With the recent opening of a rapeseed seed crushing plant in western Texas, farmers in the High Plains of New Mexico are increasingly interested in winter rapeseed as a means to diversify farm incomes. Farmer enthusiasm for rapeseed is also fueled by the potential for improved profitability in subsequent winter wheat (Triticum aestivum L.) resulting from rapeseed’s ability to reduce population densities of problematic grass weeds, disrupt life cycles of economically important crop pathogens, and mobilize nutrients critical to wheat production (Boyles et al. Reference Boyles, Bushong, Sanders and Stamm2012; Bushong et al. Reference Bushong, Griffith, Peeper and Epplin2012). Further, rapeseed can be planted and harvested with the same equipment used for wheat, which negates the need to invest in new machinery.
Current weed management options in winter rapeseed do not address the production system preferences of some farmers in New Mexico. Most notably, New Mexico farmers who prefer to use rapeseed varieties that have not been modified to tolerate specific broad-spectrum postemergence (POST) herbicides (i.e., conventional rapeseed) lack reliable and practical management strategies for Brassicaceae weeds. This deficiency in weed management partly reflects the small number of POST herbicides for broadleaf weeds in conventional rapeseed in New Mexico. Clopyralid can be used to control emerged broadleaf weeds in conventional rapeseed; however, this herbicide is not effective on Brassicaceae weeds. Emerged Brassicaceae weeds can be controlled with carfentrazone applications that require a hooded sprayer with widely spaced crop rows. However, rapeseed is typically planted with a row spacing of 10 to 38 cm (Boyles et al. Reference Boyles, Bushong, Sanders and Stamm2012; O’Donovan Reference O’Donovan1994), which makes carfentrazone applications difficult in rapeseed. Ethametsulfuron-methyl offers opportunities to control Brassicaceae weeds in conventional rapeseed; however, ethametsulfuron-methyl is not labeled for use on rapeseed in New Mexico. With few herbicide options, Brassicaceae weeds are difficult to control in conventional rapeseed grown in New Mexico.
Flixweed is a Brassicaceae weed that is indigenous to Europe and can now be found throughout the United States and Canada (Mitich Reference Mitich1996). Like rapeseed, flixweed is a winter annual that emerges in fall, overwinters as a rosette, resumes growth in early spring, and flowers by late spring. Flixweed’s phenology means that it occurs as a rosette during the two periods when weed management is crucial in winter rapeseed: early fall, before the rapeseed canopy closes, and spring, just after rapeseed growth resumes. Individual flixweed plants can produce over 75,000 seeds (Stevens Reference Stevens1957) that can reduce rapeseed oil and meal quality (Davis et al. Reference Davis, Brown, Brennan and Thill1999). Flixweed is also a host of the causal agent of clubroot (Plasmodiophora brassicae), which is a rapeseed disease that reduces seed yield and seed oil content (Ren et al. Reference Ren, Xu, Liu, Chen, Sun, Li and Fang2016). There have been reports from Kansas of flixweed developing resistance to acetolactate synthase–inhibiting herbicides, which would make flixweed even more difficult to control (Heap Reference Heap2016). The undesirable effects of flixweed in rapeseed, combined with the lack of herbicides for flixweed in conventional rapeseed, compels a search for nonherbicidal management strategies for flixweed in rapeseed.
Nonherbicidal strategies for weed management include increasing the crop seeding rate to reduce transmission of light through the canopy (Blackshaw Reference Blackshaw1993; Tharp and Kells Reference Tharp and Kells2001). An increased crop seeding rate has been shown to suppress smooth pigweed (Amaranthus hybridus L.) plant growth in crops including cowpea [Vigna unguiculata (L.) Walpers], sunn hemp (Crotalaria juncea L.), and velvet bean [Mucuna deeringiana (Bort) Merr.] (Collins et al. Reference Collins, Chase, Stall and Hutchinson2008). Increased crop seeding rate has also been shown to limit plant growth of rigid ryegrass (Lolium rigidum Gaudin) in wheat (Lemerle et al. Reference Lemerle, Cousens, Gill, Peltzer, Moerkerk, Murphy, Collins and Cullis2004), and moderately suppress wild-proso millet (Panicum miliaceum L.) in sweet corn (Zea mays L.) (Williams and Boydston Reference Williams and Boydston2013). In addition to inhibiting growth of weeds, increased crop seeding rate can also improve crop yield under weed-free conditions (Mohler Reference Mohler2007); however, increased crop seeding rate might reduce crop yield as intraspecific competition potentially decreases growth of crop plants (Lemerle et al. Reference Lemerle, Cousens, Gill, Peltzer, Moerkerk, Murphy, Collins and Cullis2004).
