1. Introduction
Ever since Jane Goodall's observations of ant-dipping chimpanzees some 50 years ago, the idea that human tool use evidences “humanique” cognitive ability has lost popularity. To date, chimpanzees have been demonstrated to use “complex toolkits” (Fowler & Sommer Reference Fowler and Sommer2007; Sanz & Morgan Reference Sanz and Morgan2007), “complex toolsets” (Sanz et al. Reference Sanz, Schning and Morgan2009; Boesch et al. Reference Boesch, Head and Robbins2009), and “composite technologies” (Carvalho et al. Reference Carvalho, Biro, McGrew and Matsuzawa2009) – behaviors that, according to many, indicate that chimpanzees share with humans at least the cognitive machinery for dealing with their physical world (see, e.g., Hrdy Reference Hrdy2009; Tomasello & Hermann Reference Tomasello and Herrmann2010). To the extent that human tool use still evidences cognitive superiority, that superiority is said to be found in the social domain: The remarkable complexity of human technologies attests to more sophistication in matters of imitation, teaching, and participation in collaborative activities. It is these capacities (rather than individual brainpower) that, through cumulative evolution, gave rise to our astonishing technological achievements (see e.g., Boyd & Richerson Reference Boyd and Richerson1996; Herrmann et al. Reference Herrmann, Call, Hernández-Lloreda, Hare and Tomasello2007; Richerson & Boyd Reference Richerson and Boyd2005; Tomasello et al. Reference Tomasello, Carpenter, Call, Behne and Moll2005).
In this paper, I show that it is a mistake to discard tool use as a hallmark of human cognition. Human tool use reflects higher social intelligence (indeed), but just as much greater non-social wit. In support of my argument, I offer a systematic comparison between humans and nonhuman primates with respect to nine cognitive capacities (both social and non-social) deemed crucial to tool use: enhanced hand-eye coordination (sect. 2), body schema plasticity (sect. 3), causal reasoning (sect. 4), function representation (sect. 5), executive control (sect. 6), social learning (sect. 7), teaching (sect. 8), social intelligence (sect. 9), and language (sect. 10).Footnote 1 Because striking differences between humans and great apes stand firm in eight out of nine of these domains (see Table 2, for a more detailed and balanced overview), I conclude that human tool use still marks a major cognitive discontinuity between us and our closest relatives; and relatedly, that no individual cognitive trait can be singled out as the key trait differentiating humans from other animals.Footnote 2
As a second aim of the paper, I make clear how several of the cognitive traits reviewed help to explain our unique ability for cumulative culture, as well as the astonishing technological complexity this has produced (sect. 12). I show how some traits enable high-fidelity cultural transmission, yielding preservation of traits across successive generations; and how others, by facilitating individual learning, further the introduction of new cultural variants, necessary for incremental change. Given that chimpanzees lack many of these traits, much of the vast discrepancy between human and chimp technologies is thereby explained.
2. Hand-eye coordination
Chimpanzees display quite complex manual skills. Byrne (Reference Byrne, Russon and Begun2004), for example, notes that chimpanzees share with humans the use of precision grips, asymmetrical and bimanual tool use, and even strong individual lateralities (preference for one hand to perform the same task).
Three lines of evidence, however, support the idea of superior hand-eye coordination in humans.Footnote 3 First, more neural tissue is devoted to the human hand than to the hand of chimpanzees; chimpanzees have much smaller amounts of gray matter controlling their limb movements (MacLarnon Reference MacLarnon1996) than humans do. This means they face more difficulties in inhibiting the contraction of muscle fibers. Instead of a successive and orderly recruitment of their motor units, chimpanzees are forced to recruit larger numbers of units at once. As Walker (Reference Walker2009) notes, this lack of cerebral inhibition endows chimpanzees with a remarkable strength, at the expense, however, of fine motor control.
The second line of evidence comes from two strands of brain research. First, Orban and colleagues (Reference Orban, Claeys, Nelissen, Smans, Sunaert, Todd, Wardak, Durand and Vanduffel2006) identified a set of functional regions in the dorsal intraparietal sulcus (IPS) of the human brain that is involved in representations of the central visual field and in the extraction of three-dimensional form from motion. Crucially, these brain regions were not found in the brains of monkeys. The regions subserve, the authors conjectured, the enhanced visual analysis necessary for the precision with which humans manipulate tools. Second, Stout and Chaminade (Reference Stout and Chaminade2007) found that parts of these regions were indeed recruited when modern human subjects engaged in Oldowan-like tool making. Importantly, no increased activation was observed when the human subjects were asked just to strike cobbles together without intending to produce flakes. Human dorsal IPS, thus, may allow for better identification of suitable targets on the core, and as such, explain in part why humans outperform other primates in matters of tool use.Footnote 4
The third line of evidence relates to handedness – a population-wide preference for one hand, such as the 85–90% right-handedness in the human population (e.g., Raymond & Pontier Reference Raymond and Pontier2004). Although it is true that chimpanzees exhibit individual and population-level hand biases for some tasks,Footnote 5 the fact remains that, in the light of current evidence, the ratio of right- to left-handedness is much lower in great apes compared with humans; and that ambidextrousness is much more common in chimpanzee than human populations.Footnote 6
How do handedness and enhanced hand-eye coordination relate? There are two plausible ways. First, lateralization enhances manual precision. McGrew and Marchant (Reference McGrew and Marchant1999), for example, observe that exclusively lateralized chimpanzees are more able termite fishers than are weakly handed or ambidextrous individuals. Second, handedness probably facilitates motor coordination in social learning tasks. When all individuals in a population are handed alike, a learner can directly copy the model's hand configuration (i.e., without having to project it to the opposing hand) (e.g., Michel & Harkins Reference Michel and Harkins1985; Uomini Reference Uomini2009).
Summary: Enhanced hand-eye coordination relates to the fact that (1) in humans, more neural tissue is devoted to the hands; (2) humans possess brain structures for higher-order visual analysis, involved in affordance discovery and exploitation; and (3) our species' handedness makes for higher manual precision and smoother social learning.
3. Body schema plasticity
To guide actions in space, the brain needs to keep track of any changes in body shape and posture, and it does so by updating its representation of the body – aka the body schema. It long has been suggested that the body schema is plastic, in the sense that it can incorporate external objects.Footnote 7 A hand-held tool, for example, may become so familiar to the user that it at least feels as if it is a natural extension of the hand. The ramifications for tool use are evident: Better tool assimilations should yield more fluent tool use. Body schema plasticity, then, might be another factor making human tool use unique.
Now there is strong evidence that the human brain indeed can and does represent external aids as belonging to the body. The evidence comes from (1) crossmodal interference tasks in healthy humans and from (2) studies on patients with unilateral spatial neglect or (3) extinction.Footnote 8 In all these experiments, subjects were asked to operate simple tools, such as canes, rakes, and golf clubs. Interestingly, it appears that tool assimilation is contingent on the functional properties of the tool. Farnè and colleagues (Reference Farnè, Iriki and Làdavas2005), for example, show that only the effective length of a tool gets incorporated, not its absolute length. So, if a 60-cm-long tool has its functional part (say, a hook for grasping) at 20 cm (making the other 40 cm of the tool functionally redundant), only the first 20 cm of the tool gets incorporated.Footnote 9
Although all of these experiments are fascinating (and in many cases extraordinarily ingenious), I will not detail them any further, mainly because body schema plasticity does not appear to be a distinctively human trait. That is, tool-using monkeys have also been shown to extend their body schema when using simple tools. The evidence in monkeys is in fact even more direct than in humans: Recordings of neuronal activity in Japanese macaques indicate that neurons originally picking out stimuli near the hand may, after just 5 minutes of tool use, come to respond to stimuli near the tool (Iriki et al. Reference Iriki, Tanaka and Iwamura1996).Footnote 10
Of course, it still might be that humans outperform monkeys. For example, the capacity for tool assimilation might be inborn in humans, whereas a period of training might be needed to get it expressed in monkeys. In their review paper, Maravita and Iriki (Reference Maravita and Iriki2004) put forward this idea as a conjecture; but to the best of my knowledge, there is no direct evidence confirming it.
Summary: Body schema plasticity might be an important cognitive trait, even so important that without it, fluent tool use is not possible. We share the trait with our closest relatives, however. By implication, we cannot invoke it to explain what makes human technological abilities unique.
4. Causal reasoning
Causal understanding involves more than just noticing (e.g., through trial and error) the covariance between a cause (e.g., an action with a tool) and an effect (e.g., retrieval of a food item). One also needs to infer a mechanism relating the two – a causal relation explaining the occurrence of the covariance (e.g., Ahn et al. Reference Ahn, Kalish, Medin and Gelman1995; Ahn & Kalish Reference Ahn, Kalish, Wilson and Keil2000). Typically, such relations hold more generally than just in the context of discovery, and they can, therefore, once discovered, be exploited more widely. Knowledge that objects always fall under gravity, for example, is just as applicable to the manufacture and usage of deadfalls as of water butts and gallows. Are chimpanzees capable of discerning and flexibly putting to use such general causal principles?
