Introduction
Kochia is one of the most troublesome summer annual broadleaf weed species across the U.S. Great Plains, including Kansas (Friesen et al. Reference Friesen, Beckie, Warwick and Van Acker2009; Kumar et al. Reference Kumar, Jha, Jugulam, Yadav and Stahlman2018). It is a highly competitive weed in agronomic crops. For instance, season-long kochia interference reduced sugarbeet (Beta vulgaris L.) yield by up to 95% (Weatherspoon and Schweizer Reference Weatherspoon and Schweizer1969). Favorable biological attributes, including early seedling emergence, low seed dormancy and persistence, tolerance to biotic and abiotic stresses, prolific seed production (>100,000 seeds per plant), and long distance seed dispersal via wind make kochia the most successful weed in the Great Plains region (Baker et al. Reference Baker, Withrow, Brown and Beck2010; Dille et al. Reference Dille, Stahlman, Du, Geier, Riffel, Currie, Wilson, Sbatella, Westra, Kniss, Moechnig and Cole2017; Friesen et al. Reference Friesen, Beckie, Warwick and Van Acker2009; Kumar et al. Reference Kumar, Jha, Jugulam, Yadav and Stahlman2018). Additionally, kochia exhibits high outcrossing potential and pollen-mediated gene flow due to its monoecious and protogynous flowering nature (Beckie et al. Reference Beckie, Blackshaw, Hall and Johnson2016; Mengistu and Messersmith Reference Mengistu and Messersmith2002; Stallings et al. Reference Stallings, Thill, Mallory-Smith and Shafii1995), thereby maintaining a high genetic diversity within and among field populations (Mengistu and Messersmith Reference Mengistu and Messersmith2002; Stallings et al. Reference Stallings, Thill, Mallory-Smith and Shafii1995).
Kochia possesses a high propensity for the evolution of herbicide resistance. Kochia populations resistant to one or more of the following herbicide sites of action (SOAs), including photosystem II (PS II) inhibitors (Weed Science Society of America [WSSA] Group 5), acetolactate synthase (ALS) inhibitors (WSSA Group 2), synthetic auxins (WSSA Group 4), and 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS) inhibitors (WSSA Group 9) have been reported in the U.S. Great Plains (Heap Reference Heap2020). Furthermore, multiple resistance to all these four herbicide SOAs has also been reported in Kansas (Varanasi et al. Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015).
The herbicides that inhibit PS II have historically played a key role in facilitating the adoption of no-tillage production practices in the U.S. Great Plains by providing effective weed control since the early to mid-1970s (Wicks and Smika Reference Wicks and Smika1973; Wicks et al. Reference Wicks, Martin and Mahnken1993). However, the widespread adoption of glyphosate-resistant (GR) crops, after first commercialization in 1996, along with a dramatic increase in glyphosate use to control weeds in fallow fields, precrop seeding and postharvest scenarios have substantially reduced the use of PS II-inhibiting herbicides (Givens et al. Reference Givens, Shaw, Kruger, Johnson, Weller, Young, Wilson, Owen and Jordan2009; Kniss Reference Kniss2018). Nevertheless, the selection pressure imposed by the extensive and repeated use of glyphosate has led to widespread evolution of GR kochia in the region; several GR populations have been reported across the region since it was first reported in 2007 in western Kansas (Godar et al. Reference Godar, Stahlman, Jugulam and Dille2015; Heap Reference Heap2020). Additionally, heavy reliance on POST applications of dicamba for controlling GR kochia over the last decade has also resulted in increasing reports of dicamba-resistant (DR) kochia populations in the region (Kumar et al. Reference Kumar, Currie, Jha and Stahlman2019a, 2019b; LeClere et al. Reference LeClere, Wu, Westra and Sammons2018; Westra et al. Reference Westra, Nissen, Getts, Westra and Gaines2019). This has resulted in a resurgence of interest in using PS II-inhibiting herbicides for controlling GR and DR kochia populations (Kumar and Jha Reference Kumar and Jha2015; Kumar et al. Reference Kumar, Engel, Currie, Jha, Stahlman and Thompson2019b). This necessitates evaluating the response of GR or DR kochia populations to PS II-inhibiting herbicides, predominantly atrazine and metribuzin.