The degree to which increased crop seeding rate is an effective weed management strategy depends, in part, on the capacity of the targeted weed to tolerate shade. Plants that tolerate shade exhibit specific physiological and morphological characteristics that are advantageous for survival under low-light conditions. As irradiance levels decrease, shade-tolerant plants reduce allocation of biomass to roots and increase the proportion of total biomass allocated to leaves (Qin et al. Reference Qin, Mao, Quan, Zhang, Xie and Ditommaso2012); thus the leaves become broader and thinner as light diminishes (Hunt and Burnett Reference Hunt and Burnett1973; Patterson Reference Patterson1979; Regnier and Harrison Reference Regnier and Harrison1993). Shading also causes a shade-tolerant plant to decrease its light compensation point, which is the level of light at which the rate of CO2 uptake is equal to the rate of CO2 release in respiration and photorespiration (Aleric and Kirkman Reference Aleric and Kirkman2005; Qin et al. Reference Qin, Mao, Quan, Zhang, Xie and Ditommaso2012). The physiological and morphological changes that are observed in shade-tolerant species facilitate plant survival under low irradiance levels because these traits conserve limited photosynthates while maximizing light interception. Knowledge of shade effects on the growth and photosynthesis of targeted weeds provides insight on the potential effectiveness of increased crop seeding rate as a weed management strategy.
Recommendation and use of increased rapeseed seeding rate for flixweed management are hindered by the lack of information on rapeseed seeding rate effects on canopy light transmittance and crop yield, as well as uncertainty regarding flixweed’s capacity for acclimation to shading. The objectives of this study were 1) to determine if increased crop seeding rate reduces the transmittance of light through the rapeseed canopy without causing yield reduction from increased intraspecific competition and 2) to assess flixweed for shade-tolerance by measuring photosynthetic and morphological responses of flixweed rosettes to decreasing irradiance levels.
Materials and Methods
Field Study
A field experiment was conducted at the New Mexico State University (NMSU) Agricultural Science Center at Clovis, New Mexico (34.4048°N, 103.2052°W) from September 2014 to June 2015 and repeated from September 2015 to June 2016. Annual experimental runs were conducted in different fields, but both fields featured an Olton clay loam soil (fine, mixed, superactive, thermic Aridic Paleustolls). For the 2014/2015 experimental run, soil pH was 7.6 and organic matter content was 1.7%. For the 2015/2016 experimental run, soil pH was 7.8 and organic matter content was 1.4%. In the growing season prior to 2014, the study site was fallow. The 2015/2016 experimental run was planted into a field previously used for wheat production. One month prior to planting the 2015/2016 experimental run, the field was tilled using a disc plow to remove wheat stubble. For both experimental runs, fields were tilled to the 10-cm depth using a Sunflower 6333 Land Finisher (AGCO, Duluth, GA) one day prior to planting.