Seminal experiments by Povinelli (Reference Povinelli, Reaux, Theall and Giambrone2000) and colleagues suggest that they are not. In one of Povinelli's experiments, chimpanzees that were trained to use rakes for food retrieval failed to differentiate between functional rakes (made of stiff tubing) and nonfunctional ones (with their tops made of a thin strip of flimsy rubber) (see Fig. 1a). In another, the chimpanzees failed to appreciate that the food item would fall into a trap before being pulled to within reach (see Fig. 1b).Footnote 11 Finally, the animals failed to see that rakes with tines down were ineffective for capturing the reward (see Fig. 1c).

Figure 1. Three tasks used by Povinelli (Reference Povinelli, Reaux, Theall and Giambrone2000) and colleagues to test causal reasoning in chimpanzees in the context of tool use. (a) The flimsy-tool problem, wherein chimpanzees had to choose between an ineffective rake (tines made of flimsy rubber) and an effective one (tines made of stiff tubing) to retrieve a food item. (b) The table-trap problem, in which the chimpanzees had to choose between pulling a rake that would cause the food item to fall into a trap (left) or one with which the food item could be successfully retrieved (right). (c) The inverted-rake problem, wherein chimpanzees had to choose between an upright rake and one that had been inverted, making the latter ineffective for food retrieval. (By permission of Oxford University Press, Inc.)
An important note: The chimpanzees could learn to avoid the causally unfavorable conditions. For example, after 25–125 (!) trials, all but one chimpanzee avoided the table-trap (in Fig. 1b). Hence, Povinelli's experiments suggest that chimpanzees learn about causality (or rather, about cause-effect covariances) through associative learning (dependent on contiguity and repetition) rather than through causal reasoning (i.e., inferring from the presence of a trap that the table containing the trap should be avoided).
Martin-Ordas and Call (Reference Martin-Ordas and Call2009) and Seed and colleagues (Reference Seed, Call, Emery and Clayton2009) qualified and refined these observations. Both groups of researchers found that chimpanzees could avoid (often already at the first trial) causally unfavorable conditions in tasks without tools, suggesting that the cognitive load associated with tool use is too high and blocks chimpanzees' ability to properly assess a task's causal set-up.
Furthermore, Martin-Ordas and colleagues (Reference Martin-Ordas, Call and Colmenares2008; Reference Martin-Ordas and Call2009) examined the extent to which chimpanzees could transfer their causal knowledge across tasks. Their subjects consecutively completed different, but functionally equivalent trap tasks (involving a tube-trap, a table-trap, a platform-trap, and a gap-trap). It appeared that performances across tasks were not robustly correlated, which indicates that the apes did not make broad generalizations about the principles governing traps – an intuitive “trap physics” that can be applied to all kinds of traps.
Interestingly, transfer of causal knowledge across tasks plausibly depends on two related, yet distinct cognitive mechanisms.Footnote 12 The first is inferential causal reasoning: inferring the cause responsible for an observed cause-effect covariance (e.g., that this particular kind of trap causes food rewards to fall beyond reach). The second is analogical causal reasoning: appreciating that the causal principles governing this particular kind of trap are analogous to the principles governing other kinds of traps. Chimpanzees, the studies above suggest, face substantial difficulties in both inferential and analogical causal reasoning tests (although in the case of the former, only when tools are involved).
Humans, in contrast, are not just very proficient at, but, in fact, very fond of figuring out how things work.Footnote 13 From early on in their lives, they exhibit a remarkable drive for explanation (e.g., Gopnik Reference Gopnik, Keil and Wilson2000; Penn & Povinelli Reference Penn and Povinelli2007a; Povinelli & Dunphy-Lelii Reference Povinelli and Dunphy-Lelii2001; Premack & Premack Reference Premack and Premack1994; Visalberghi & Tomasello Reference Visalberghi and Tomasello1998). If a novel object behaves somewhat unexpectedly, for example, human children (from age 3 onward) seek causal explanations; whereas apes will explore the object in a way identical to that when exploring any other novel object they come across (Povinelli & Dunphy-Lelii Reference Povinelli and Dunphy-Lelii2001).
In many cases, humans establish causal inferences by diagnosing the unexpected outcomes of their goal-directed actions. Humans intervene nonrandomly in a situation to find out why anomalies occur; they run “tests” to confirm or disconfirm their initial hypotheses and updates thereof (Lagnado et al. Reference Lagnado, Waldmann, Hagmayer, Sloman, Gopnik and Schulz2007). Schulz and colleagues (Reference Schulz, Gopnik and Glymour2007) ran tests demonstrating that even preschool children (mean age: 56 months) can use information from interventions to distinguish between causal chains (A→B→C), common causes (A←B→C), and interactive causal structures (A←B→C and A⇆C). This kind of diagnostic learning is, in light of current scientific evidence, a uniquely human trait.
Intuitively, it is evident in what sense a grasp of causality has helped Homo faber. The more one knows about the causes of an event, the more likely that one can intervene and change the course of nature in one's own favor. Arguably, no light bulbs, phones, or spacecraft would exist without the causal relations established in science and engineering. For the moment, though, I leave the impact of causal reasoning on the evolution of human technologies intuitive, discussing it more fully in section 12.1.
What neural mechanisms are responsible for our capacity for causal reasoning in tool use contexts? In a short review, Frey (Reference Frey2003) remarks that neuroscience has largely ignored the question. The author mentions that to date there are some clues that processing of causal relations between self, tool, and goal object depends on higher-level temporal cortex, whereas the use of unfamiliar tools seems to involve parietal and/or joint parietotemporal mechanisms. On a more general level, for contexts without tools, Roser and colleagues (Reference Roser, Fugelsang, Dunbar, Corballis and Gazzaniga2005) found that causal inferential reasoning is likely left-lateralized. Analogical causal reasoning, by comparison, has not been studied as such. Inasmuch as it is a subtype of a more general capacity for analogical reasoning, research by Morrison and colleagues (Reference Morrison, Krawczyk, Holyoak, Hummel, Chow, Miller and Knowlton2004) suggests that it implicates brain regions related to working memory, inhibitory control, and semantic memory.
Summary: Causal thought involves both the ability to infer causal mechanisms relating cause-effect covariances (i.e., inferential causal reasoning) and the ability to recognize that such mechanisms underpin causally analogous events (i.e., analogical causal reasoning). Current evidence suggests that chimpanzees perform rather modestly in both respects. Humans, in contrast, have a drive for seeking and generalizing causal explanations, and often learn about causality through their own diagnostic interventions – a behavior not yet observed in the great apes.
5. Function representation
Primates do not attach particular functions to particular objects. For example, when trained to use a certain rake for food retrieval, a monkey will not stick to it when alternatives become available (e.g., Cummins-Sebree & Fragaszy Reference Cummins-Sebree and Fragaszy2005; Hauser Reference Hauser1997). The monkey switches opportunistically, using for food retrieval whatever it comes across. Likewise, there is no evidence for permanent function attribution in primates in the wild. After production and a one-time usage, chimpanzees typically discard their tools.Footnote 14 So instead of creating more permanent function-bearers, primates always manufacture tools anew and on the fly.
Humans, in contrast, use hammers for hammering, nutcrackers for nutcracking, cherry pitters for cherry pitting; and during their lifespan, these tools typically remain for what they originally were for. Once having conceptualized a tool as being for a particular purpose, humans find it even difficult to use a tool for something other than its designated function – a phenomenon called functional fixedness.Footnote 15
A traditional explanation of this phenomenon relies on associative learning. Repeated exposure to a tool's design function causes motor programs associated with that function to be activated whenever the tool is encountered, blocking alternative, more creative uses (e.g., Kaplan & Simon Reference Kaplan and Simon1990; Smith Reference Smith, Sternberg and Davidson1995). As chimps are capable of associative learning, the fact that functional fixedness occurs in us and (presumably) not in them just attests to our much more frequent engagement with technologies.
However, this traditional explanation has difficulties explaining the observation of Defeyter and German (Reference Defeyter and German2003) that functional fixedness in humans also occurs without repeated exposure to a tool. Being informed just once about a tool's conventional usage is sufficient to hinder non-conventional usage. From this, the authors infer the existence of a conceptual system – presumably unique to humans – for organizing and storing functional information.Footnote 16
Additional evidence for such a conceptual system comes from neuropsychological observations of brain-injured patients suffering from apraxia – a disorder affecting the purposeful execution of learned behaviors.Footnote 17 Fluent tool use in apraxics may be disrupted in two ways: conceptual and motoric errors. In the case of conceptual errors, apraxics perform tool use actions skillfully, but out of context. A patient may, for example, eat with a toothbrush and brush his teeth with a spoon (Ochipa et al. Reference Ochipa, Rothi and Heilman1992). So although the patient's relevant motor programs are intact, he is unable to associate them with the correct functions of toothbrush and spoon. Inversely, in the case of motoric errors, the patient knows about the function of a tool, but cannot activate the associated motor program to use it. For example, she may know that a spoon is for eating soup yet, when asked to use it for that purpose, grasp the spoon with the entire hand, instead of exhibiting the learned finger position associated with spoon use (Sirigu et al. Reference Sirigu, Cohen, Duhamel, Pillon, Dubois and Agid1995; also Buxbaum et al. Reference Buxbaum, Sirigu, Schwartz and Klatzky2003). In conclusion, fluent tool use relies on the intactness of two separate systems: (1) a conceptual system, which stores information about familiar tools and their usage; and (2) a production system, representing learned tool use skills.Footnote 18
It is important to appreciate the relationship between functional knowledge and causal reasoning (as discussed in the previous section). Functional knowledge regulates usage of familiar tools (however causally opaque). If one encounters a familiar tool – say, a hammer – one can afford to stop reasoning about its possible uses and straightforwardly grasp it by its shaft. By so favoring a particular kind of usage, a functional representation may hinder causal assessments of situations in which the tool should be deployed in an atypical way (cf. functional fixedness). The point generalizes: Whenever conditions are unfamiliar (the tool or the task), the importance of causal reasoning increases.