Currently, 107 weed species have been reported to exhibit resistance to PS II-inhibiting herbicides worldwide (Heap Reference Heap2020). Kochia that is resistant to atrazine was first reported in 1976 in Kansas corn fields and along railroads in Iowa (Heap Reference Heap2020). Subsequently, atrazine resistance in kochia populations was reported along railroad rights-of-way from eight additional states (Friesen et al. Reference Friesen, Beckie, Warwick and Van Acker2009; Heap Reference Heap2020). Both target-site and nontarget-site mechanisms of resistance have been reported among weed populations resistant to PS II-inhibiting herbicides (Jugulam and Shyam Reference Jugulam and Shyam2019; Trebst Reference Trebst1996). The target-site mechanism of resistance to PS II inhibitors involves a point mutation in the psbA gene, leading to an altered D1 protein (Mengistu et al. Reference Mengistu, Christoffers and Lym2005). The most prevalent target-site mutations in the psbA gene causing resistance to PS II inhibitors in weed populations are Ser264Thr, Ser264Gly, or Val219Ile (Mengistu et al. Reference Mengistu, Christoffers and Lym2005; Trebst Reference Trebst1996). The target-site mutation Ser264Gly in the psbA gene has previously been reported in kochia populations from Kansas and Illinois conferring resistance to POST atrazine (Foes et al. Reference Foes, Liu, Vigue, Stoller, Wax and Tranel1999; Varanasi et al. Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015). In addition, the Ser264Gly point mutation in the psbA gene has also been reported to confer a high level of resistance to metribuzin in wild radish (Raphanus raphanistrum) and common lambsquarters (Chenopodium album L.; Lu et al. Reference Lu, Yu, Han, Owen and Powles2019; Thiel and Varrelmann Reference Thiel and Varrelmann2014). In contrast, a Val219Ile substitution in the psbA gene causing a high level of resistance to diuron and tebuthiuron and a moderate level of resistance to metribuzin and atrazine has been reported in kochia populations from railroad rights-of-way in North Dakota and Minnesota (Mengistu et al. Reference Mengistu, Christoffers and Lym2005). The nontarget-site mechanisms of resistance to PS II inhibitors include reduced absorption, translocation, and increased metabolism via enhanced activity of GST or cytochrome P450 (CYP450) enzymes (Evans et al. Reference Evans, O’Brien, Ma, Hager, Riggins, Lambert and Riechers2017; Jugulam and Shyam Reference Jugulam and Shyam2019).
In 2017, kochia accessions with multiple resistance (referred to as MHR) to dicamba, fluroxypyr, and glyphosate were reported from two different corn fields near Garden City, KS (Kumar et al. Reference Kumar, Currie, Jha and Stahlman2019a). Due to repeated use of PS II inhibitors for kochia control in those fields, the collected MHR accessions were also suspected as being resistant to PS II inhibitors (R. Currie, personal observation). In addition, limited information exists on alternative POST herbicide options for controlling these MHR kochia accessions. The main objectives of this research were 1) to confirm the existence of cross-resistance to PRE- and POST-applied atrazine and metribuzin in MHR kochia accessions using dose–response assays, 2) investigate the underlying mechanism of resistance, and 3) evaluate the effectiveness of alternative POST herbicides for controlling these MHR kochia accessions.
Materials and Methods
Plant Material
Seeds of individual MHR kochia plants (designated as KS-4A, KS-4D, KS-4H, KS-10A, KS-10G, and KS-10H accessions) with multiple resistance to dicamba (3.1-fold to 9.4-fold), fluroxypyr (3.0-fold to 8.6-fold), and glyphosate (3 to 13 EPSPS gene copies) were originally collected in autumn 2017 from two separate fields near Garden City, KS (Kumar et al. Reference Kumar, Currie, Jha and Stahlman2019a). Due to limited seed availability of other accessions, only KS-4A and KS-4H kochia accessions were included in this research. The field in which KS-4A and KS-4H accessions were obtained was under a wheat-fallow-wheat rotation for >6 yr followed by corn (38.0011°N, 100.4854°W) with a frequent use of dicamba, fluroxypyr, glyphosate, and/or atrazine (Kumar et al. Reference Kumar, Currie, Jha and Stahlman2019a). In a preliminary study, about 10 plants from each selected kochia accession (KS-4A and KS-4H) surviving a field-use rate (1,120 g ha−1) of atrazine POST were allowed to produce seeds in two separate greenhouse rooms at Kansas State University Agricultural Research Center (KSU-ARC) near Hays, KS, during autumn 2018. Progeny seeds of all 10 plants from each accession were hand threshed, cleaned, and composited into one sample and stored in paper bags at 4 C until used. Similarly, seeds of an herbicide-susceptible kochia accession (KS-SUS) were collected during autumn 2018 from a pasture field at KSU-ARC with no previous history of PS II inhibitor use and stored at 4 C. Before the experiments began the seed viability of all three accessions (KS-4A, KS-4H, and KS-SUS) was tested using a 1% tetrazolium solution at 25 C and was found to be ≥97% (data not shown).