Experimental units were plots (1.83 m by 9.14 m) that were arranged in a randomized complete block design with four replications. Treatments were rapeseed seeding rates of 0.5, 1.0, 1.5, 2.0, and 2.5 times the recommended seeding rate of 3.6 kg ha−1 (Boyles et al. Reference Boyles, Bushong, Sanders and Stamm2012). ‘Saffran’, a hybrid winter rapeseed variety that was previously determined to be suitable for the region (Sangu Angadi, personal communication, August 2014), was seeded into rows spaced 15.2 cm apart. Seeding was performed using a plot drill equipped with a double disc opener (Great Plains 3P600, John Deere, Moline, IL) and took place on September 10, 2014, and September 9, 2015. Immediately after seeding, trifluralin (Triflurex HFP®, Makhteshim Agan of North America, Raleigh, NC) was broadcast applied at 0.140 kg aiha−1 using a tractor-mounted sprayer calibrated to deliver 234 L ha−1 at 276 kPa using flat-fan XR TeeJet 8002 spray tips (TeeJet Technologies, Wheaton, IL). Herbicide was incorporated with the Sunflower 6333 Land Finisher, and rapeseed germination was stimulated with overhead, center-pivot irrigation. After planting, plots were hand-hoed as needed to maintain weed-free conditions. Plots were irrigated via center pivot as needed from September to late November and from early February to late April.
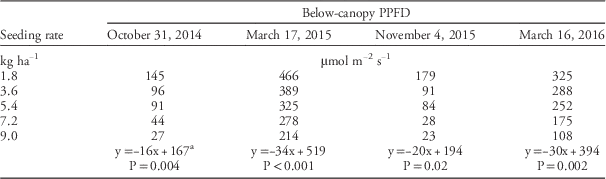
Rapeseed stand counts (plants per meter) and photosynthetic photon flux density (PPFD) were measured in fall (October 31, 2014, and November 4, 2015) and spring (March 17, 2015, and March 16, 2016), from a central location within each plot. At the time that PPFD measurements were taken, rapeseed rosettes were at the 12 to 16 growth stage on the Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie (BBCH) scale (Lancashire et al. Reference Lancashire, Bleiholder, Boom, Langelüddeke, Stauss, Weber and Witzenberger1991). On each measurement day, PPFD values were determined within one hour of solar noon. Above-canopy PPFD (PPFD above ) was determined with a quantum sensor (LI 190r Quantum Sensor, Li-Cor Inc., Lincoln, NE) and below-canopy PPFD (PPFD below ) was measured with a quantum sensor that determined PPFD across 1 m (LI 191 Line Quantum Sensor, Li-Cor Inc., Lincoln, NE). Within a plot, PPFD above and PPFD below were recorded simultaneously. Photosynthetic photon flux density data were used to calculate light transmittance (%) through the canopy:
To accelerate desiccation, rapeseed was treated with diquat five days prior to harvest (Reglone, Syngenta Crop Protection, Greensboro, NC) at 0.36 kg aiha−1. Rapeseed was harvested on June 29 and 30, 2015, and June 16 and 17, 2016. Prior to harvesting, each plot was divided into two sections. One section was used to collect data on harvest index, which is the proportion of the aboveground biomass allocated to seeds (Hay Reference Hay1995). At the time of harvest index measurements, rapeseed plants were at the 99 growth stage on the BBCH scale (Lancashire et al. Reference Lancashire, Bleiholder, Boom, Langelüddeke, Stauss, Weber and Witzenberger1991). Harvest index was determined by first hand-harvesting aboveground biomass from quadrats (1 m by 1 m), then drying biomass for 5 days at 65 C and separately weighing dried seeds and dry vegetative biomass. Following the collection of aboveground biomass for harvest index, crop yield was measured in the remaining section of the plot (15.7 m2) using a plot combine (Nurserymaster Elite, Wintersteiger Inc., Salt Lake City, UT). Seed oil content was determined from combine-harvested subsamples and was adjusted to an 8.5% seed moisture content by the Brassica Breeding and Research Program Oilseed Quality Lab at the University of Idaho using nuclear magnetic resonance spectroscopy methods from Hammond (Reference Hammond1991) and Howard and Daun (Reference Howard and Daun1991).
Greenhouse Study
A greenhouse study was conducted to determine shading effects on flixweed photosynthesis and growth. The study occurred from November 14, 2015, to December 18, 2015, and was repeated from March 19, 2016, to April 30, 2016. The greenhouse was located at the NMSU main campus at Las Cruces, New Mexico, and was set to maintain an air temperature of 24 C (±3 C). Each experimental run arranged in a randomized complete block with five replications. Treatments used reduced-PPFD enclosures that allowed light-transmittance levels equal to 100%, 60%, and 30% of ambient light.