Stable function representations plausibly facilitate ease of (re)use of a much wider diversity of specialized tools. Furthermore, given that it is general-purpose, functional knowledge may inform both tool production and usage, allowing alignment of the two in case they, as is common nowadays, are divorced.
Summary: There is converging evidence that human tool use depends on a conceptual system representing functional knowledge. Nonhuman primates, in contrast, do not attach particular functions to particular objects, which hinders (re)use of complex technologies.
6. Executive control
In outline, executive control refers to the voluntary control over actions. More specifically, executive systems subserve (1) inhibition, the capacity to suppress current drives (e.g., sex) for the attainment of long-term goals (e.g., a nulliparous life); (2) autocuing, the capacity to trigger certain behaviors autonomously; that is, in the absence of external stimuli (e.g., daily taking a contraceptive pill); (3) foresight, the capacity to form long-term goals (e.g., a nulliparous life), by prospecting needs other than those experienced in the immediate present; and (4) monitoring ongoing action, the capacity to monitor whether actions are indeed leading to the desired long-term goal (e.g., directing my attention to the contraceptives on the pharmacy shelf, away from distractors such as annoying background music).Footnote 19 Let me first consider how these four features bear on human tool use, and then assess the extent to which nonhuman primates display similar capacities.
According to Wynn (Reference Wynn1981; Reference Wynn2002), monitoring ongoing action must have been present to allow for the emergence of Acheulean industries, some 1.5 million years ago. An exemplar of this industry is the Acheulean hand ax, which is produced by removing several flakes from a core so as to yield a sharpened, standardized, teardrop shape (see Fig. 2). In a first stage, the basic shape of the tool is achieved by detaching flakes using a hammer stone. Next, the artifact is finished by using a soft hammer (from antler, wood, or bone). While removing the flakes, Wynn argues, early toolmakers needed to keep in mind the desired end shape and monitor whether flake removal properly affected the overall shape of the tool.

Figure 2. The production of an Acheulean hand ax. (1), (2) A hard hammer is used to achieve the basic shape of the ax by removing flakes from both sides of the core. (3) A soft hammer (made of bone, antler, or wood) is used to remove “thinning” flakes to achieve the final form of the ax. (Figure redrawn from Mithen Reference Mithen1996; courtesy of Lies Mertens.)
A recent neuro-imaging study by Stout and colleagues (Reference Stout, Toth, Schick and Chaminade2008) lends some support to Wynn's hypothesis. Stout and colleagues found that when modern humans, after the requisite training, engaged in Acheulean hand ax production, regions of ventrolateral prefrontal cortex were recruited. Increase of activation in these regions was observed during Acheulean-style, but not Oldowan-style, tool manufacture, reflecting the higher cognitive demands of the former. Importantly, these regions of prefrontal cortex are thought to indeed be involved in the coordination of ongoing hierarchical action sequences that are directed toward a higher-order goal (such as flake removal toward a standardized tool form).
Foresight (and inhibition; see below) is often linked to the advent of multi-component tools, such as the Levallois spears of 250,000 years ago (Coolidge & Wynn Reference Coolidge and Wynn2005; Wynn & Coolidge Reference Wynn, Coolidge, Mellars, Boyle, Bar-Yosef and Stringer2007). The thought is that intermediate goals (associated with each individual component) have to be brought into accord prospectively; the stone blade needs to fit the future wooden handle (or vice versa), and the binding material needs to fit both. One task (say, handle production) is put in abeyance (in working memory) until another (say, blade production) is completed (Aunger Reference Aunger2010).
If these sorts of multi-step action indeed depend on an ability for off-line planning, one can expect impairments of the human executive system to result in an inability to perform multi-step action. But that does not appear to hold unambiguously.
Goldenberg and colleagues (Reference Goldenberg, Hartmann-Schmid, Sürer, Daumüller and Hermsdörfer2007a), for example, studied multi-step action in patients with dysexecutive syndrome, involving the disruption of executive functions following damage to the frontal lobe. Dysexecutive patients may exhibit disinhibition of behavior (hence, produce inappropriate aggression, sexual behavior, and the like), attentional deficits, perseveration and utilization behavior, or lack of drive and initiative. The patients, when given the required items and a set of distractor items (teabags, a fork, and a bottle of milk), could take perfectly all steps needed to make coffee with a drip coffeemaker. So they managed to bring into alignment several items (water, water container, coffee, filter, coffeepot), each item having its own functional goal. Put differently, they managed to properly sequence actions with the relevant items, all to the attainment of an overall goal. The patients were able to “foresee,” for example, that pouring in coffee before inserting a filter would not make the plan work.
On the other hand, the patients did perform poorly on two other multi-step action tasks. First, they failed on a pure problem-solving task (i.e., the Tower of London task; see Fig. 3) – where they had to work out a novel solution for themselves, rather than falling back on an established action routine (as in the coffee-making task). The default way of solving Tower of London problems is not through trial and error, but by mental planning ahead, indeed a capacity typically associated with executive function.
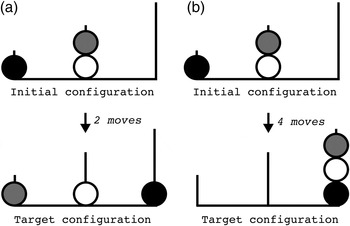
Figure 3. The Tower of London task: two initial configurations (a) and (b) with corresponding target configurations. Two moves are needed to get from initial configuration (a) to target configuration (a); four moves are needed to get from initial configuration (b) to target configuration (b).
Second, patients faced substantial difficulties when asked to pack both a lunch box and a schoolbag with items specified by an instruction. Some of the items were lying on a table; others needed to be retrieved from a drawer, which also contained distractor items. The challenge was to keep both assignments in mind while switching between table and drawer, and while being distracted by unnecessary items such as toothbrushes and screwdrivers. Moreover, in contrast to the coffee-making assignment, the equipment here did not provide an external reminder of the goal of the task, so that the goal had to be maintained internally in working memory.
The upshot of Goldenberg's study is that executive functions are involved in preplanning innovative multi-step action and in monitoring ongoing action in the absence of external reminders, but not in the case of routine activities. Whether the advent of multi-component technologies really required a capacity for foresight, then, seems to depend on whether we conceive the event as one of innovation or of slow, haphazard adjustments to a set of established tool-making routines (more on this in sect. 12.2).
More convincing evidence of foresight are technologies such as traps and deadfalls. There one needs to predict how current actions will affect future events, without the environment providing immediate cues about how these events will unfold and without the environment serving as a reminder about which purposes the actions are supposed to serve (Coolidge & Wynn Reference Coolidge and Wynn2005). Unfortunately, traps and deadfalls do not preserve well in the archaeological record; some authors estimate their occurrence some 12,000 years ago (Bar-Yosef Reference Bar-Yosef2002; Moore et al. Reference Moore, Hillman and Legge2000; both cited in Coolidge & Wynn Reference Coolidge and Wynn2005).
Usually, planning ahead is successful on the condition that current drives are ignored. In trap building, for example, the delay in the reward is compensated for by the reward's higher nutritional value. So the trap-builder must inhibit actions on current appetite and postpone nourishment until the trap is filled. Likewise, in agriculture, it requires considerable inhibition to not consume the whole harvest, but save a portion for future planting instead (Coolidge & Wynn Reference Coolidge and Wynn2005). And finally, the more time and labor it costs to produce a certain tool, the more demands put on the inhibitory system (Coolidge & Wynn Reference Coolidge and Wynn2005).
According to Donald (Reference Donald1993; Reference Donald, Corballis and Lea1999), just as crucial as inhibition is its inverse: autocuing. Humans can “cue themselves” to act in certain ways, ways for which the environment does not provide any direct stimuli. Humans can voluntarily activate action schemes, for example, to engage in deliberate practice. Doing so leads to greater skill, which in turn allows for more complex tool use. Rossano (Reference Rossano2003) conjectures that because skilled Acheulean toolmaking requires considerable practice, the Acheulean industry might provide us with the first evidence of an early form of autocuing.
How do apes fare with respect to (1) inhibition, (2) autocuing (3) foresight, and (4) monitoring ongoing action? To start with (2), autocuing has received little attention in the comparative psychology literature. None of the great apes has been reported to exhibit deliberate training in the wild, and no experiments have been conducted to test autocuing in captive nonhuman primates. So for now, we do not really know whether nonhuman primates can cue themselves to activate learned motor programs, offering them much broader windows of opportunity for improving skill.
