11.5 mg/L), and 2.9% (n = 30, mean 73 mg/L). The limit of detection of the hsCRP method is 0.02 mg/L and the functional sensitivity was better than 0.1 mg/L. Glycoprotein acetyls (mmol/L) were analyzed using a high-throughput nuclear magnetic resonance metabolomics platform (1HNMR spectra, Nightingale Ltd.; Espoo, Finland) (Soininen et al., Reference Soininen, Kangas, Wurtz, Suna and Ala-Korpela2015).
Depression
We derived depression diagnoses from the Care Register for Healthcare (HILMO), comprising diagnoses of all inpatient hospitalizations in Finland since 1969 and outpatient hospitalizations and specialized treatments since 1998; participants were born 1959–1989. Depression diagnoses were identified with the International Classification of Diseases, Tenth-Revision (ICD-10) codes F32–F33, F341 since 1996 and with ICD-9 codes 2961, 2968A, and 3004A in 1987–1995. No women had bipolar disorder in our sample. The median time interval between the last hospital discharge with depression and conception was 3.1 years (interquartile range = 1.9–6.7 years).
In early pregnancy, the women answered the question ‘Have you ever been diagnosed by a physician with depression?’ followed by a question on when they were diagnosed.
Starting from 12–13 gestational weeks, the women completed the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, Reference Radloff1977). The 20 CES-D questions describe depressive symptoms during the past week, rated from none (0) to all (3) of the time. The women completed the CES-D biweekly up to 14 times until 38–39 gestational weeks or delivery. This allowed us to identify the measurements that matched the closest to the three blood samplings for inflammatory biomarkers; for each sampling, we identified two CES-D scores closest to the sampling date. We used the average of these two scores at the three sampling points in our analyses.
Higher CES-D scores indicate more depressive symptoms, and 16 points or more represent probable clinical depression (Radloff, Reference Radloff1977). The CES-D is a well-established measure of depression, and it has been validated in pregnant women (Lahti et al., Reference Lahti, Savolainen, Tuovinen, Pesonen, Lahti, Heinonen, Hamalainen, Laivuori, Villa, Reynolds, Kajantie and Raikkonen2017).
Covariates and moderators
Early pregnancy BMI, calculated from weight [kilograms (kg)] and height [meters (m)] measurements verified at the first antenatal clinic visit [mean = 8.5, standard deviation (s.d.) = 1.5 gestational weeks)], was derived from medical records [overweight (25–29.99 kg/m2)/obese (⩾30 kg/m2)/normal weight (⩽24.99 kg/m2) (WHO, 2000)]. Diagnoses of diabetes (type 1 diabetes/gestational diabetes/no diabetes) and hypertensive (chronic hypertension/pre-eclampsia/gestational hypertension/normotension) pregnancy disorders were derived from medical records and the diagnoses were verified by a clinical jury. Additional covariates included age (<40 v. ⩾40 years) and smoking during pregnancy (did not smoke v. quit during first trimester/smoked throughout pregnancy), derived from medical records and Finnish Medical Birth Register, and antenatal alcohol use (yes/no) and education level (basic/secondary v. tertiary), which were reported in early pregnancy.
Statistical analyses
The primary data analytic tool was linear mixed-model regression with hsCRP and glycoprotein acetyls at the three sampling points during pregnancy, analyzed in separate models, as time-varying within-person outcomes. History of depression diagnoses before pregnancy from HILMO and self-reports were treated as time-invariant between-person predictors, and depressive symptoms at the three points matching the blood sampling points as a time-varying within-person predictor. In addition to treating depressive symptoms during pregnancy as continuous, we conducted analyses treating the symptoms as a binary variable indicating probable clinical depression (CES-D ⩾ 16). All depression indicators were assessed in separate mixed models, which included a gestational week at blood sampling as a time-varying within-person predictor and those covariates that were significantly associated with hsCRP and/or glycoprotein acetyls. Interactions of depression (diagnoses or symptoms) × gestational week at blood samplings were added into the models to test if depression predicted changes in hsCRP or glycoprotein acetyls during pregnancy.