Dose-Response Experiments
PRE Atrazine and Metribuzin Dose Response
Greenhouse experiments were conducted at the KSU-ARC near Hays, KS, to characterize the response of KS-4A, KS-4H, and KS-SUS kochia accessions to PRE-applied atrazine and metribuzin herbicides. For each PRE dose-response assay, plastic trays (each 25 × 25 × 10 cm) containing sterilized field soil (filled up to a depth of 9 cm) were used. Soil (Roxbury silt loam, pH 7.6, 1.9% organic matter) used in these experiments was collected from a research field (with no history of residual herbicide use) at KSU-ARC near Hays, KS. Separate dose-response experiments were conducted for atrazine and metribuzin, respectively, in a randomized complete block design (RCBD) with four replications (one tray = one replication) during autumn 2019 and repeated in spring 2020. One day before these experiments were initiated, plastic trays containing field soil were saturated with tap water. Fifty randomly selected seeds of each kochia accession were uniformly spread on the soil surface in each tray and covered with a fine layer of soil particles covering the seed with approximately 0.5 to 1 mm of soil. Doses of PRE-applied atrazine (AAtrex® 4L, Syngenta Crop Protection, LLC, Greensboro, NC), including 0, 280, 560, 1,120 (1× = field-use rate), 2,240, and 4,480 g ai ha−1 were tested. The tested PRE metribuzin (Sencor®, Bayer Crop Science, Saint Louis, MO) doses included 0, 157.5, 315, 630 (1× = field-use rate), 1,260, and 2,520 g ai ha−1. All selected doses for each herbicide were applied using a stationary cabinet spray chamber (Research TrackSprayer, DeVries Manufacturing, Hollandale, MN), equipped with an even flat-fan nozzle tip (TeeJet® 8001EXR, Spraying Systems, Wheaton, IL) calibrated to deliver 112 L ha−1 of spray solution at 241 kPa. Immediately after herbicide applications, the spray chamber was used to apply a mist of water to simulate a 0.2-mm irrigation to incorporate the herbicide into the soil. Greenhouse conditions were set at 25/23 ± 3 C day/night temperatures and 16/8-h photoperiod supplemented with metal-halide lamps (560 μmol m−2 s−1). Trays were kept moist by subsurface watering as needed. Emerged kochia seedlings from each tray were counted at 28 d after treatment (DAT). Immediately after seedling counts, the aboveground shoot biomass of kochia seedlings was hand-harvested from each tray and oven-dried at 65 C for 96 h to obtain shoot dry weights.
POST Atrazine and Metribuzin Dose Response
Similar to PRE dose-response, greenhouse experiments were conducted to characterize the putative cross-resistance in selected MHR (KS-4A, KS-4H) kochia accessions to POST-applied atrazine and metribuzin using dose–response assays. Plants from each selected accession were grown in square plastic pots (10 × 10 cm) containing a commercial potting mixture (Miracle-Gro® Moisture Control® Potting Mix, Miracle-Gro Lawn Products, 14111 Scottslawn Road, Marysville, OH). Separate dose-response assays were conducted for POST atrazine and metribuzin in autumn 2019 and were repeated in spring 2020. Each dose-response study was conducted in an RCBD with 12 replications (each replication = 1 plant per pot). Young actively growing plants (8- to 10-cm tall) from each accession were treated with different doses of atrazine applied POST: 0, 280, 560, 1,120 (1× = field-use rate), 2,240, 4,480, and 8,960 g ha−1. Doses of POST metribuzin included 0, 157.5, 315, 630 (1× = field-use rate), 1,260, 2,520, and 5,040 g ha−1. All atrazine and metribuzin doses also included 1% vol/vol crop oil concentrate adjuvant. All selected doses were applied using a stationary cabinet spray chamber as described above. Similar greenhouse conditions were maintained throughout the experiments as mentioned in the paragraph above that explains the PRE dose-response assays. For each kochia accession and herbicide dose, the treated plants were visually rated for visible injury on a scale of 0% (no injury) to 100% (complete plant death) at 14 and 28 DAT. At 28 DAT, the aboveground biomass of each treated plant was harvested and oven-dried at 65 C for 96 h to obtain shoot dry weights.
Mechanism of Cross-Resistance to Atrazine and Metribuzin
Sequencing the psbA Gene
Nucleotide sequences of the psbA genes from KS-4A, KS-4H, and KS-SUS kochia accessions were analyzed to determine the presence of any known target-site mutation(s) conferring resistance to PS II inhibitors. Fresh young leaf tissue samples (200 mg) from three untreated plants of each kochia accession were collected and shipped (overnight) to the weed research laboratory at the Colorado State University, in Fort Collins, CO. The genomic DNA was extracted using a DNeasy Plant Mini kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. Previously reported primer sequences specific to the kochia psbA gene were used (Varanasi et al. Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015). The forward and reverse primer sequences were 5′-CTCCTGTTGCAGCTGCTACT-3′ and 5′-TAGAGGGAAGTTGTGAGC-3′, respectively. These primer sequences were used to amplify a 575-base pair (bp) fragment of the psbA gene in MHR and susceptible kochia plants.