Much more literature is available for (1), (3), and (4). Regarding (4), Stokes and Byrne (Reference Stokes and Byrne2001) have argued that chimpanzees engage in complex, flexible hierarchical action patterns, in a non–tool use context; namely, when processing leaves of the tree Broussonettia papyrifera. The authors document that while processing the foods, the chimpanzees need to monitor the progress they are making, as different intermediate results may require different types of follow-up action. The process is best represented by means of a standard decision tree, with multiple pathways and loops leading to and serving the overall goal (viz., processed food). In a tool use context, Boesch and colleagues (Reference Boesch, Head and Robbins2009) have observed something similar: They found that chimpanzees used five different objects – in the correct functional order – to obtain honey.
Quite a few studies have recently attempted to find evidence for (1) and (3). The study coming closest to proving something like inhibition and foresight is by Osvath and Osvath (Reference Osvath and Osvath2008).Footnote 20 The authors report on two chimpanzees and an orangutan that seemed to select a tool (viz., a drinking straw) to retrieve a larger delayed reward (half a liter of highly favored fruit soup), instead of selecting an instant smaller reward (a grape). But, as Suddendorf and Corballis (Reference Suddendorf and Corballis2009) remark, because the apes went through a substantial training phase, the results can still be explained in terms of simple associative learning, with the apes' repeated experience with straw and fruit soup forging a strong connection between the two. What is more, Suddendorf and Corballis (Reference Suddendorf and Corballis2009; also, e.g., Reference Suddendorf and Corballis1997; Reference Suddendorf and Corballis2007) rightly point out that human foresight and inhibition are not just a matter of “sitting out” a current desire, biting the bullet until satiation; human forethought also involves foreseeing and acting on a future drive, which might be qualitatively different from current ones (e.g., not drinking too much wine now, to be fit tomorrow). Because grapes and fruit soup tap the same desire (viz., hunger), Osvath and Osvath's study – supposing for a moment that associative learning can be excluded – gives at best evidence of apes being in control of a current drive, not of planning a future one.
In fact, to have current and future goals diverge, one must be able to entertain more than two qualitatively different goals to begin with. Although chimpanzees certainly have more than one drive (food, mating, shelter, territorial protection), it is uncontroversial that the range of goals that chimps pursue pales in comparison with the range of human goals (e.g., Csibra & Gergely Reference Csibra and Gergely2007; McGrew Reference McGrew, Gibson and Ingold1993). Humans even may take as a goal the satisfaction of someone else's anticipated goals – as happens when one individual produces a tool for another (often thereby tapping theory of mind abilities; see sect. 9).
Summary: Executive control is the capacity for (1) inhibition; (2) autocuing; (3) foresight; and (4) monitoring hierarchically structured, ongoing action. Comparative evidence for (2) is lacking; humans and chimpanzees both seem to perform well on (4). In light of current evidence, however, humans still appear to have unique abilities for (1) and (3).
7. Social learning
In Reference Tomasello, Davis-Dasilva and Camak1987, Tomasello and colleagues observed that chimps were able to learn from a model that a T-shaped rake affords food retrieval, yet ignored the exact way in which the model operated the rake. Tomasello (Reference Tomasello, Parker and Gibson1990) took this to be the characteristic difference in social learning between apes and humans. Apes are capable of emulation, reproducing the goal achieved by the model; humans, in contrast, are imitators, copying not just goals, but also the means towards them.
Tomasello's proposal remains controversial (although it is often presented as settled). The most systematic challenge comes from a series of experiments conducted by Whiten and colleagues, three of which are particularly forceful.
First, Whiten and colleagues (Reference Whiten, Horner and de Waal2005; also Horner et al. Reference Horner, Whiten, Flynn and de Waal2006; Whiten et al. Reference Whiten, Spiteri, Horner, Bonnie, Lambeth, Schapiro and de Waal2007) showed that chimpanzees can sustain technologies within the population through mechanisms of social transmission.Footnote 21 For example, Whiten et al. (Reference Whiten, Horner and de Waal2005; Reference Whiten, Spiteri, Horner, Bonnie, Lambeth, Schapiro and de Waal2007) experimentally introduced two different foraging techniques into two different captive groups, by training one individual of each group then reintroducing it to its respective group. Under the influence of their local expert, most chimpanzees adopted the method seeded in their group. Moreover, even if a chimpanzee individually learned the alternative foraging technique, it nevertheless continued to deploy the technique used by most of its peers; as such, conforming to the cultural norm of the group.
If emulation is default among chimpanzees, and they learn about results (emulation) rather than about actions (imitation), one could expect them to be able to learn also in the absence of action-information. In a second study, Whiten and colleagues (Hopper et al. Reference Hopper, Spiteri, Lambeth, Shapiro, Horner and Whiten2007) tested this hypothesis using ghost conditions; that is, conditions in which the tool task was not demonstrated by a model, but rather was performed automatically/mechanically. If environmental effects are sufficient to learn about a task, then chimpanzees should be able to perform the task also after having been exposed to the ghost condition. This, however, was not observed. Chimpanzees appeared not to learn in the ghost condition, although they got skilled after having observed another chimpanzee performing the task. This suggests that the model's actions are needed for transmission to ensue.
In a third study, Horner and Whiten (Reference Horner and Whiten2005) found that chimpanzees are able to switch between emulative and imitative strategies. When causal information regarding the operation of a tool remains opaque, chimpanzees tend to imitate, as well as copy the causally irrelevant actions performed by the model. When this causal information becomes available, though, they emulate, omitting causally irrelevant behaviors, individually devising a suitable – and more straightforward – method to attain the goal attained by the model.
In light of these studies, Tomasello (Reference Tomasello, Laland and Galef2009) has recently qualified his original position. He grants that apes indeed appear responsive to both goals and means. Nonetheless, Tomasello (also Whiten and colleagues, for that matter) still notes remarkable differences between chimps and humans, be it in degree rather than in kind. He rightly notes, for example, that Horner and Whiten's observation of imitation/emulation switching in chimpanzees contrasts starkly with their observations in human infants. In particular, Horner and Whiten (Reference Horner and Whiten2005) report that infants imitate in the causally opaque condition (like the chimps), but also in the causally transparent condition (unlike the chimps). So even when given information about the irrelevance of an action, human infants copy it; as such, engaging in what is often called overimitation (e.g., Hernik & Csibra Reference Hernik and Csibra2009; Lyons et al. Reference Lyons, Young and Keil2007; McGuigan et al. Reference McGuigan, Whiten, Flynn and Horner2007; Whiten et al. Reference Whiten, McGuigan, Marshall-Pescini and Hopper2009). Given their relative ignorance, it apparently pays children to faithfully copy by default, correcting errors later in life, once they are capable of reading the minds of models (see sect. 9), of being receptive to instruction, and/or of understanding the causal structure of tool use tasks.Footnote 22
Two other features are characteristic of human social learning – although they are more intuitive than corroborated experimentally. First, humans can socially acquire not just means, but also a remarkably wide variety of nonbasic goals; that is, goals that are not directly linked to drives such as food and sex and shelter (piety, a healthy old age, a unique collection of stamps).Footnote 23 Social learning in chimps, in contrast, is typically triggered by the prospect of one of the not-too-distant goals in the ape's innate goal repertoire – usually a food reward. At present, there is little evidence suggesting that chimps can extend that repertoire by copying parts of someone else's.
Second, humans are not just better imitators, they plausibly are better at emulating, too.Footnote 24 As long as problems are not too complex, a chimp may try-and-err its way to an emulated goal state. Higher complexity, however, increases the range of possibilities for reaching the goal, making random trial and error ineffective. Humans are fairly good emulators also in these circumstances. For many of us, for example, the default way of learning how to put to use new and complex electronic devices is through emulation. Instead of carefully carrying out all steps described in the manual, we are able to infer proper usage from function – perhaps because our grasp of causality constrains the range of possible actions we consider and try out or because we notice analogies with the usage of other devices (see also sect. 12.1).
Summary: Human social learning is special in three respects. First, humans start as faithful, nonselective, default imitators, developing more selective modes of imitation over the years. Second, humans commonly acquire through social learning not just means, but also a remarkably wide variety of nonbasic goals. Third, emulation learning, too, is plausibly much more powerful in humans than in the great apes.
8. Teaching
Social learning can moreover be facilitated by a trait not so much of the imitator as of the model being imitated. More specifically, learning proceeds more smoothly if the model has the willingness and a capacity to teach. For example, if the model actively shows which actions are relevant and which ones can safely be ignored, the imitator does not need a causal grasp of the situation to be successful. The imitator may be ignorant because she is too young, or because the ultimate causal effects of the action are opaque. For example, as Csibra and Gergely (Reference Csibra, Gergely, Johnson and Munakata2006; Reference Csibra and Gergely2009) point out, if there is a divorce between the production and use of a tool, as in making a tool for a tool, there are no perceptual rewards that can be used as immediate clues concerning the relevance of certain actions.Footnote 25 From your observation today of someone affixing a spear point to a shaft by means of a rope, you cannot infer the action's relevance for killing a boar tomorrow. In such circumstances, it helps if the model assists you; for example, by emphasizing salient actions through repetition, by pointing to the functional features of a tool, by contrasting proper versus improper usage, and so forth.