We then tested if overweight/obesity, diabetes, or hypertensive pregnancy disorders accounted for any effects of depression on inflammation by including the main effects of these conditions into separate mixed-model equations. If the effect sizes of depression attenuated after adjustment for these conditions, we further tested for mediation with the bootstrapping method using 5000 resamples and bias corrected 95% confidence intervals. These analyses were performed only if the other criteria for mediation were also met: (1) the depression indicator was associated with the condition that attenuated the association and (2) the condition in question was associated with the inflammation marker in question. Finally, to study if depression added to the inflammatory effects of overweight/obesity, diabetes or hypertensive pregnancy disorders, we included interaction terms depression × normal weight/overweight/obesity, depression × diabetes disorders, and depression × hypertensive disorders into the mixed-model equations.
For mixed-models, we used variance components covariance structure and defined a random intercept and random slope for time, i.e. gestational week at blood sampling. Because hsCRP and CES-D distributions were skewed, we normalized hsCRP with logarithm and CES-D with square root transformations. To facilitate interpretation, we transformed all continuous variables to standard deviation (s.d.) units (for time-varying variables we used the mean of the three data points during pregnancy and its s.d. to retain within-person variation). To facilitate clinical interpretation, we also provide test statistics in raw units of hsCRP and glycoprotein acetyls.
We conducted sensitivity analyses by excluding measurements of hsCRP and glycoprotein acetyls taken within a month preceding or following acute infectious disease diagnoses derived from HILMO to ascertain that acute infection did not affect our results. The sensitivity analyses included 879–1112 hsCRP and 808–1020 glycoprotein measurements out of the 885–1125 available samples. Infectious illnesses were identified with diagnostic codes as described elsewhere (Lund-Sorensen et al., Reference Lund-Sorensen, Benros, Madsen, Sorensen, Eaton, Postolache, Nordentoft and Erlangsen2016; Kohler et al., Reference Kohler, Petersen, Mors, Mortensen, Yolken, Gasse and Benros2017).
Results
Background characteristics
Table 1 shows the sample characteristics. HsCRP and glycoprotein acetyls were inter-correlated (Pearson r's ⩾ 0.38, p < 0.001) and showed high rank-order stability across pregnancy (r ⩾ 0.75 for hsCRP and r ⩾ 0.72 for glycoprotein). Online Supplementary Figure ST1 shows that levels of hsCRP (panel A) and glycoprotein acetyls (panel B) changed during pregnancy; change in hsCRP was A-shaped, whilst glycoprotein acetyls increased linearly across pregnancy. HILMO and self-reported history of depression diagnosis before pregnancy showed concordance (κ = 0.47, p < 0.001), and both were associated with higher levels of depressive symptoms during pregnancy [diagnosis from HILMO: mean difference(MD) = 1.00 s.d., 95% CI 0.44–1.56, p = 0.001; diagnosis from self-reports: MD = 1.09 s.d., 95% CI 0.64–1.53, p < 0.001) and with higher prevalence of probable clinical depression during pregnancy (diagnosis from HILMO: 66.7% v. 19.4%, p < 0.001; diagnosis from self-reports: 57.9% v. 18.4%, p < 0.001).
Online Supplementary Table ST4 shows that women with lower education, who were overweight or obese in early pregnancy or had chronic hypertension, pre-eclampsia, or gestational diabetes had higher overall hsCRP and glycoprotein acetyl levels. HsCRP levels were also higher and changed less across pregnancy in women younger than 40 years (β = 0.013 in older and β = −0.006 in younger women; p = 0.01 for age × time interaction). Glycoprotein acetyls increased more across pregnancy in overweight than normal weight women (β = 0.08 in overweight and β = 0.07 in normal weight women; p = 0.01 for normal weight v. overweight × time interaction). Smoking, alcohol use during pregnancy or type 1 diabetes was not associated with hsCRP or glycoprotein acetyls (online Supplementary Table ST4).