A PCR assay was performed in a T100 thermal cycler (Bio-Rad, Hercules, CA) using EconoTaq PLUS 2XPCR master mix (Lucigen, Middleton, WI). Each reaction contained 10 μl of Master Mix, 2.5 μl each of the forward and reverse primers (5 μM), 5 μl of genomic DNA template (10 ng μl−1), and 5 μl of nuclease-free water. For polymerase chain reaction (PCR) amplification the following thermal conditions were used: 95 C for 3 min; 30 cycles at 95 C for 30 s, 58 C for 30 s, and 72 C for 90 s; followed by 72 C for 7 min. To confirm the amplicon size, PCR products were examined on a 1.0% agarose gel stained with GelRed™ nucleic acid gel stain (Biotium, Fremont, CA). Purified PCR products were sequenced by Sanger sequencing (Genewiz, South Plainfield, NJ). The sequence reads of the psbA genes were aligned to a reference kochia psbA sequence using Geneious Prime (https://www.geneious.com) to analyze the presence of any known target-site mutations that confer resistance to the PS II inhibitors.
Effect of Malathion on Atrazine and Metribuzin Resistance
The role of CYP450 monooxygenases (the enzymes involved in herbicide detoxification) in cross-resistance to atrazine and metribuzin in KS-4A and KS-4H kochia accessions was investigated using a malathion test. As reviewed by Powles and Yu (Reference Powles and Yu2010), malathion is an organophosphate insecticide that inhibits the activity of CYP450 monooxygenases and can indirectly detect the role of these enzymes in herbicide resistance. Kochia plants from KS-4A, KS-4H, and KS-SUS accessions were grown in plastic pots in the greenhouse at KSU-ARC as described in the section that described the POST dose-response experiments, and were sprayed with malathion at 1,000 g ha−1 when they were 8- to 10-cm tall. About 1 h after the malathion treatment, plants from each accession were either kept as such (malathion only) or treated with POST atrazine at 1,120 g ha−1 (malathion followed by atrazine) or POST metribuzin at 630 g ha−1 (malathion followed by metribuzin). Malathion and herbicide treatments were applied by using a cabinet spray chamber as previously described. Experiments were conducted in RCBD with 12 replicates during autumn 2019 and repeated in spring 2020. Visual estimates of percent injury and shoot dry weights of all treated plants were recorded at 21 DAT.
Effectiveness of Alternative POST Herbicides
Greenhouse experiments were conducted at the KSU-ARC in spring 2019 and repeated in autumn 2019 to determine the effectiveness of alternative POST herbicides for controlling MHR kochia accessions. Kochia plants from KS-4H and KS-SUS accessions were grown in square plastic pots (10 × 10 cm) containing the commercial potting mixture previously described. Actively growing kochia plants (8- to 10-cm tall) from each accession were treated with the labeled field-use rates of alternative POST herbicides (Table 1) using the stationary spray chamber. Experiments were conducted in an RCBD with 12 replications (one rep = one plant per pot). Similar greenhouse conditions were maintained throughout the experimental period as mentioned in the section on dose-response assays. Data on percent visible injury estimates (on a scale of 0% to 100%) were recorded at 21 DAT. Immediately after visual ratings, the aboveground shoot biomass were hand-harvested and oven-dried at 65 C for 96 h to obtain shoot dry weights. Shoot dry weights of treated plants were expressed as percent reduction relative to that of the nontreated control for each accession using the following equation:
 $$Y = {\frac{{DWc - DWt}}\over{{DWc}}}{\rm{\;}}x{\rm{\;}}100$$
$$Y = {\frac{{DWc - DWt}}\over{{DWc}}}{\rm{\;}}x{\rm{\;}}100$$
Table 1. List of alternative POST herbicides tested for controlling KS-SUS and KS-4H kochia accessions in a greenhouse study at the Kansas State University Agricultural Research Center near Hays, KS, in 2019.

where Y is the percent shoot dry weight reduction, DW c is the shoot dry weight of the nontreated control, and DW t is the shoot dry weight of treated plants at 21 DAT.
Statistical Analyses
All data were subjected to ANOVA using the PROC MIXED procedure in SAS (version 9.3, SAS Institute, Cary, NC) to test the significance of fixed effects (i.e., accession, treatment [atrazine or metribuzin doses in dose-response, malathion-based treatment, or herbicide(s) in alternative POST efficacy experiments]) and their interactions. Random effects in the model included experimental run and replication (nested within experimental runs; SAS v.9.3). There was no significant interaction between experimental run and herbicide treatments in all greenhouse studies; therefore, data were combined across experimental runs for each experiment. Data were checked for the ANOVA assumptions using the the PROC UNIVARIATE procedure in SAS, and all data met both assumptions except for the alternative POST herbicide study. Data on percent visible injury and shoot dry weight reduction (%) from the alternative POST herbicide study were square root–transformed before analysis to improve the normality of residuals and homogeneity of variance. Nontransformed means are presented based on the interpretation from the transformed data. Shoot dry weights (% of nontreated) of each accession from PRE and POST dose-response assays were regressed against atrazine or metribuzin doses using a three-parameter log-logistic model (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015):
where y is the shoot dry weight reduction (% of nontreated), d is the maximum shoot dry weight, e is the atrazine or metribuzin dose needed for 50% reduction in shoot dry weight (referred as GR50 values, respectively), x is the herbicide dose (atrazine or metribuzin), and b represents the slope for each curve. All nonlinear regression parameters were estimated using the drc package in R software. The resistance index (R/S ratio) was estimated by dividing the GR50 value for each MHR kochia accession by the GR50 value of the KS-SUS accession.