Seeds for this study were collected on May 27, 2015, from plants growing in field margins at the NMSU Agricultural Science Center at Clovis, New Mexico. Flixweed inflorescences were clipped by hand and dried the laboratory for 14 d in the absence of direct sunlight. Dried inflorescences were hand-threshed, and sequential combinations of sieving and forced-air separation were used to separate seeds from chaff. Seeds were stored in airtight glass jars at 4 C.
To reduce physiological seed dormancy (Baskin et al. Reference Baskin, Milberg, Andersson and Baskin2004), seeds were incubated on moistened filter paper for 4 wk in darkness at 25/15 C (12 hr cycles). To stimulate germination, seeds were transferred to prehydrated Jiffy-7 Peat® pellets (Ferry-Morse Seed Company, Fulton, KY) and placed in a plant growth chamber at 20/10 C (12 hr cycles) for 5 to 10 d. When they had reached the one-leaf stage, seedlings in pellets were transferred to 1.67 L pots (15-cm diameter, 15-cm height; 1 plant per pot) filled with potting mix (Metro-Mix® 350, Sun Gro Horticulture, Agawam, MA) and moved onto a greenhouse bench with reduced-PPFD enclosures. Each reduced-PPFD enclosure (38-cm height, 33-cm length, 33-cm width) featured four corner posts to which a single layer of shade cloth was fixed to create upper and side walls. The bottoms of side walls were elevated 14 cm from the greenhouse bench. Shade cloths were purchased from a supplier (AM Leonard Inc., Piqua, OH) and were manufactured to provide 60% and 30% light transmittance. Structures for the 100% light transmittance treatment were not wrapped with shade cloth. Before the plants were transferred to the greenhouse, PPFD in the reduced-PPFD enclosures was measured using a quantum sensor (LI 190r Quantum Sensor, Li-Cor Inc., Lincoln, NE). Results indicated that light-transmittance levels were within 1% of expected values. Moisture levels in pots were visually evaluated daily. When potting mix surfaces appeared dry, individual pots were watered to saturation. At 14 d after transfer to the greenhouse, each pot received 250 mL of a 0.4% aqueous solution containing water-soluble fertilizer (20 parts nitrogen, 20 parts potassium, and 20 parts phosphorus by weight; Jacks Classic All Purpose 20-20-20, JR Peters Inc., Allentown, PA).
Each reduced-PPFD enclosure contained two plants: one plant for photosynthetic measurements and one plant for measurements of morphology. Morphological characteristics of flixweed plants were measured 28 d after plant transfer to the greenhouse. At the time of measurement, plants were in rosette stages. Flixweed leaves are two or three times pinnately compound. In this study, surface areas of leaflets, rachises, and petioles were simultaneously measured using a leaf area meter (LI 3000, Li-Cor Inc., Lincoln, NE) immediately after harvest. Hereafter, the surface area of combined leaf parts will be referred to as foliage area. Foliage area in place of leaf area was previously used to study several Acacia species, which, like flixweed, have pinnately compound leaves (Atkin et al. Reference Atkin, Schortemeyer, McFarlane and Evans1998). As for belowground parts, roots were hand-washed with gentle agitation in a basin of water. Aboveground and belowground parts were separately bagged and dried at 65 C for 7 days. Dried plant material was weighed to determine aboveground biomass, belowground biomass, and total biomass. In this study, aboveground biomass is synonymous with foliage biomass because harvested plants were rosettes. Biomass and foliage area measurements were used to calculate the root mass ratio (RMR), which is the root mass divided by the total plant mass; foliage area ratio (FAR), which is the foliage area divided by the total biomass; and specific foliage area (SFA), which is the foliage area divided by the aboveground biomass.