Depression and inflammation during pregnancy
Table 2 shows that hsCRP levels were 0.69 s.d. [mean difference in raw units (MD) = 4.11, 95% confidence interval (CI) 2.54–5.69 mg/L] and 0.56 s.d. (MD = 2.44, 95% CI 1.12–3.77 mg/L) higher in women with compared to those without a history of depression diagnosis before pregnancy derived from HILMO and self-reports, respectively; hsCRP levels were also 0.28 s.d. (MD = 1.02, 95% CI 0.17–1.88 mg/L) higher in women with compared to those without probable clinical depression during pregnancy, and 0.06 s.d. higher per each s.d. increase in these symptoms during pregnancy. Glycoprotein acetyls were 0.52 s.d. (MD = 1.02, 95% CI 0.17–1.88 mg/L) higher in women with compared to those without a history of depression diagnosis from HILMO and 0.25 s.d. (MD = 0.05, 95% CI 0.003–0.09 mg/L) higher in women with compared to those without probable clinical depression during pregnancy. All associations, except for probable clinical depression during pregnancy with glycoprotein acetyls, remained significant when adjusted for age and education (Table 2) and when adjusted for diabetes and hypertensive pregnancy disorders (online Supplementary Table ST5). However, all associations became non-significant when adjusted for early pregnancy BMI (Table 2). In the models where depression no longer associated with hsCRP, overweight (MD = 0.54 s.d. between normal weight v. overweight, 95% CI 0.31–0.97) and obesity (MD = 1.01 s.d. between normal weight v. obesity, 95% CI 0.80–1.22) remained significant predictors of hsCRP (respective values for glycoprotein acetyls were MD = 0.73 s.d., 95% CI 0.51–0.97 and MD = 0.93 s.d., 95% CI 0.51–1.18). Figures 1–2 display that there were no depression × gestational week at blood sampling interactions.
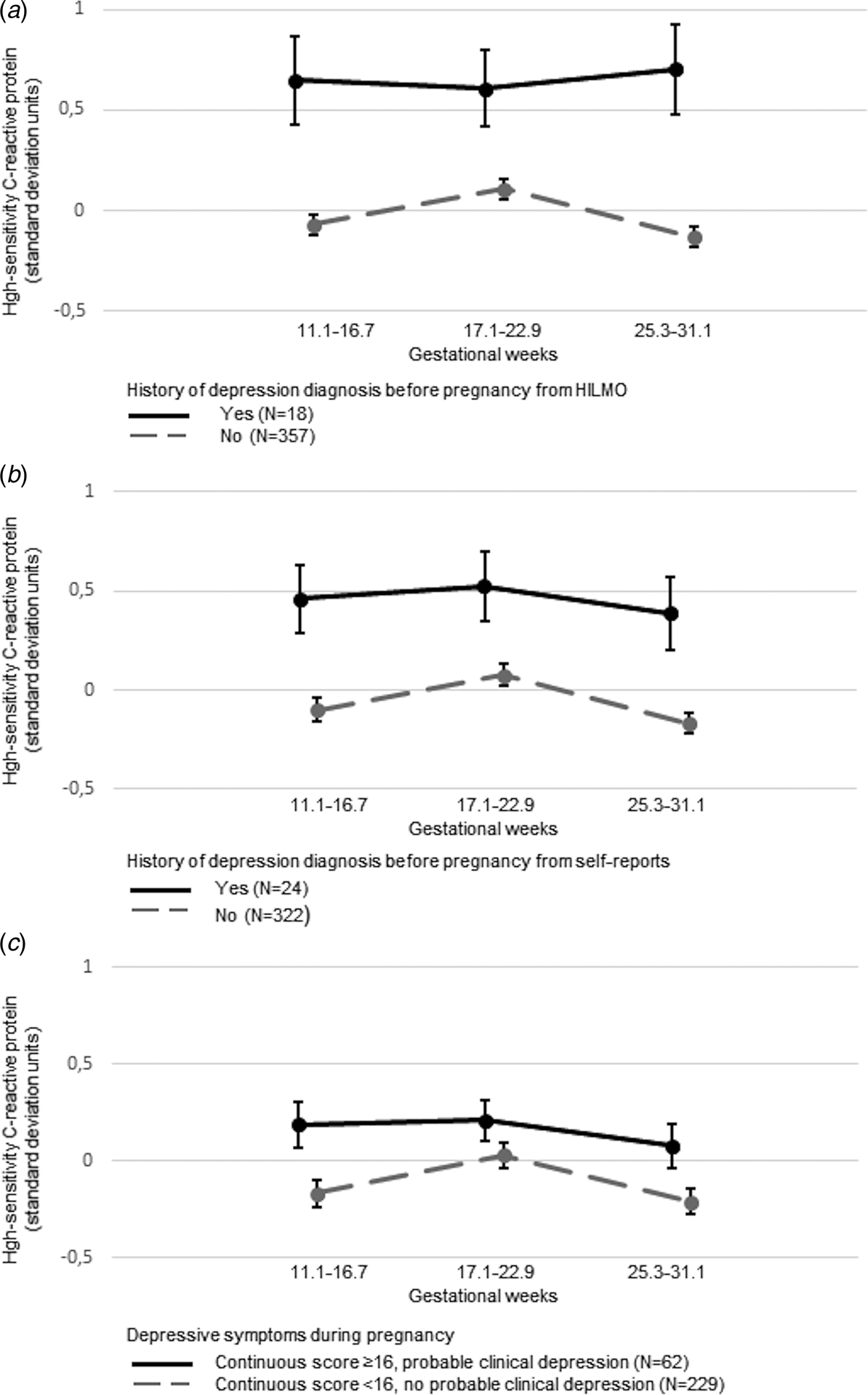
Fig. 1. Associations between history of depression diagnosis before pregnancy from the Care Register for Healthcare (HILMO) (panel A; p = 0.37 for interaction with gestational week at blood sampling) and from self-reports (panel B; p = 0.99 for interaction with gestational week at blood sampling) and probable clinical depression during pregnancy (panel C; p = 0.62 for interaction with gestational week at blood sampling) and high-sensitivity C-reactive protein across the three measurement points during pregnancy.

Fig. 2. Associations between (1) history of depression diagnosis before pregnancy from the Care Register for Healthcare (HILMO) (panel A; p = 0.60 for interaction with gestational week at blood sampling) and (2) probable clinical depression during pregnancy (panel C; p = 0.70 for interaction with gestational week at blood sampling) and glycoprotein acetyls across the three measurement points during pregnancy.