Results and Discussion
Dose-Response Experiments
PRE and POST Atrazine Dose Response
Results from PRE and POST dose-response assays confirmed that both MHR kochia accessions (KS-4A and KS-4H) had evolved resistance to atrazine. The PRE-applied atrazine doses for 50% shoot dry weight reduction (GR50 values) of KS-4A and KS-4H kochia accessions were 6,449 and 3,999 g ha−1, respectively, both of which were greater than the 134 g ha−1 for the KS-SUS accession (Table 2; Figure 1A). Similarly, GR50 values of POST-applied atrazine for KS-4A and KS-4H kochia accessions were 11,705 and 9,252 g ha−1, respectively, both of which were also greater than the 388 g ha−1 for the KS-SUS accession (Table 2; Figure 1B). Based on GR50 values, the KS-4A and KS-4H kochia accessions exhibited 29-fold to 48-fold resistance to PRE-applied atrazine and 23-fold to 30-fold resistance to POST-applied atrazine compared with the KS-SUS accession (Table 2; Figure 1 A and B). These results revealed high-level resistance to PRE- and POST-applied atrazine in both MHR kochia accessions. These results are consistent with those previously reported by Foes et al. (Reference Foes, Liu, Vigue, Stoller, Wax and Tranel1999), who documented a kochia accession from McDonough County, IL, with a high-level resistance (>500-fold) to POST-applied atrazine. In addition, Varanasi et al. (Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015) also reported an MHR kochia population from Garden City, KS, with >25% survivors after POST-applied atrazine at 2,240 g ha−1, although the resistance level was not determined in that population.
Table 2. Regression parameter estimates for shoot dry weight reductions (% of nontreated) of KS-SUS, KS-4A, and KS-4H kochia accessions from Garden City, KS, treated with PRE and POST applied atrazine in separate dose-response experiments in 2019 and 2020.

a Abbreviations: KS-SUS, susceptible kochia accession from Hays, KS; KS-4A, and KS-4H are multiple herbicide–resistant kochia accessions from research plots at the Kansas State University Southwest Research and Extension Center near Garden City, KS; CI, confidence interval.
b Data were pooled from two experimental runs before analysis.
c GR50 is the effective dose (g ha−1) of atrazine applied PRE and/or POST for 50% shoot dry weight reduction.
d R/S (resistance factor) is the ratio of the GR50 of a resistant kochia accession to the GR50 of the KS-SUS kochia accessions.

Figure 1. Shoot dry weight (% of nontreated) response of multiple herbicide-resistant (KS-4A, KS-4H) and –susceptible (KS-SUS) kochia accessions averaged across two runs of dose-response experiments with atrazine applied PRE (A) and POST (B) at 28 d after treatment at Kansas State University Agricultural Research Center near Hays, KS. Symbols represent actual values, and lines represent predicted values obtained from the three-parameter log-logistic model. Vertical bars indicate ± standard errors of the mean values.
PRE and POST Metribuzin Dose Response
Based on the fitted log-logistic model, the estimated PRE-applied metribuzin doses to obtain 50% shoot dry weight reduction (GR50 values) of KS-4A and KS-4H accessions were 250 and 119 g ha−1, values that were higher than the dose needed for the KS-SUS accession (37 g ha−1; Table 3; Figure 2A). In contrast, GR50 values of POST-applied metribuzin for KS-4A and KS-4H kochia accessions were 5,406 and 3,963 g ha−1, respectively, both higher than 301 g ha−1 for the KS-SUS accession (Table 3; Figure 2B). Based on GR50 values, the KS-4A and KS-4H kochia accessions exhibited a putative resistance to PRE metribuzin and 13-fold to 18-fold resistance to POST metribuzin compared with the KS-SUS accession (Table 3; Figure 2 A and B). It is important to note that only a few doses of metribuzin were tested in the PRE dose-response study under greenhouse conditions. Field studies with multiple doses of PRE-applied metribuzin should be conducted to confirm resistance in the two accessions tested. In comparison to our results, a kochia population from railroad rights-of-way in Minnesota has been reported with a 4-fold resistance to metribuzin applied POST (Mengistu et al. Reference Mengistu, Christoffers and Lym2005).
Table 3. Regression parameter estimates for shoot dry weight reductions (% of nontreated) of KS-SUS, KS-4A, and KS-4H kochia accessions from Garden City, KS, treated with metribuzin applied PRE and POST in separate dose-response experiments in 2019 and 2020.