For teaching, language is helpful but not requisite. Before mastering a language, human infants learn a lot by relying on nonverbal cues (e.g., of their parents): simple gestures such as pointing or showing objects; sounds of excitement or disapproval in case of success or failure; looking for eye contact, gaze shifting and capturing, or redirecting attention (Csibra & Gergely Reference Csibra, Gergely, Johnson and Munakata2006; Reference Csibra and Gergely2009).
By and large, it is agreed that such active pedagogy is uniquely human, notwithstanding two isolated observations of (supposed) teaching in chimpanzees by Boesch (Reference Boesch1991).Footnote 26 To be sure, it is common that mother chimpanzees stimulate their offspring to engage in tool use (e.g., by leaving stones and nuts in the vicinity of the anvil), and even facilitate it (e.g., by lending their own preferred tools to their infants); but active teaching is extremely (!) rare, if existent at all.Footnote 27
Summary: Human teaching is unique: Until now, no nonhuman species has been reported to engage systematically in the kind of active teaching commonly observed in Homo sapiens.
9. Social intelligence
Here I discuss four profound dependencies between sociality and tool use: (1) heuristics for selecting models for social learning; (2) theory of mind abilities; (3) contingent reciprocity; and (4) goal sharing.
Let us start with the first. To avoid adoption of maladaptive behavior, social learners should be capable of selecting as models those individuals that possess adaptive information. Humans deploy several heuristics to that end. These fall into two categories: model-based and frequency-dependent heuristics.Footnote 28
In the former, particular features of the model are used to estimate the potential benefits of social learning. As a copier, one might preferentially select particularly successful individuals (inferred from their previous successes); particularly prestigious individuals (inferred from the amount of deference shown to them by other individuals); or individuals that are simply similar to oneself (inferred from self- and other-recognition; similarity can work on different criteria, such as similar age, similar ethnicity, similar dialect, etc.). In all three cases, social cues are used to select individuals worth learning from.
The cues of single individuals still might be ambiguous. So a second set of strategies – namely, frequency-dependent strategies – exploits information aggregated over the behavior of many individuals. If most of one's group members do X, chances are high that doing X pays off, given that selective forces are at work in each individual of the population. The inverse of this conformity bias may also occur; namely, when the rarity of a behavior is used as a cue.
No such biases have been observed in great ape social learning.Footnote 29 But that both types of model selection are potent mechanisms for technological innovation is uncontroversial: If individuals in a population are able to select and learn from the most skilled individuals, ceteris paribus, the average skill level of the population increases – stated otherwise, the population as a whole innovates.
Let us turn to the second sociocognitive trait facilitating tool use: understanding the mental life of others. Thirty years of research have not solved Premack and Woodruff's (Reference Premack and Woodruff1978) question of whether chimpanzees have a theory of mind. According to Povinelli and colleagues (Penn & Povinelli Reference Penn and Povinelli2007b; Povinelli & Vonk Reference Povinelli and Vonk2003), on the one hand, evidence is still lacking for something remotely resembling chimp mental state attribution. According to Call and Tomasello (Reference Call and Tomasello2008), on the other, chimpanzees have in several experiments displayed at least some grasp of the mental life of others. Notwithstanding that fact, Call and Tomasello, too, believe that chimpanzees lack the full-fledged belief-desire psychology of humans.
Theory of mind abilities interact with human tool use in at least two ways (both hinted at already). First, social learning of complex tool-using and tool-making activities is facilitated when the learner understands the model's intentions – that is, the model's motives for executing some actions yet omitting others (e.g., Cheney & Seyfarth Reference Cheney and Seyfarth1990; Tomasello Reference Tomasello, Heyes and Huber2000). Theory of mind is one of the plausible mechanisms involved in the transition from overimitation to selective imitation. Second, theory of mind makes possible the divorce of tool production and usage. Making a tool for someone else is contingent on the recognition that the other may need the tool in question. Contemporary market research is a good exemplar of how theory of mind abilities may drive technological change.
The third dependency between sociality and tool use concerns contingent reciprocity and the way in which it supports strong divisions of labor, at both kin and non-kin levels. At kin level, a remarkable feature of humans is their sexual division of labor. In hunter-gatherers, for example, one sex is specialized in gathering, the other in hunting, and the revenues of both are shared to the benefit of the entire household (Marlowe Reference Marlowe2007). Such division of labor yields a broader and more reliable diet and an increase of skill level, which in turn leads to greater foraging success rates.Footnote 30 In chimpanzees, in contrast, males and females target different foods but do not share revenues within the sexual pair bond.
The point, in fact, generalizes: Chimpanzees are reluctant to exchange foodstuffs. Plant food sharing has been observed only sporadically outside the mother-infant dyad.Footnote 31 Male hunting chimpanzees do share meat with non-kin; but this usually evidences mutualism (in this case, male allies are given their share; see below) or one-time pay-for-sex strategies (i.e., swollen females receive a chop), not specialization or division of labor (e.g., Stanford Reference Stanford, Stanford and Bunn2001; Stanford et al. Reference Stanford, Wallis, Mpongo and Goodall1994).
Moreover, these redistributions are very local, and involve only those group members present at the kill. Bipedalism means that humans can transport gathered foodstuffs and carcasses, allowing them to develop much wider exchange networks.Footnote 32 Also, transportation of food to more permanent dwellings enables delay of consumption. And when foods can be stored, it starts to pay off to specialize in food production, because excesses can be saved for future consumption or future exchange.
Quadrupedal chimpanzees face severe limitations in this sense: Investments in specialization (say, the production of sophisticated weaponry or other tools) can be brought in balance only with immediate rewards – that is, immediate consumption or immediate exchange (followed by immediate consumption of the exchanged goods). To make it even worse, chimpanzees also face a cognitive handicap: Their limited sense of contingent reciprocity precludes the development of reliable exchange networks.
Contingent reciprocity occurs when A's helping of B is contingent on B's previous help toA.Footnote 33 Stevens and Hauser (Reference Stevens and Hauser2004) discern seven cognitive requirements for contingent reciprocity (see Table 1). I have already discussed two of them (i.e., time estimation and temporal discounting) in section 6 (both under the heading of inhibitory control). For three of them (i.e., cheater detection, punishment, and reputation recognition), there is little to no evidence in nonhuman primates. The last two are borderline cases: numerical discrimination and memory. Numerical discrimination – necessary to make exchanges equitable, for if absent, defectors give back less than a fair amount – has been observed in all of the great apes (e.g., Hanus & Call Reference Hanus and Call2007; Tomonaga Reference Tomonaga2008).Footnote 34 Regarding memory: Although chimpanzees do have reliable memories of past interactions with others when it concerns services such as grooming or support (especially de Waal Reference de Waal1989; also de Waal Reference de Waal2000; Matsuzawa Reference Matsuzawa2001), such mental score keeping has not been observed when it concerns exchanging foods for foods (Brosnan et al. Reference Brosnan, Silk, Henrich, Mareno, Lambeth and Schapiro2009; Melis et al. Reference Melis, Hare and Tomasello2008). Finally, Table 1 contains an eighth cognitive trait, not mentioned by Stevens and Hauser (Reference Stevens and Hauser2004), but proposed by Brosnan and Beran (Reference Brosnan and Beran2009) – namely, empathy. The idea is that reciprocal exchange is facilitated by an ability to recognize someone else's needs, and a willingness to act thereupon. Although such other-regarding behavior has been reported in chimpanzees, again it does not come off in food contexts.Footnote 35
Table 1. Cognitive requirements for contingent reciprocity

Note: The second column gives references to reports describing absence/presence of these requirements in chimpanzees. The symbol “?” indicates that no systematic data are available.
To sum up, expensive investments in tool use will pay off if one can share or exchange outputs exceeding one's own consumption. That, in turn, requires sophisticated mechanisms for contingent reciprocity. When limited in this sense, as is the case with chimps, strong divisions of labor and highly specialized toolkits are unlikely to evolve.
The fourth and final relation between social intellect and tool use regards goal sharing – that is, many individuals aligning their goals so as to produce group beneficial outputs. Many authors invoke such a form of mutualism to characterize some of the oldest tools found in the archaeological record. Toth and Schick (Reference Toth and Schick2009), for example, conjecture that Oldowan tool production was a social enterprise, with production sites being used by large social groups. Acheulean hand axes presumably required even more social organization. These axes were – at least according to some – thrown to knock down animals, after which a group of hunters could club the animal (Calvin Reference Calvin1990).
But even if Acheulean hand axes testify to a quite sophisticated social intellect, they do not yet indicate any superiority over nonhuman primates. After all, chimpanzees, just like humans, strongly cooperate while foraging for meat (but, unlike humans, typically go hunting unarmed) (e.g., Boesch Reference Boesch1994; Reference Boesch2002; Boesch & Boesch Reference Boesch and Boesch1989). The most salient difference in social behavior between chimp and human contemporary meat foraging is, according to Stanford (Reference Stanford, Stanford and Bunn2001), that humans (unlike chimps) coordinate hunts with vocal and gestural communication. Therefore, to the extent that prehistoric hunters can be modeled on contemporary hunter-gatherers, it would be more natural to argue that cooperative hunting depends on improved communicative abilities, rather than on goal sharing per se.