Table 2. Associations of a history of depression diagnosis before pregnancy derived from the Care Register for Healthcare (HILMO) and self-reports, and depressive symptoms and probable clinical depression reported during pregnancy with high-sensitivity C-Reactive protein and glycoprotein acetyls across the three measurement points during pregnancy

Model 1 is unadjusted for covariates but includes the gestational week when blood was sampled as a within-person time-varying predictor, Model 2 is Model 1 + age and education, Model 3 is Model 2 + body mass index in early pregnancy [categorized as normal weight (<25 kg/m2), overweight (25–29.99 kg/m2) and obese (⩾30 kg/m2)].
a Estimates and 95% confidence intervals (95% CI) reflect differences between those with and without a history of depression diagnosis before pregnancy or with and without probable clinical depression during pregnancy in high-sensitivity C-reactive protein (hsCRP) and glycoprotein acetyls in standard deviation (s.d.) units or change in s.d. units in hsCRP and glycoprotein acetyls per s.d. unit change in the continuous depressive symptom scores during pregnancy.
The exclusion of hsCRP and glycoprotein measurements taken within one month preceding or following diagnosed infectious diseases did not change the associations (online Supplementary Table ST6).
Mediation
Online Supplementary Figures ST2–ST4 show that early pregnancy BMI mediated the following effects on hsCRP: history of depression diagnosis before pregnancy from HILMO and from self-reports, and depressive symptoms reported during pregnancy. Online Supplementary Figure ST5 shows that BMI also mediated the effect of history of depression diagnosis before pregnancy from HILMO on glycoprotein acetyls. We did not test other possible mediation effects, as the criteria for mediation tests were not met.
Additive effects
We found one significant interaction: depressive symptoms during pregnancy interacted significantly with normal weight v. obesity in the analysis of hsCRP (p = 0.006 for interaction; p = 0.57 for depressive symptoms × normal weight v. overweight interaction). Figure 3 shows that higher depressive symptoms during pregnancy were associated with higher hsCRP levels in obese women, but not in overweight or normal weight women. This may reflect that below BMI 30 kg/m2 hsCRP increased with increasing BMI, but at BMI 30 kg/m2 and above hsCRP plateaued showing no further increase (online Supplementary Fig. ST6).