To determine light compensation point (LCP), photosynthetic measurements were taken within 1 hr of solar noon from December 14 to 18, 2015, and April 26 to 30, 2016. Data were collected to create a photosynthetic light-response curve, which is a mathematical function fitted to the relationship between photosynthetic rate and irradiance (Lambers et al. Reference Lambers, Charin and Pons2008). Photosynthetic rates at specific irradiance levels (0, 20, 80, 150, 300, 500, 700, 1,000, and 1,500 µmol m−2 s−1) were measured using the Li-6400 Portable Photosynthesis System (Li-Cor, Inc., Lincoln, NE). Irradiance levels were achieved using a Li-6400-02B LED light source attached to the Li-6400 Portable Photosynthesis System that provided light at 470 and 665 nm. The abundance of low irradiance levels (≤150 µmol m−2 s−1) in this study facilitated accurate measurements of LCPs, which are widely regarded as indicators of shade tolerance (Aleric and Kirkman Reference Aleric and Kirkman2005; Qin et al. Reference Qin, Mao, Quan, Zhang, Xie and Ditommaso2012). Procedures for fitting photosynthetic light-response curves are described in the statistical analyses section. On each measurement day, photosynthetic data were collected from all plants within one replicate. Photosynthetic data were collected from the youngest fully expanded leaf (one leaf per plant). Following photosynthetic data collection, leaves were harvested and scanned into digital images that were used to determine the leaf surface areas for photosynthesis. Leaf surface areas were determined from digital images using image analysis software (Schneider et al. Reference Schneider, Rasband and Eliceiri2012).
To determine if shading inadvertently influenced thermal conditions in pots, temperatures at the 4-cm depth were measured in pots that were filled with potting mix but were without plants (three pots per light-transmittance treatment). Temperature pots were irrigated and shaded following the procedures for the pots with plants described above. Temperature data were recorded using sensors equipped with data loggers (HOBO Micro Station, Onset Computer Corporation, Bourne, MA) programmed to simultaneously record soil conditions every 60 min throughout the duration of the study.
Statistical Analyses
All statistical analyses were performed with the statistical software R, version 3.3.0 (R Core Team 2016). For field study experimental runs, aboveground biomass, crop seed yield, harvest index, rapeseed seed oil content, rapeseed stand counts, and canopy light-transmittance responses to crop seeding rate were analyzed with linear regression models. Following the method described by Gomez and Gomez (Reference Gomez and Gomez1984), regressions were fitted using mean values for each crop seeding rate. Tests of the null hypothesis, that regressions for experimental runs estimated the same population regression model, were made using the following F test for coincidental regression (Zar Reference Zar1999):
 $$F{\equals}{{{{SS_{t} {\minus}SS_{p} } \over {2(k{\minus}1)}}} \over {{{SS_{p} } \over {DF_{p} }}}},$$
$$F{\equals}{{{{SS_{t} {\minus}SS_{p} } \over {2(k{\minus}1)}}} \over {{{SS_{p} } \over {DF_{p} }}}},$$
where SS t is the total residual sums of squares of the combined data set, SS p is the pooled residual sums of squares, k is the number of samples being compared, and DF p is the pooled degrees of freedom of the pooled regressions.
For the greenhouse study, photosynthetic light-response curves were developed for each light-transmittance treatment using the following nonlinear regression equation (Aleric and Kirkman Reference Aleric and Kirkman2005):
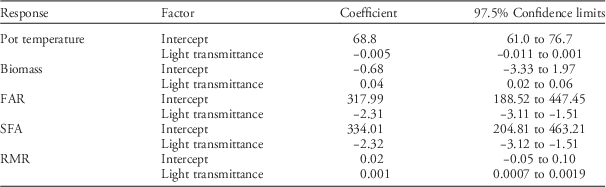
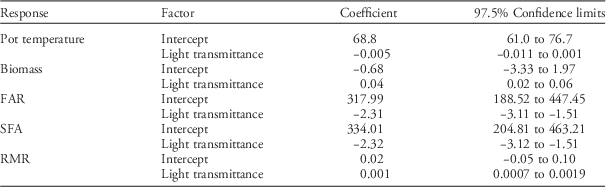
where A is the photosynthetic rate (µmol CO2 m−2 s−1), A Max is the maximum photosynthetic rate, A qe is the initial slope of the light-response curve, PPFD is the photosynthetic photon flux density, and LCP is the light compensation point. Using the experimental run means for each irradiance level, photosynthetic light-response curves were fitted using the nls library in R (Crawley Reference Crawley2007). Starting values for nonlinear regressions were determined by visually inspecting plots with preliminary curves of Equation 3 overlaid on actual photosynthetic rates. For the response variables pot temperature, total biomass, FAR, SFA, and RMR, linear mixed-effects models were produced using the R library lme4 (Crawley Reference Crawley2007). These models treated light-transmittance treatment as a fixed effect and hierarchal structures of sampling, run, and replicate within run as random effects.