Better evidence for increased demands on social intelligence would come from technologies that are produced by many individuals, or from technologies the use of which is unequivocally more socially involving. One contender is the colonization of Sahul (or Pleistocene Australia–New Guinea) some 45,000 years ago. That event arguably required cooperation in production and usage, of marine modes of transportation in particular. Another contender is the decorative use of beads (110 kya), implying early modes of communication and perhaps even trade. It also might be that the first multi-component tools already marked a difference in sociality. That is, it might be that the first hafted tools (250–200 kya) were produced by many – each individual responsible for (or even specialized in) the production of one component, complementing one another to achieve a common goal. Indeed, the attainment of that particular common goal would strongly depend on a careful coordination between team members.
Summary: Four sociocognitive traits in particular are to the advantage of Homo faber: (1) recognition and assessment of social cues as a proxy for a model's copy-worthiness; (2) theory of mind abilities, facilitating selective social learning and the divorce of production and usage; (3) strong forms of contingent reciprocity, which enable profound divisions of labor and specialization; and (4) goal sharing, which helps to distribute the costs of complex technologies among collectives of individuals. Our nearest relatives score (much) lower in all four domains.
10. Language
There are some obvious ways in which language facilitates advanced tool use: Thanks to language, processes of social learning and teaching and cooperation proceed far more efficiently; technological knowledge is more easily preserved in linguiform format and therefore can accumulate over longer periods of time, distributed over larger groups of individuals; linguistic and other representational artifacts (from symbol systems, sketches and books to computers and models) speed up the cognitive process of technological innovation; language paves the way for more symbolic forms of cultural behavior.
Yet, it is far from certain that language was necessary to make human technologies diverge from those used by our closest relatives some 2–3 million years ago.Footnote 36 On the contrary, numerous scholars have argued exactly the opposite: Early advances in human tool use played a causal role in the evolution of language. It is this point, rather than the more mundane contribution of language to modern tool use, that is widely investigated and debated. This section sketches the contours of the debate.
Roughly, there are two plausible ways of spelling out the evolutionary transition from tool use to language. Sequence A is as follows (e.g., Arbib Reference Arbib2005; Bradshaw & Nettleton Reference Bradshaw and Nettleton1982; Corballis Reference Corballis2010; Gibson Reference Gibson, Gibson and Ingold1993; Stokoe Reference Stokoe2001): (1) Advanced tool use gave good control of arm and hand; (2) such manual dexterity automatically made for increased gestural capacity; that is, it was exapted for communicative purposes; (3) a similar form of fine control was later applied to oral movements, leading to speech.Footnote 37
Arbib (Reference Arbib2005) remarks that tool use actually offers an excellent opportunity for the kind of gestural communication implied in step 2: When teaching an infant to use a tool, pantomiming becomes salient (also Rizzolatti & Arbib Reference Rizzolatti and Arbib1998). While the infant holds the tool, the model acts out what the infant is supposed to do. In the pantomime, the model performs an action that is instrumental in origin, but communicative in the context of teaching. That is, the resources needed to perform the instrumental act can be simply recruited to engage in communication – it “just” requires a slightly different mind-set.
Sequence A is mainly inferred from observations of patients with local left-hemispheric brain lesions, which at the same time affect linguistic, gestural, and object-manipulation capacities. Patients suffering from aphasia, for example, often fail to perform easy actions with tools, such as unlocking and opening a door (e.g., Kimura Reference Kimura, Steklis and Raleigh1979); and defective pantomiming (e.g., of tool use) is almost always associated with language deficits such as aphasia.Footnote 38
However, the finding that regions for language and tool use overlap does not suffice to justify the particular chronology suggested by sequence A. For that, one needs to make an extra assumption; namely, that gestural communication indeed preceded vocal communication. It is a plausible assumption to the extent that one believes that our closest relatives exhibit greater gestural than vocal capacities; for under that condition, our common ancestor must also have possessed better gestural than vocal skill.
The second way of spelling out the transition from tool use to language draws on the exaptation not so much of fine motor control as of resources for processing complex hierarchical structures (e.g., Bradshaw & Nettleton Reference Bradshaw and Nettleton1982; Gibson Reference Gibson, Parker and Gibson1990; Greenfield Reference Greenfield1991; Higuchi et al. Reference Higuchi, Chaminade, Imamizua and Kawato2009). Version B of the causal-temporal sequence, then, is as follows: (1) Advanced tool use gave humans the capacity to combine and integrate lower-order elements (viz., actions) into higher-order units; (2) resources initially devoted to structuring manual hierarchies were exapted for linguistic purposes (viz., for combining phonemes into words, words into meaningful sentences, and so forth).
Section 6 already discussed (B1). I showed there that Acheulean toolmaking requires the ability to organize and execute a sequence of manual operations (viz., different sorts of flake removal) in such an order that a higher-level goal is achieved (viz., a standardized hand ax). Put differently, the overall action (viz., Acheulean toolmaking) consists of an ordered set of subactions. A similar hierarchical organization is observed in human languages. Sentences consist of lower-level units such as clauses, which also consist of lower-level units such as phrases, words, and eventually, phonemes.
What (B2) suggests, now, is not just an analogy between the organizations of tool use and language but a common origin (with the hierarchical organization of tool use evolving first). Evidence for (B2) comes from three quarters: developmental studies and lesion and neuro-imaging studies.
With respect to the first, Greenfield (Reference Greenfield and Lock1978; Reference Greenfield1991) observes remarkable parallels in the development of skills for organizing manual actions and words. For example, children start to be able to pair objects (e.g., putting a smaller cup in a bigger cup) around the same time that they learn how to pair words. In a similar vein, but somewhat later, they develop strategies to combine more than two objects (e.g., putting a cup in a cup, both of which are then put in a third, even bigger cup) around the same time at which they learn how to nest words into sentence-like structures.
For a proof of common origin, concurrent development is insufficient, though. It still might be that both types of organizational skills develop in parallel coincidentally and are regulated by two separate neurological structures. Now lesion studies – the second type of evidence for B2 – complement the developmental data in support of a common origin. Grossman (Reference Grossman1980; reviewed by Greenfield in Walker Reference Walker2009), for example, reports on agrammatic patients with Broca's aphasia. These patients lack hierarchical organization in their syntactic production; they just sequence individual words, without relating them so as to produce meaningful overarching structures (phrases, clauses, sentences). Grossman found that Broca's aphasics also face substantial difficulties when reproducing hierarchically ordered drawings, such as tree structures (like those used to represent genealogies or phylogenies). He concludes the existence of a domain-general hierarchical processor, which can be used to organize both basic linguistic and nonlinguistic elements into complex constructs.
Finally, neuro-imaging studies in support of (B2) point to the recruitment of particular regions in Broca's area during both tool use and linguistic tasks. Higuchi and colleagues (Reference Higuchi, Chaminade, Imamizua and Kawato2009), for example, found increased activation in Brodmann's area BA44 when subjects manipulated common tools (scissors, pencils, and chopsticks) and when they were listening to a narrator reading a Japanese fairy tale. The perception of the hierarchical structure of a set of utterances (as in the fairy tale comprehension task) therefore appears to be regulated by the same neural resources governing the hierarchical organization of manual movements.
Moreover, Higuchi and colleagues (Reference Higuchi, Chaminade, Imamizua and Kawato2009) make plausible on neurological grounds that computational principles for processing complex hierarchical structure originally evolved to support tool use and were exapted for grammatical purposes later. Because F5, the region in the monkey brain thought to be homologous to BA44, is involved in tool use just as well, and because monkeys lack syntactically structured language, it is reasonable to think that only humans have put to use their capacity for hierarchical organization (subserved by BA44) outside the domain of its origin.
As a final remark, it is noteworthy that sequences A and B are not mutually exclusive. On the contrary, it is likely that tool use has given rise both to fine gestural/oral control and to a capacity for hierarchical organization. Of course, even if this is right, an important question remains; namely, whether these two abilities coevolved (i.e., A and B happening concurrently) or one of them evolved first, thereby, perhaps, triggering the emergence of the other.
Summary: Although language has contributed enormously to the sophistication of human technologies, the prehistoric divergence between humans and other primates with respect to tool use is most likely not attributable to linguistic ability. On the contrary, accumulating evidence suggests that human tool use has played a causal role in the evolution of human language, rather than the other way around. Still, many of the details of the causal pathway from tool use to language remain uncertain.
11. Overview
Let me briefly summarize my findings up until here. My comparison has revealed striking differences between humans and great apes – roughly, for eight of the nine cognitive domains discussed (for a more detailed and balanced assessment, see the overview in Table 2). Humans benefit from, roughly, better hand-eye coordination (sect. 2); a unique system for causal thought (sect. 4); a unique system for representing functional knowledge (sect. 5); remarkable inhibitory control and foresight (sect. 6); a suite of sophisticated social learning strategies (sect. 7); a unique disposition for teaching (sect. 8); increased social intelligence (sect. 9); and all the fruits of language (easing social learning and teaching, knowledge preservation, cooperation, innovation, and the like; sect. 10).