Fig. 3. Associations between depressive symptoms during pregnancy and high-sensitivity C-reactive protein during pregnancy in women who in early pregnancy were normal weight [body mass index (BMI) < 25 kg/m2), overweight (BMI 25–29.99 kg/m2), or obese (BMI ⩾ 30 kg/m2).
Discussion
We found that depression was associated with higher levels of hsCRP and glycoprotein acetyls during pregnancy. The findings for hsCRP were consistent and significant across the different information sources of depression; whether history of depression diagnosis before pregnancy was derived from HILMO or self-reports, or whether depressive symptoms were reported during pregnancy concurrent to the three consecutive blood samplings, and treated either as a continuous or a binary variable, the latter indicating probable clinical depression during pregnancy. The pattern of findings on glycoprotein acetyls was also consistent across the different information sources, but reached conventional significance levels for the history of depression diagnosis before pregnancy derived from HILMO and for the probable clinical depression reported during pregnancy.
While hsCRP and glycoprotein acetyl levels changed modestly during pregnancy, the associations between depression and these inflammatory biomarkers did not vary across pregnancy. The level of these inflammatory biomarkers has, however, been shown to be markedly higher among women who are than who are not pregnant (Wang et al., Reference Wang, Würtz, Auro, Mäkinen, Kangas, Soininen, Tiainen, Tynkkynen, Jokelainen, Santalahti, Salmi, Blankenberg, Zeller, Viikari, Kähönen, Lehtimäki, Salomaa, Perola, Jalkanen, Järvelin, Raitakari, Kettunen, Lawlor and Ala-Korpela2016). In line, another study has reported that in pregnant women the mean hsCRP levels were above 10 mg/L at 10.6 gestational weeks (Berggren et al., Reference Berggren, Roeder, Boggess, Moss, Offenbacher, Campbell and Grotegut2015), and yet another study has reported that over 50% of non-pregnant 31-year-old women have hsCRP values below 1.0 mg/L (Liukkonen et al., Reference Liukkonen, Rasanen, Jokelainen, Leinonen, Jarvelin, Meyer-Rochow and Timonen2011).
Our findings associating depression with higher inflammation among pregnant women correspond with meta-analytic findings from the general population showing longitudinal associations between depression and higher hsCRP and IL-6 levels (Valkanova et al., Reference Valkanova, Ebmeier and Allan2013). Furthermore, in our study, the degree of inflammation related to depression was of comparable magnitude to the inflammation associated with early pregnancy overweight, gestational diabetes, and pre-eclampsia. Only the effects of early pregnancy obesity exceeded the degree of depression-related inflammation during pregnancy. In raw units, mean differences in hsCRP levels between women with and without depression diagnosis before pregnancy and with and without probable clinical depression during pregnancy were between 1.02 and 4.11 mg/L. This magnitude of inflammation is comparable to the degree of inflammation that has been suggested to increase cardiovascular disease risk moderately in the general population (Li et al., Reference Li, Zhong, Cheng, Zhao, Zhang, Hong, Wan, He and Wang2017). These findings suggest that depression is associated with a higher proinflammatory state during pregnancy, bearing at least moderate clinical relevance to maternal health and possibly fetal development. To our knowledge, our prospective study is the largest on this topic in sample size thus far, and the first to show such associations using the information on depression derived from different sources and three consecutive stages during pregnancy.
The associations between the different depression measures with hsCRP and glycoprotein acetyls were independent of age, education, diabetes, and hypertensive pregnancy disorders. However, early pregnancy BMI accounted for and mediated the effects of depression diagnosis before pregnancy and depressive symptoms during pregnancy on inflammation. The mediation via BMI is not surprising, since early pregnancy overweight/obesity and antenatal depression are highly interrelated (Molyneaux et al., Reference Molyneaux, Poston, Ashurst-Williams and Howard2014; Kumpulainen et al., Reference Kumpulainen, Girchenko, Lahti-Pulkkinen, Reynolds, Tuovinen, Pesonen, Heinonen, Kajantie, Villa, Hamalainen, Laivuori and Raikkonen2018). Nevertheless, since depression and obesity show continuity across time (Simmonds et al., Reference Simmonds, Llewellyn, Owen and Woolacott2016; Kumpulainen et al., Reference Kumpulainen, Girchenko, Lahti-Pulkkinen, Reynolds, Tuovinen, Pesonen, Heinonen, Kajantie, Villa, Hamalainen, Laivuori and Raikkonen2018), and the depression-BMI-association is bi-directional (Luppino et al., Reference Luppino, de Wit, Bouvy, Stijnen, Cuijpers, Penninx and Zitman2010), we cannot disentangle whether overweight/obesity preceded depression, or vice versa. Therefore, the mediation findings must be interpreted with caution.