Results and Discussion
Field Study
Increasing rapeseed seeding rate caused an increase in rapeseed stand counts (Table 1). Increased seeding rate also caused a significant decrease in below-canopy irradiance levels in the fall and spring of both experimental runs (Table 2). As for rapeseed seeding rate effects on canopy light transmittance, the F-test for coincidental regression indicated that the individual regressions for experimental runs estimated the same population for both fall (F2,4=1.02; P=0.44 ) and spring (F2,4=1.14 ; P=0.41). Light transmittance decreased linearly as rapeseed seeding rate increased from 1.8 to 9.0 kg ha−1 (Figure 1). The results of this study were consistent with those of previous studies that showed decreases in canopy light transmittance caused by increased seeding rate for corn (Tharp and Kells Reference Tharp and Kells2001) and safflower (Carthamus tinctorius L.) (Blackshaw Reference Blackshaw1993). Moreover, the results of the current study indicate that below-canopy irradiance can be manipulated by altering the rapeseed seeding rate.

Figure 1 Light transmittance through the rapeseed canopy in (A) fall (October 31, 2014, and November 4, 2015) and (B) spring (March 17, 2015, and March 16, 2016). Seeding rates were 0.5, 1.0, 1.5, 2.0, and 2.5 times the recommended seeding rate of 3.6 kg ha−1.
Table 1 Mean rapeseed seed yield (kg ha−1), aboveground biomass (kg ha−1), harvest index (%), rapeseed oil content (%), and rapeseed stand counts (plants m−2) for rapeseed planted at five different seeding rates in 2014/2015 and 2015/2016. Rapeseed was planted on September 10, 2014, and September 9, 2015, at New Mexico State University Agricultural Science Center at Clovis, New Mexico

a Linear regression model for the effect of seeding rate on the indicated response variable.
b F test for the null hypothesis that both experimental runs are from the same population.
Table 2 Below-canopy photosynthetic photon flux density (PPFD) during fall and spring for two experimental runs (September 2014 to June 2015 and September 2015 to June 2016)
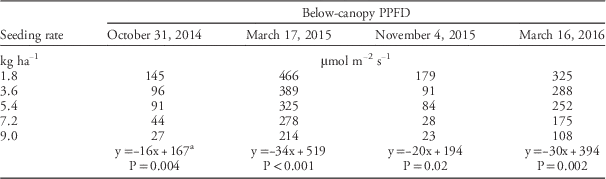
a Linear regression model for the effects of seeding rate on below-canopy PPFD.
Increased seeding rate neither increased nor decreased crop yield (Table 1). Similar results were reported by Kutcher et al. (Reference Kutcher, Turkington, Clayton and Harker2013) who showed that altering rapeseed seeding rates from 3.2 to 9.6 kg ha−1 caused no change in crop yield. Additional studies have indicated that rapeseed yields were not influenced by seeding rates between 3 and 14 kg ha−1 (Christensen and Drabble Reference Christensen and Drabble1984; Degenhardt and Kondra Reference Degenhardt and Kondra1981; Taylor and Smith Reference Taylor and Smith1992). In contrast, increased yields with greater seeding rates have been reported for other crops, including wheat (Arduini et al. Reference Arduini, Masoni, Ercoli and Mariotti2006) and soybean [Glycine max (L.) Merr.] (Butts et al. Reference Butts, Norsworthy, Kruger, Sandell, Young, Steckel, Loux, Bradley, Conley, Stoltenberg, Arriaga and Davis2016).