Table 2. Cognitive capacities subserving complex tool use.

Note: The symbol “++” indicates that the trait is highly more pronounced in humans than in chimpanzees. The symbol “+” indicates that the trait is more pronounced in humans. The symbol “=” indicates similar capacities in humans and in chimpanzees. The symbol “?” implies that little comparative evidence is available. Symbols are attributed in light of current scientific evidence; more detailed explanations are given in the text.
Apparently, therefore, human tool use attests to a major cognitive discontinuity between us and our closest relatives. And relatedly, apparently no individual cognitive trait can be singled out as the key trait differentiating humans from other animals. In other words, my overview should be an antidote to single-trait explanations of “humaniqueness.”
Bearing this in mind, I now turn to the second part of the paper. I consider how the cognitive traits discussed add up, producing the technological complexity characteristic of our lineage.
12. Cumulative culture and the complexity of human technologies
The complexity of human technologies is tightly linked to our remarkable ability for cumulative culture: Humans have been able to build complex systems by accumulating modifications over successive generations, gradually improving on previous achievements.Footnote 39
Key in recent explanations of this phenomenon is high-fidelity cultural transmission: Accumulation will take place when innovations are passed on to subsequent generations without degenerating too much (e.g., Boyd & Richerson Reference Boyd and Richerson1985; Reference Boyd and Richerson1996; Henrich Reference Henrich2004; Henrich & McElreath Reference Henrich and McElreath2003; Richerson & Boyd Reference Richerson and Boyd2005; Tennie et al. Reference Tennie, Call and Tomasello2009; Tomasello et al. Reference Tomasello, Kruger and Ratner1993). The thought is intuitive, but not without problems.
First, there is the classic problem of the Acheulean. The continuity of Acheulean hand axes on vast scales of time (1 million years) and space (from Africa to India to Wales) must have been sustained by very accurate mechanisms of cultural transmission (e.g., Lycett & Gowlett Reference Lycett and Gowlett2008; Mithen Reference Mithen1996; Reference Mithen, Box and Gibson1999; Petraglia et al. Reference Petraglia, Shipton, Paddayya, Gamble and Porr2005; Shipton Reference Shipton2010; Toth & Schick Reference Toth, Schick, Gibson and Ingold1993). Yet, there is no clear sense in which later generations of Acheulean assemblages built further on previous ones; first signs of genuinely cumulative culture are found in much more recent times, with, for example, gradual refinements of prepared-core and multi-component technologies. In light of this, it is reasonable to suppose that cumulation requires more than high-fidelity transmission alone.
Second, even if one grants that high-fidelity transmission is just a necessary condition for cumulative culture, the question remains of how it is implemented. Most seem to agree that sophisticated mechanisms for social learning and active teaching are essential.Footnote 40 However, these labels also black-box much of the cognitive machinery implied. The research described in section 7, for example, suggests that human infants copy more faithfully than chimpanzees do when it concerns relatively simple tasks, such as retrieving food from a box by means of a stick. But it is doubtful that this basic, and apparently fairly blind, disposition neatly scales up to learning much more intricate behaviors of the kind needed for sustaining incrementally complex technologies. Rather, it is more likely that other cognitive capacities need to be added; for example, that causal thought is part and parcel of human social learning, and hence, of high-fidelity cultural transmission.
These two issues are addressed below. I reconsider four cognitive traits that do not directly link to high-fidelity cultural transmission and show how: (1) by improving individual learning, they may complement processes of high-fidelity transmission (accommodating the insufficiency objection); (2) by facilitating social learning and/or teaching, they may subserve high-fidelity transmission (accommodating the black-box objection). Because at various points my arguments remain speculative, I also identify a set of issues in need of empirical validation.
The four traits in question are causal reasoning (as discussed in sect. 4), executive control (as discussed in sect. 6), and the capacities for contingent reciprocity and for goal sharing (both discussed in sect. 9).Footnote 41
12.1. Causal reasoning and cumulative culture
Boyd and Richerson (Reference Boyd and Richerson1995) point out that social learning is adaptive when social learning makes individual learning more effective; in particular, if it allows individuals to learn selectively: individually, if cheap and accurate; socially, if individual learning is difficult and error-prone. By implication, for cumulation to take place, individual learning costs must remain low. If not, all will switch to social learning – everyone imitating everyone – with cultural stasis or decline as a result.
Consider now the assumption that individual learning costs increase proportionally to the complexity of technologies. Indeed, labor-intensive technologies quite plausibly raise the costs of, for example, individual random trial-and-error learning. Whereas a one-minute experiment (say, in Oldowan flake production) may be excusable, executing month-long random trials (say, in boat production) is a cost that no single individual should be willing to bear. Likewise, the causal structure of a technology can be so intricate that the likelihood of success of a random adjustment approaches nil. So even if an individual is prepared to spend her valuable resources on arbitrary interventions, her result will almost always be inferior to that obtained through social learning. Eventually, that strategy is the one she adopts, and cumulation comes to a halt.
So if I am right, the accumulation of complexity is constrained by the cost-effectiveness of individual learning strategies.Footnote 42 At some level of complexity, more sophistication in individual (rather than social) learning is required to produce further complexity.
How can, in the face of accruing complexity, the cost of individual learning be reduced? A capacity for causal reasoning helps, for it makes individual learning targeted. Causal thought allows individuals to consider and learn directly about the salient features of a problem, not wasting resources on the limitless array of irrelevant factors. Suppose a particular deadfall is effective for catching small amphibians (e.g., frogs). One can easily learn how to apply the deadfall to larger animals (e.g., rabbits) – if one appreciates that it is the deadfall's dimensions and bait, rather than its operational principles, that are in need of experimentation. Rather than random and difficult, individual learning gets targeted and affordable.
Even if early technologies owed little to scientific theory, and they are consistent with being the product of trial and error, the kind of trial and error involved was plausibly reasoned rather than random: Given a folk understanding of the reasons for success or failure of an action, some interventions were tried, but not others. Causes inferred from diagnostic learning and causal analogies (e.g., that hafts are useful not just for spades, but also for rakes and hoes) were brought to bear in order to structure and delineate ill-structured design problems and spaces. This happened even more so towards the late 18th century, when science really began to have an influence on technology (although it is true that science is not a good example of strict individual learning; but more on this in sect. 12.3) (Mokyr Reference Mokyr2002; Wolpert Reference Wolpert2003).
We do not know whether by boosting individual learning, an improved grasp of causality forced the deadlock of the Acheulean. For what it is worth, by the time cumulative culture really got off the ground (say, in the Middle Stone Age), human brain size had increased substantially (e.g., Rightmire Reference Rightmire2004). Yet, whether this improved our causal reasoning capacities is uncertain, especially given our uncertainties regarding the neural mechanisms underlying causal thought (see my discussion in sect. 4).
Fortunately, my argument is general enough not to suffer from our ignorance about the precise whenabouts of the evolution of causal thought. If increased complexity indeed discourages individual learning, and a capacity for causal thought can ease the burden of such learning, then causal thought (whenever it emerged) is a plausible explanans for why cumulative culture evolved so markedly in humans and so modestly in apes.
Apart from reducing individual learning costs, causal reasoning abilities may also positively affect social learning. In particular, causal reasoning may facilitate: emulation learning (e.g., when putting to use a new device without consulting the user's manual, or when reverse-engineering)Footnote 43 ; selective imitation (i.e., copying only causally salient actions) (e.g., Want & Harris Reference Want and Harris2001); and proper model selection (e.g., copy X's doing of Y, because X's success is attributable to her doing of Y).
12.1.1. Outstanding questions
1. Almost all studies concerning inferential causal reasoning, analogical causal reasoning, and diagnostic learning use only Westerners as human subjects.Footnote 44 Are these forms of causal thought universal and observable cross-culturally?
2. Can the intuitive link between causal thought and individual inventiveness be empirically validated?
3. Although inferential causal reasoning in chimpanzees has received considerable attention, how do chimpanzees fare with respect to analogical causal reasoning and (especially) diagnostic learning?
4. To what extent does causal thought smooth social learning – in any of the three ways described above?
12.2. Executive control and cumulative culture
Improved executive control may positively affect cumulative culture in four ways. First, it may facilitate social learning. On this hypothesis, social learning of complex action sequences requires extra resources for representing interactions with the model; the social learner must inhibit her current drives longer, pay attention to the model's relevant behaviors only, and put into accord various actions (past, current, and future) of both model and herself.Footnote 45 To the best of my knowledge, no one has systematically addressed the impact of executive functions on social learning.
Second, improved executive functions may contribute to cumulative culture by lowering the costs of individual learning. The study of Goldenberg and colleagues (Reference Goldenberg, Hartmann-Schmid, Sürer, Daumüller and Hermsdörfer2007a; discussed in sect. 6) lends some support to this thought, for the authors show that dysexecutive patients perform poorly on innovative tasks, such as Tower of London tasks. For this sort of problem solving, Goldenberg and colleagues argue, it is apparently necessary to mentally plan ahead the steps to take. On this account, executive control would lower individual learning costs in largely the same way as causal reasoning does: by making individual learning reasoned rather than random. Innovation is easy for farsighted individuals, cumbersome for the arbitrary trial-and-error learner.