We also found that depressive symptoms during pregnancy added to the inflammatory effects of obesity: among obese women, who had already approximately 1 s.d. higher hsCRP levels throughout pregnancy, hsCRP increased further by 0.19 s.d. by each s.d. increase in depressive symptoms during pregnancy. In overweight and normal weight women, this was not true. Based on the nature of the association we found between BMI and hsCRP, we speculate that the strong linear association between BMI and hsCRP between 20 and 30 kg/m2 leaves no room for depression to independently predict hsCRP in normal weight and overweight women. However, our data suggest that in obese women hsCRP reaches a ceiling: at 30 kg/m2 and above hsCRP levels plateau, remain consistently high, no longer increasing with increasing BMI. This leaves room for the effects of depressive symptoms, which increase inflammation in obese women even further. Corresponding interactions between obesity and depression on inflammation have also been reported in non-pregnant populations (Ladwig et al., Reference Ladwig, Marten-Mittag, Lowel, Doring and Koenig2003), but our findings are inconsistent with findings from one study of pregnant women that were ethnically diverse from our sample (Cassidy-Bushrow et al., Reference Cassidy-Bushrow, Peters, Johnson and Templin2012).
Obesity is a well-known proinflammatory state (Choi et al., Reference Choi, Joseph and Pilote2013; Pantham et al., Reference Pantham, Aye and Powell2015) with the perturbation of intestinal microbiota and changes in intestinal permeability being potential triggers of inflammation (Cox et al., Reference Cox, West and Cripps2015). The secretion of inflammatory cytokines from adipose tissue leads to overexpression of pro-inflammatory cytokines (Hotamisligil, Reference Hotamisligil2006). Obesity indeed mediated most effects of depression on inflammation in our study. However, since inflammation levels increased even further in obese women with higher depressive symptoms during pregnancy, also other factors associated with both depression and inflammation may have contributed to our findings. Genetics and epigenetics and their interactions may contribute, since depression has been associated with both the single-nucleotide polymorphisms and expression of genes regulating inflammatory function (Barnes et al., Reference Barnes, Mondelli and Pariante2017; Mahajan et al., Reference Mahajan, Vallender, Garrett, Challagundla, Overholser, Jurjus, Dieter, Syed, Romero, Benghuzzi and Stockmeier2018). These factors may also contribute to the interactions between obesity and depression on inflammation, since evidence suggests shared genetic origins of obesity and depression (Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui, Adams, Agerbo, Air, Andlauer, Bacanu, Baekvad-Hansen, Beekman, Bigdeli, Binder, Blackwood, Bryois, Buttenschon, Bybjerg-Grauholm, Cai, Castelao, Christensen, Clarke, Coleman, Colodro-Conde, Couvy-Duchesne, Craddock, Crawford, Crowley, Dashti, Davies, Deary, Degenhardt, Derks, Direk, Dolan, Dunn, Eley, Eriksson, Escott-Price, Kiadeh, Finucane, Forstner, Frank, Gaspar, Gill, Giusti-Rodriguez, Goes, Gordon, Grove, Hall, Hannon, Hansen, Hansen, Herms, Hickie, Hoffmann, Homuth, Horn, Hottenga, Hougaard, Hu, Hyde, Ising, Jansen, Jin, Jorgenson, Knowles, Kohane, Kraft, Kretzschmar, Krogh, Kutalik, Lane, Li, Li, Lind, Liu, Lu, MacIntyre, MacKinnon, Maier, Maier, Marchini, Mbarek, McGrath, McGuffin, Medland, Mehta, Middeldorp, Mihailov, Milaneschi, Milani, Mill, Mondimore, Montgomery, Mostafavi, Mullins, Nauck, Ng, Nivard, Nyholt, O'Reilly, Oskarsson, Owen, Painter, Pedersen, Pedersen, Peterson, Pettersson, Peyrot, Pistis, Posthuma, Purcell, Quiroz, Qvist, Rice, Riley, Rivera, Saeed