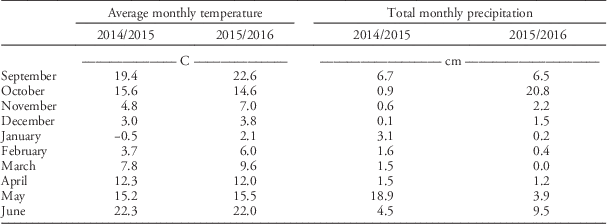
In the current study, harvest index was not affected by rapeseed seeding rate (Table 1). Similar results were observed by Angadi et al. (Reference Angadi, Cutforth, McConkey and Gan2003), who showed that increased seeding rate for spring rapeseed did not affect harvest index. The lack of seeding rate effects on harvest index reflects the fact that at lower seeding rates there are larger spaces between plants, and thus individual plants grow larger and produce more seeds per plant. Rapeseed seed oil content was not affected by seeding rate in the 2014/2015 experimental run, but was negatively affected by seeding rate in the 2015/2016 experimental run (Table 1). Taylor and Smith (Reference Taylor and Smith1992) showed that seeding rates between 4.6 and 14 kg ha−1 did not have an effect on rapeseed seed oil content, whereas Harker et al. (Reference Harker, O’Donovan, Smith, Johnson, Peng, Willenberg, Gulden, Mohr, Gill and Grenkow2015) determined that rapeseed seed oil content increased when seeding rate rose from 7.5×105 seeds ha−1 to 15×105 seeds ha−1. Results in the current study suggest that seeding rate effects on seed oil content are affected by the year in which the crop is grown. Year effects were also observed for aboveground biomass, seed yield, and harvest index, as these response variables were significantly lower in the 2015/2016 experimental run than they were in the 2014/2015 experimental run. This could have been caused by the large amount of precipitation that occurred during seed fill (May) in the 2014/2015 experimental run (Table 3).
Table 3 Average monthly temperature and total precipitation for two experimental runs recorded at the New Mexico State University Agricultural Science Center at Clovis, New Mexico

Greenhouse Study
A greenhouse study was chosen over a field study because greenhouse conditions facilitate measurements of biomass partitioning between above- and belowground parts, which is thought to be related to shade tolerance (Valladares and Niinemets Reference Valladares and Niinemets2008). Prior to flixweed transfer to the greenhouse, it was determined that PPFD levels (average±SE) were 1,350±50 µmol m−2 s−1 for the 100% light–transmittance treatment, 815±30 µmol m−2 s−1 for the 60% light–transmittance treatment, and 390±20 µmol m−2 s−1 for the 30% light–transmittance treatment. There was no effect of light-transmittance treatment on pot temperature, and the average pot temperature was 20.5±1.9 C (Table 4).
Table 4 Summary of linear mixed-effects models for pot temperature, flixweed total biomass, foliage area ratio (FAR), specific foliage area (SFA), and root mass ratio (RMR) responses to light-transmittance levels. Significant factors are indicated by coefficients with confidence limits that do not overlap zero.
For each of the three light-transmittance treatments, photosynthetic light-response curves (Equation 3) summarize the effects of increasing irradiance on net photosynthetic rate (Table 5). Photosynthetic light-response curves indicated that LCP decreased from 141.0 to 77.2 μmol m−1 s−1 as light transmittance declined from 100% to 30%. This was similar to results observed in Palmer amaranth (Amaranthus palmeri S. Wats) (Jha et al. Reference Jha, Norsworthy, Riley, Bielenberg and Bridges2008) and annual ragweed [Ambrosia artemisiifolia L. var. elatior (L.) Descourtils] (Qin et al. Reference Qin, Mao, Quan, Zhang, Xie and Ditommaso2012), in which LCPs decreased as light transmittance was reduced from 100% to 10%. LCP reductions that occur in response to declining light levels suggest some degree of shade tolerance (Valladares and Niinemets Reference Valladares and Niinemets2008).