Third, better executive control may allow the representation of increasingly complex behaviors (however learned). But, as explained in section 6, this idea is questioned by the finding of Goldenberg and colleagues (Reference Goldenberg, Hartmann-Schmid, Sürer, Daumüller and Hermsdörfer2007a) that dysexecutive patients are able to execute quite complex routine action sequences (such as making coffee with a drip coffeemaker). Perhaps even more complex routine tasks put higher demands on executive functions; those not involving external reminders are likely to. At any rate, the relationship between executive control and complex action representation, however intuitive, has not been established yet.Footnote 46
Fourth, inhibition of immediate drives makes room for other motives, and those are remarkably diverse in humans. Two nonbasic motivations in particular can be expected to act on cumulative culture: the motivation to be like others, and conversely, the motivation to be unlike others (better than, different from). These act in opposite directions: the former furthering conformity and preservation of traits, the latter promoting competition and the introduction of new cultural variants. The former has been claimed to be characteristic of small-scale egalitarian societies (or of humanity, even) (e.g., Henrich & McElreath Reference Henrich and McElreath2003); the latter is usually associated with modern cultures valuing personal achievement and the strive for excellence (e.g., McClelland Reference McClelland1985). The speed of technological progress in capitalist societies since the Industrial Revolution seems to indeed be correlated with motivations to excel and outperform others. True, the rapid spread of many of our inventions may well attest to strong norms of conformity; their enormous diversity is more consistent with pronounced motivations not to be like others.
Incidentally, as was the case for causal thought, it is unknown whether improved executive functions, in any of the four ways described above, were implied in the Acheulean–Middle Stone Age transition.
12.2.1. Outstanding questions
1. Does social learning of complex behaviors really increase the demands on executive functions? (For example, does dysexecutive syndrome affect social learning?)
2. Goal maintenance and planning ahead appear to be critical for innovative tasks, such as solving Tower of London problems. Does the same hold for other innovative acts, especially those involving tools?
3. Models of cultural evolution have largely ignored the effects of nonconformity.Footnote 47 Questions related to nonconformity include: How do individual achievement motives favor individual learning? Increase variation? Speed up innovation rates? How does the resulting competition affect cumulation?Footnote 48
12.3. Social intelligence and cumulative culture
Section 9 discussed four sociocognitive skills: (1) heuristics for assessing a model's imitation-worthiness; (2) theory of mind abilities; (3) strong forms of contingent reciprocity; and (4) goal sharing. Cultural evolution theory has amply discussed the effects on cumulative culture of the former two. In this section, I consider the role of the latter two.
12.3.1. Contingent reciprocity
Contingent reciprocity supports cumulative culture, first, by allowing nonvertical modes of social learning and, second (again), by lowering individual learning costs.
First, when active teaching is involved, nonvertical modes of social learning are more difficult to establish than are vertical transmission modes. As teachers have no genetic interest in helping non-kin apprentices, their help is dependent on other forms of return on investment. These may be indirect (e.g., increasing the teacher's prestige) or direct (e.g., receiving favors from the apprentice or apprentice's close kin). In the case of the latter, mechanisms for contingent reciprocity seem crucial.
There is much discussion about how important nonvertical modes of transmission have been in initiating human cumulative culture.Footnote 49 On the other hand, the role of oblique and horizontal transmission in sustaining cumulative processes, especially in the modern era, is undeniable.Footnote 50 Equally undeniable is the role of contingent reciprocity in it – teaching has become an economic good, just like butter and bread.
Second, the effect of contingent reciprocity on individual learning relates to the fact that contingent reciprocity supports strong divisions of labor, also when learning is concerned. Consider, for example, a subsistence farmer who tries to learn individually which crop to plant.Footnote 51 The crop's eventual yield is dependent on numerous factors (e.g., climatic variations, soil conditions, pests), not just crop choice; so the farmer would need to experiment quite a bit before knowing which crop truly is best. Because she is unlikely to be willing to bear the costs of these experiments alone, she can be expected to just plant whatever crop other farmers plant(ed). Other farmers, too, will prefer social learning over individual efforts, and cumulation will come to a halt.
The stalemate can be broken if the farmer's learning costs are distributed: that is, if the farmer spends her resources on optimizing crop choice while others carry out the remaining tasks in return. The farmer specializes in farming, others in weaving rugs, baking pottery, grinding flour, and so forth. Goods are exchanged, and cumulation occurs in all domains. Unlike causal thought and executive control, contingent reciprocity thus lowers individual learning costs relatively rather than absolutely: Instead of making individual learning less laborious, contingent reciprocity ensures the labor of learning does not preclude the satisfaction of the other necessities of life.
There are some intuitive interconnections between contingent reciprocity, on the one hand, and causal thought and executive control, on the other. Contingent reciprocity makes teaching profitable. The effect of teaching on cumulative culture, in turn, can be expected to increase if the teaching not only transmits certain domain-specific skills (as in traditional transmissions of crafts), but also promotes capacities for individual learning (e.g., by passing on domain-general causal knowledge; see sect. 12.1) and motivates learners to put these capacities to use (e.g., by insisting on virtues of personal achievement and, popular since the Enlightenment, of thinking for oneself; see sect. 12.2). These reinforcement relations, however, still lack empirical and/or model-theoretic bite.
12.3.2. Goal sharing
Individual learning costs can be distributed in yet another way: by, as it were, making individual learning social. Now several individuals bundle their efforts to produce group-beneficial learning outputs. Cognitively speaking, the individuals display goal sharing in a learning context.
Several farmers, for example, may invest in a small, collective experimental patch of land where they can try out different crop varieties. The outcome of these experiments is to the benefit of the collective; each group member can now plant the crop the group jointly has identified as best.
At a certain level of behavioral complexity, individual specialization insufficiently reduces individual learning costs, and joint learning strategies (of the sort just described) need to arise for adding further complexity. Take, for example, the fairly modest transition from CD to DVD technologies. Even this small incremental improvement was initially the product of a joint effort of four major electronics corporations. Later, the technology was developed further by an even much larger consortium, the DVD Forum, consisting of 195 member companies in 2007. In other words, no single individual could have invented the DVD, even if, as cultural evolution theorists are fond of stressing, much of the work had been already done by previous generations.
As said (see Note 50), we don't know whether the evolution of nonvertical modes of transmission terminated the Lower Paleolithic, nor whether the evolution of joint learning had anything to do with it.
12.3.3. Outstanding questions
1. What are the conditions under which costly, nonvertical modes of teaching evolve? Which of the cognitive presuppositions of contingent reciprocity (see Table 1) can be expected to be crucial?
2. Cultural evolution theory has studied the preconditions, interactions, and effects of individual and social learning. What are the preconditions and effects of joint learning strategies, and their interactions with individual and social learning strategies?Footnote 52
13. Conclusion
Only human animals have been able to produce complex technologies such as wireless communication networks, satellite-driven navigation systems, and devices for arthroscopic and nanobot surgery. I have shown that this remarkable feature reflects a profound discontinuity between us and nonhuman primates in matters of social and non-social intelligence. And I have explained, albeit tentatively, in what sense our social and non-social cognitive sophistication has contributed to the technological accumulation characteristic of our species.
ACKNOWLEDGMENTS
Research by Krist Vaesen was supported by the Netherlands Organization for Scientific Research (NWO). He would like to thank six anonymous reviewers, Alex Mesoudi, Wybo Houkes, Marieke van Holland, and Auke Pols for useful comments on previous versions of this text.








Target article
The cognitive bases of human tool use
Related commentaries (30)
An area specifically devoted to tool use in human left inferior parietal lobule
Brain structures playing a crucial role in the representation of tools in humans and non-human primates
Can object affordances impact on human social learning of tool use?
Cathedrals, symphony orchestras, and iPhones: The cultural basis of modern technology
Childhood and advances in human tool use
Cultural intelligence is key to explaining human tool use
Evidence from convergent evolution and causal reasoning suggests that conclusions on human uniqueness may be premature
Evidence of recursion in tool use
Foresight, function representation, and social intelligence in the great apes
Human tool behavior is species-specific and remains unique
Human tool-making capacities reflect increased information-processing capacities: Continuity resides in the eyes of the beholder
Language and tool making are similar cognitive processes
Look, no hands!
Motor planning in primates
Neurocognitive anthropology: What are the options?
Not by thoughts alone: How language supersizes the cognitive toolkit
Prosthetic gestures: How the tool shapes the mind
So, are we the massively lucky species?
Technological selection: A missing link
The dual nature of tools and their makeover
The key to cultural innovation lies in the group dynamic rather than in the individual mind
The limits of chimpanzee-human comparisons for understanding human cognition
The role of executive control in tool use
Thinking tools: Acquired skills, cultural niche construction, and thinking with things
Tool innovation may be a critical limiting step for the establishment of a rich tool-using culture: A perspective from child development
Tool use and constructions
Tool use as situated cognition
Tool use induces complex and flexible plasticity of human body representations
Unique features of human movement control predicted by the leading joint hypothesis
What exists in the environment that motivates the emergence, transmission, and sophistication of tool use?
Author response
From individual cognition to populational culture