Mirza, Saxena, Schoevers, Schulte, Shen, Shi, Shyn, Sigurdsson, Sinnamon, Smit, Smith, Stefansson, Steinberg, Stockmeier, Streit, Strohmaier, Tansey, Teismann, Teumer, Thompson, Thomson, Thorgeirsson, Tian, Traylor, Treutlein, Trubetskoy, Uitterlinden, Umbricht, Van der Auwera, van Hemert, Viktorin, Visscher, Wang, Webb, Weinsheimer, Wellmann, Willemsen, Witt, Wu, Xi, Yang, Zhang, Arolt, Baune, Berger, Boomsma, Cichon, Dannlowski, de Geus, DePaulo, Domenici, Domschke, Esko, Grabe, Hamilton, Hayward, Heath, Hinds, Kendler, Kloiber, Lewis, Li, Lucae, Madden, Magnusson, Martin, McIntosh, Metspalu, Mors, Mortensen, Muller-Myhsok, Nordentoft, Nothen, O'Donovan, Paciga, Pedersen, Penninx, Perlis, Porteous, Potash, Preisig, Rietschel, Schaefer, Schulze, Smoller, Stefansson, Tiemeier, Uher, Volzke, Weissman, Werge, Winslow, Lewis, Levinson, Breen, Borglum and Sullivan2018). Hypothalamic–pituitary–adrenal (HPA) axis activity may also be involved. Glucocorticoids regulate inflammation by exacerbating the secretion of pro-inflammatory cytokines and acute phase proteins (Pariante, Reference Pariante2017) and have both pro- and anti-inflammatory effects in the brain (Walker and Spencer, Reference Walker and Spencer2018). Glucocorticoid functioning is also closely associated with depression and obesity (Stetler and Miller, Reference Stetler and Miller2011; Boggero et al., Reference Boggero, Hostinar, Haak, Murphy and Segerstrom2017; Milaneschi et al., Reference Milaneschi, Simmons, van Rossum and Penninx2019). Findings in smaller subsamples of the PREDO study suggest that depressive symptoms during pregnancy are associated with placental mRNA level changes in genes regulating HPA axis function (Raikkonen et al., Reference Raikkonen, Pesonen, O'Reilly, Tuovinen, Lahti, Kajantie, Villa, Laivuori, Hamalainen, Seckl and Reynolds2015; Reynolds et al., Reference Reynolds, Pesonen, O'Reilly, Tuovinen, Lahti, Kajantie, Villa, Laivuori, Hamalainen, Seckl and Raikkonen2015). The gut microbiota-brain axis functioning is also intertwined with inflammatory processes, and changes in its function are associated with depression (Alam et al., Reference Alam, Abdolmaleky and Zhou2017). Furthermore, depression, obesity and inflammation are each also associated with poorer nutrition, insufficient sleep, physical inactivity, and substance use (Lai et al., Reference Lai, Hiles, Bisquera, Hure, McEvoy and Attia2014; Lai et al., Reference Lai, Cleary, Sitharthan and Hunt2015; Ironson et al., Reference Ironson, Banerjee, Fitch and Krause2018; Stubbs et al., Reference Stubbs, Vancampfort, Firth, Schuch, Hallgren, Smith, Gardner, Kahl, Veronese, Solmi, Carvalho and Koyanagi2018; Milaneschi et al., Reference Milaneschi, Simmons, van Rossum and Penninx2019). A large Mendelian randomization study found that while CRP concentrations were associated with depression, genetic variation regulating CRP was not (Wium-Andersen et al., Reference Wium-Andersen, Orsted and Nordestgaard2014). This finding argues against a causal pathway from inflammation to depression and suggests that a common ‘residual confounding’ factor may possibly underlie the associations found. Hence, the proinflammatory effects of depression and obesity likely stem from multiple contributory factors. Our findings emphasize the need for further studies on these pathways specifically during pregnancy.