a Abbreviations: KS-SUS, susceptible kochia accession from Hays, KS; KS-4A, and KS-4H are multiple herbicide–resistant kochia accessions from research plots at the Kansas State University Southwest Research and Extension Center near Garden City, KS; CI, confidence interval.
b Data were pooled from two experimental runs before analysis.
c GR50 is the effective dose (g ha−1) of metribuzin applied PRE and/or POST for 50% shoot dry weight reduction.
d R/S (resistance factor) is the ratio of the GR50 of a resistant kochia accession to the GR50 of the KS-SUS kochia accessions.

Figure 2. Shoot dry weight (% of nontreated) response of multiple herbicide-resistant (KS-4A, KS-4H) and –susceptible (KS-SUS) kochia accessions averaged across two runs of dose-response experiments with metribuzin applied PRE (A) and POST (B) at 28 d after treatment at Kansas State University Agricultural Research Center near Hays, KS. Symbols represent actual values, and lines represent predicted values obtained from the three-parameter log-logistic model. Vertical bars indicate ± standard errors of the mean values.
Mechanism of Resistance
Mutation in the psbA Gene
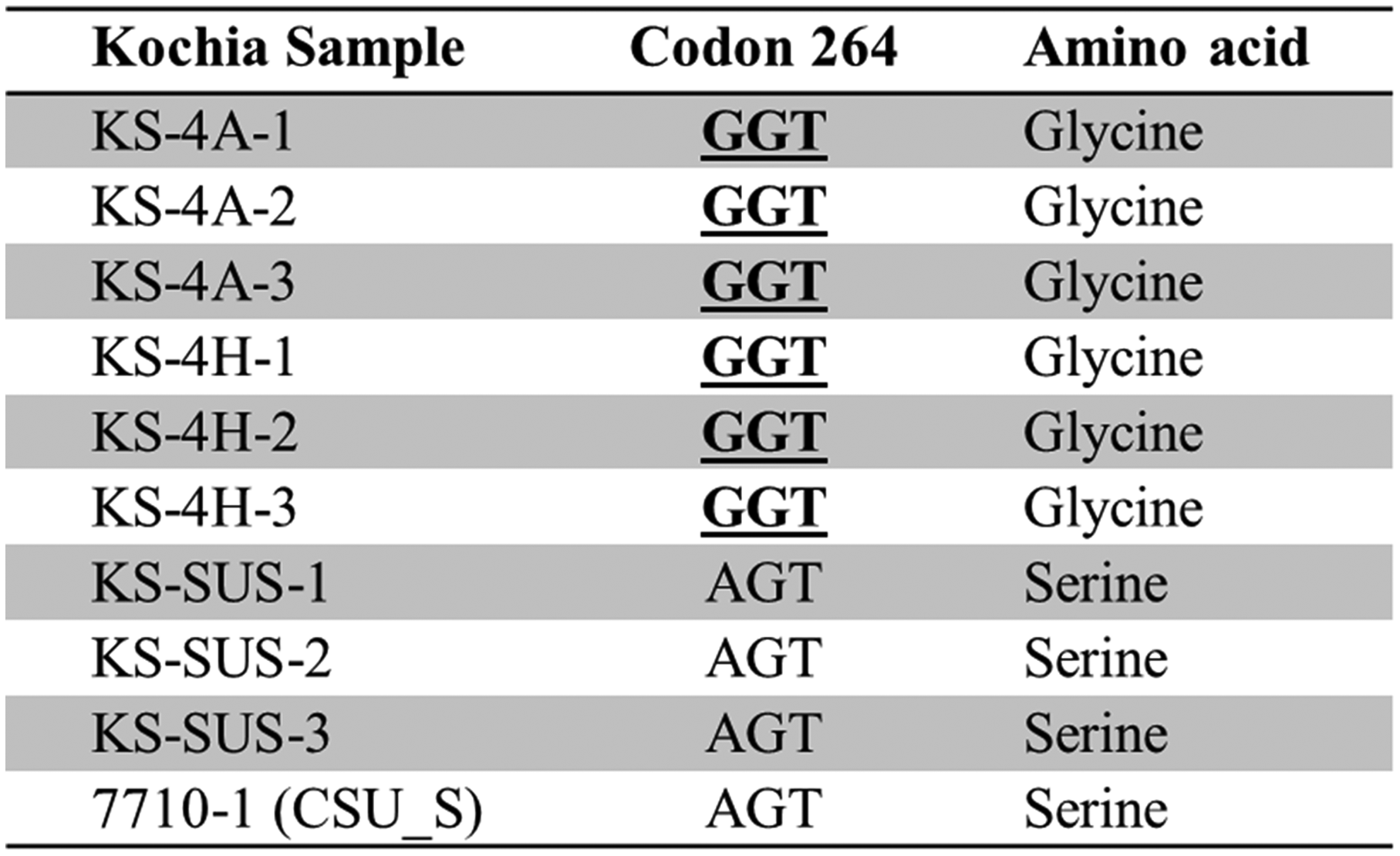
The sequence analyses of the psbA gene (approximately 550 bp) in both MHR kochia accessions (KS-4A and KS-4H) showed a point mutation at the Ser264 position (Figure 3). All the nucleotide sequences for the psbA gene are deposited in GeneBank (GeneBank accession numbers BankIt2399244 KS-4A, MW241628; BankIt2399244 KS-4H, MW241629; and BankIt2399244 KS-SUS, MW241630). More specifically, a nucleotide substitution of AGT to GGT at codon 264 was observed in all KS-4A and KS-4H accessions relative to KS-SUS plants (Figure 3). This nucleotide change resulted in a substitution of amino acid from serine to glycine at residue 264 (Figure 3). Our results are consistent with those of Varanasi et al. (Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015) who reported that the same mutation (Ser264Gly) in a MHR kochia population from Garden City conferred resistance to atrazine. In contrast, Mengistu et al. (Reference Mengistu, Christoffers and Lym2005) reported a different point mutation (Ile219Val) causing moderate levels of resistance to metribuzin in kochia populations from railroad rights-of-way in Minnesota. No other known point mutations in the psbA gene conferring resistance to PS II inhibitors were detected in MHR or SUS accessions.