Table 5 Photosynthetic light-response curve parameters (±SE) for flixweed plants subjected to light-transmittance treatments under greenhouse conditions
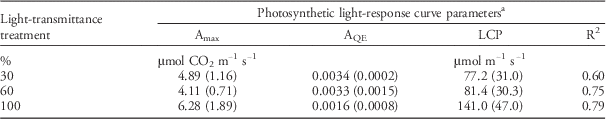
a Photosynthetic light response curve, A=A max [1−eAQE(PPFD−LCP)], where A is the photosynthetic rate, A Max is the maximum photosynthetic rate, A QE is the initial slope, PPFD is the photosynthetic photon flux density, and LCP is the light compensation point.
Reduced light transmittance caused a significant decrease in both total biomass and RMR, whereas FAR and SFA increased as light transmittance decreased (Table 4). The morphological responses of flixweed to shading suggest shade tolerance (Valladares and Niinemets Reference Valladares and Niinemets2008) and are generally consistent with reduced light effects on morphologies of other weed species. Shade-induced reduction in RMR was previously observed in common ragweed in a greenhouse setting with irradiance levels ranging from 100% to 10% of ambient conditions (Qin et al. Reference Qin, Mao, Quan, Zhang, Xie and Ditommaso2012). Shade-induced increases in leaf area ratio, which is similar to FAR, were previously detected for itchgrass (Rottboellia exaltata [L.] L. f.) (Patterson Reference Patterson1979), tumble pigweed (Amaranthus albus L.) (Stoller and Myers Reference Stoller and Myers1989), and perennial ryegrass (Lolium perenne L.) (Hunt and Burnett Reference Hunt and Burnett1973). Regnier and Harrison (Reference Regnier and Harrison1993) found that decreasing light transmittance from 100% to 5% caused significant increases in specific leaf area (SLA), which is similar to SFA, for common cocklebur (Xanthium strumarium L.) and velvetleaf (Abutilon theophrasti Medik.).
Reports of weeds acclimating to low irradiance are consistent with frameworks for weediness that emphasize phenotypic plasticity (Baker Reference Baker1974). Combinations of decreased LCP, reduced biomass, and diminished RMR, along with increased FAR and SFA, promote plant survival as irradiance levels decrease. This is because shade-tolerant species allocate more of their reduced growth to the above-ground portion of the plant rather than root mass, to maximize uptake of the limited photosynthetically active radiation that is available to the shaded plant (Stoller and Myers Reference Stoller and Myers1989). To our knowledge, this is the first report of shade tolerance in flixweed.
Implications for Management
The results of this study indicate that rapeseed seeding rate can be increased to manipulate canopy light transmittance without incurring a rapeseed seed yield penalty from intraspecific competition. The field study did not address the effects of interspecific competition, which can influence rapeseed yield responses to increasing seeding rate (Lemerle et al. Reference Lemerle, Luckett, Koetz, Potter and Wu2016). However, by growing rapeseed alone, seeding rate effects on light transmittance were determined without the confounding effects of weed interference.
It is difficult to draw direct conclusions about the effectiveness of increasing rapeseed seeding rate to control flixweed because this study did not investigate competition components such as antagonistic interactions for belowground resources and canopy-induced changes in light quality. Nonetheless, reduction in flixweed biomass in the greenhouse study suggests that reduced light transmittance caused by increased seeding rate can suppress growth of flixweed plants. Thus, increasing rapeseed seeding rate may be a promising tactic for flixweed suppression and might be especially useful for managing herbicide-resistant flixweed. Increasing rapeseed seeding rate alone will not be an effective way to eliminate flixweed because this weed species shows some degree of shade tolerance. Management programs for flixweed in rapeseed should consider increased crop-seeding rate in concert with other tactics.
Acknowledgments
Salaries and research support were provided by state and federal funds appropriated to the New Mexico Agricultural Experiment Station. Mention of a trademark or proprietary product does not constitute a guarantee or endorsement of the product by New Mexico State University. We gratefully acknowledge Israel Marquez, Edward Morris, and Joseph Wood for their assistance with data collection.