Strengths of our study include a large sample size compared to the previous studies, which often included less than 100 participants. We had data on depression from different sources and hsCRP and glycoprotein acetyls were measured at three consecutive stages during pregnancy, which no previous study has had. Furthermore, many previous studies on depression and inflammation during pregnancy utilized very rapidly degrading inflammatory markers, most commonly IL-6. HsCRP is an acute-phase protein with a longer half-life than IL-6 (Wirtz et al., Reference Wirtz, Heller, Miltner, Zilkens and Wolff2000) and glycoprotein acetyls display even slower kinetics than hsCRP. Thus, we were able to obtain more stable estimates of the participants' inflammatory state across pregnancy (Ritchie et al., Reference Ritchie, Würtz, Nath, Abraham, Havulinna, Fearnley, Sarin, Kangas, Soininen, Aalto, Seppälä, Raitoharju, Salmi, Maksimow, Männistö, Kähönen, Juonala, Ripatti, Lehtimäki, Jalkanen, Perola, Raitakari, Salomaa, Ala-Korpela, Kettunen and Inouye2015). While the increases in hsCRP and glycoprotein acetyls in pregnancy (Wang et al., Reference Wang, Würtz, Auro, Mäkinen, Kangas, Soininen, Tiainen, Tynkkynen, Jokelainen, Santalahti, Salmi, Blankenberg, Zeller, Viikari, Kähönen, Lehtimäki, Salomaa, Perola, Jalkanen, Järvelin, Raitakari, Kettunen, Lawlor and Ala-Korpela2016) suggest they are suitable markers of antenatal inflammation, having data also on other inflammatory biomarkers would have given further insight on the associations of depression and antenatal inflammation. Since glycoprotein acetylation is a mix of a range of proteins (Ritchie et al., Reference Ritchie, Würtz, Nath, Abraham, Havulinna, Fearnley, Sarin, Kangas, Soininen, Aalto, Seppälä, Raitoharju, Salmi, Maksimow, Männistö, Kähönen, Juonala, Ripatti, Lehtimäki, Jalkanen, Perola, Raitakari, Salomaa, Ala-Korpela, Kettunen and Inouye2015), we would also have benefited from data on the specific protein levels. It would also have been informative to have cortisol data to indicate HPA axis activity and other biomarkers that are triggered by inflammation.
The study limitations also include that our sample comprised women at risk for pre-eclampsia and IUGR and that blood samples were available only for a subsample. Furthermore, although diagnostic data from HILMO were available for 99.5% of women with three blood samples, self-reported diagnostic data were available for 91.6% and depressive symptoms were reported by 77.8% of the women. The analytic samples comprised women who were younger and less often self-reported a history of depression diagnoses before pregnancy. These factors limit generalizations of our findings to other samples.
In conclusion, our study showed that depression is associated with a proinflammatory state during pregnancy. These associations are mediated by early pregnancy BMI, and depressive symptoms during pregnancy aggravate the inflammation related to obesity.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719001909.
Acknowledgements
The authors would like to thank the participating women for their contribution to the study, and the University of Helsinki and the participating study hospitals for their support in the conduction of this study.
Financial support
The PREDO study is funded by the Academy of Finland (grant number 285324, 12848591, 1284859, 1312670, 269925), European Union's Horizon 2020 Award SC1-2016-RTD-733280 for RECAP, European Commission Dynamics of Inequality Across the Life-course: structures and processes (DIAL) No 724363 for PremLife, EVO (a special state subsidy for health science research), University of Helsinki Research Funds, the Signe and Ane Gyllenberg Foundation, Emil Aaltonen Foundation, Finnish Diabetes Research Foundation, Foundation for Cardiovascular Research, Foundation for Pediatric Research, Jane and Aatos Erkko Foundation, Novo Nordisk Foundation, Päivikki and Sakari Sohlberg Foundation, Sigrid Juselius Foundation, and Finnish Medical Foundation. The sponsors played no role in the design or conduct of this study.
Conflict of interest
None.