Figure 3. Change in nucleotide base (bold and underlined) in the psbA gene of KS-4A and KS-4H kochia samples compared to KS-SUS samples showing an amino acid substitution from serine to glycine at codon 264.
Effect of Malathion on Resistance
A pretreatment with malathion followed by POST applications of atrazine at 1,120 g ha−1 or metribuzin at 633 g ha−1 did not reverse the resistance phenotypes of KS-4A and KS-4H compared to the KS-SUS kochia accession (data not shown). Furthermore, no differences in shoot dry weights of KS-4A and KS-4H accessions were observed among malathion only, malathion followed by atrazine, or malathion followed by metribuzin treatments (Table 4). In contrast, a significant reduction in shoot dry weights of KS-SUS kochia accession was observed between malathion only and malathion followed by atrazine or malathion followed by metribuzin treatments. These results may rule out the possibility of an enhanced metabolism of atrazine or metribuzin via CYP450 monooxygenases in these MHR kochia accessions. It is important to note that GST inhibitors were not tested for their role in metabolism-based resistance in this study. Future research is needed to explore the role of GSTs for conferring resistance (if any) to PS II inhibitors in these MHR accessions.
Table 4. Averaged shoot dry weights of KS-SUS, KS-4A, and KS-4H kochia accessions at 21 d after treatment with malathion alone or malathion followed by atrazine or metribuzin in greenhouse experiments conducted at Kansas State University Agricultural Research Center near Hays, KS.

a Means for a malathion treatment within a column followed by similar lowercase letters are not significantly different based on Fisher’s protected LSD test at P < 0.05; means for a kochia accession within a row followed by similar uppercase letters are not significantly different based on Fisher’s protected LSD test at P < 0.05 for shoot dry weights. Data were pooled from two experimental runs before analyses.
b Abbreviations: KS-SUS, susceptible kochia accession from Hays, KS; KS-4A, and KS-4H are multiple herbicide–resistant kochia accessions from research plots at the Kansas State University Southwest Research and Extension Center near Garden City, KS.
c Malathion alone, 1,000 g ha−1.
d Malathion followed by atrazine, 1,120 g ha−1.
e Malathion followed by metribuzine, 600 g ha−1.
Effectiveness of Alternative POST Herbicides
Percent Visible Injury
An interaction of POST herbicides by kochia accessions was significant for percent visible injury at 21 DAT, indicating a differential response of KS-4H and KS-SUS accessions to some of the POST herbicides tested (Table 5). Among all POST herbicides tested, bicyclopyrone + bromoxynil; bromoxynil + pyrasulfotole; paraquat alone or in combination with atrazine, metribuzin, 2,4-D, or saflufenacil; and saflufenacil alone or in combination with 2,4-D provided excellent control (≥98% injury) of KS-SUS and KS-4H accessions at 21 DAT (Table 5). Wicks et al. (Reference Wicks, Martin, Haack and Mahnken1994) documented 96% control of triazine-resistant kochia with paraquat applied as a burndown treatment prior to sorghum planting. Similarly, excellent control (>97%) of GR kochia has been reported with bromoxynil + pyrasulfotole, bromoxynil + fluroxypyr, paraquat, and saflufenacil alone or in combination with 2,4-D in two separate studies (Kumar et al. Reference Kumar, Jha and Reichard2014;Sbatella et al. Reference Sbatella, Adjesiwor, Kniss, Stahlman, Westra, Moechnig and Wilson2019). In the current study, glufosinate and a premix of 2,4-D + bromoxynil + fluroxypyr resulted in greater injury (96% vs. 84% averaged) to the KS-SUS than the KS-4H accession. Furthermore, visible injury of both accessions averaged 86% with bromoxynil + fluroxypyr and a premix of 2,4-D + dicamba + fluroxypyr.
Table 5. Visible injury and shoot dry weight reduction of multiple herbicide–resistant and –susceptible kochia accessions at 21 d after treatment of various alternative POST herbicides in a greenhouse study conducted in 2019 at the Kansas State University Agricultural Research Center in Hays, KS. a,b

a Abbreviations: KS-SUS, susceptible kochia accession from Hays, KS; KS-4H is a multiple herbicide–resistant kochia accession from Kansas State University Southwest Research and Extension Center near Garden City, KS.
b All herbicides were applied to 8- to 10-cm-tall kochia plants. Means for a kochia accession within a column followed by similar lowercase letters are not significantly different based on Fisher’s protected LSD test at P < 0.05; means for an herbicide within a row followed by similar uppercase letters are not significantly different based on Fisher’s protected LSD test at P < 0.05 for percent of visible injury and shoot dry weight reduction separately.
c Crop oil concentrate at 1% (vol/vol) was included.
d Nonionic surfactant at 0.5 (vol/vol) was included.
e Ammonium sulfate at 2% (wt/vol) was included.
f Methylated seed oil at 1% (vol/vol) was also included.
Shoot Dry Weight Reduction
In general, shoot dry weight reduction of both kochia accessions followed the pattern of percent visible injury for all alternative POST herbicides tested. For instance, bicyclopyrone + bromoxynil; bromoxynil + pyrasulfotole; paraquat alone or in combination with atrazine, metribuzin, 2,4-D, or saflufenacil; and saflufenacil alone or in combination with 2,4-D reduced shoot dry weights of KS-SUS and KS-4H accessions by 96% to 99% at 21 DAT (Table 5). Comparatively, the reduction in shoot dry weight of the KS-4H accession with glufosinate and premix of 2,4-D + bromoxynil + fluroxypyr was lower (77% vs. 92% averaged) than that of the KS-SUS kochia accession (Table 5). Bromoxynil + fluroxypyr and a premix of 2,4-D + dicamba + fluroxypyr reduced shoot dry weights of both kochia accessions by 81% to 87%.
Practical Implications
Results from PRE and POST dose-response assays confirmed the coexistence of cross-resistance to atrazine (high level) in two MHR kochia accessions (KS-4A and KS-4H) from Garden City, KS. Furthermore, both the MHR accessions exhibited moderate levels of resistance to POST-applied metribuzin and were putatively resistant to PRE-applied metribuzin. Sequence analysis of the psbA genes in MHR kochia plants further revealed a single point mutation (Ser264Gly) conferring resistance to these PS II inhibitors. The malathion test most likely ruled out the possibility of a CYP450 monooxygenases in metabolism-based mechanism in these accessions. However, the role of GST-based metabolic resistance to PS II inhibitors was not explored in this study and warrants future research work. Although the same point mutation conferring resistance to POST-applied atrazine has previously been documented in a four-way resistant (resistance to glyphosate, dicamba, chlorsulfuron, and atrazine) kochia population in this region (Varanasi et al. Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015), it was unknown whether this mutation also conferred resistance to PRE-applied atrazine and cross-resistance to POST-applied metribuzin. To our knowledge, this research reports the first case of kochia with cross-resistance to PRE-applied atrazine and POST-applied metribuzin. Several studies have previously reported a fitness penalty associated with the target site mutation in triazine-resistant weed species (Conard and Radosevich Reference Conard and Radosevich1979; Vila-Aiub et al. Reference Vila-Aiub, Neve and Powles2009). It is also important to note that these two MHR kochia accessions are resistant to dicamba and fluroxypyr (Kumar et al. Reference Kumar, Currie, Jha and Stahlman2019a). Studies have also documented a fitness cost in kochia populations with resistance to dicamba and/or fluroxypyr (Kumar and Jha Reference Kumar and Jha2016; LeClere et al. Reference LeClere, Wu, Westra and Sammons2018). The underlying mechanism(s) conferring cross-resistance to dicamba and fluroxypyr and fitness attributes of KS-4A and KS-4H kochia accessions from Kansas are still unknown. Nevertheless, controlling these MHR kochia accessions would be a challenging task for producers in the region. Our greenhouse study on alternative POST herbicides further indicated that bicyclopyrone + bromoxynil; bromoxynil + pyrasulfotole; paraquat alone or in combination with atrazine, metribuzin, 2,4-D, or saflufenacil; and saflufenacil alone or in combination with 2,4-D can provide effective control of MHR kochia.
A multistate field survey for monitoring the presence of MHR (resistant to glyphosate, dicamba, fluroxypyr, atrazine, and metribuzin) kochia across the south-central Great Plains is currently underway. A multilocation study has been planned to investigate the integrated effect of crop competition, cover crops, harvest weed seed control, and targeted tillage on long-term soil seedbank dynamics of these MHR kochia accessions; information that is greatly needed for developing ecologically based weed management strategies in the U.S. Great Plains region.
Acknowledgments
No conflicts of interest have been declared. This work was supported by the U.S. Department Agriculture–National Institute of Food and Agriculture (Hatch project accession number 1019671). This publication is contribution o. 21-045-J from the Kansas Agricultural Experiment Station, Manhattan, KS.