INTRODUCTION
Of the parasitic platyhelminths, monogeneans are generally considered to be among the most host specific, with many species reported to be strictly host specific i.e. infecting a single host species (Whittington et al. 2000). However, for host specificity to develop and be maintained over time, sufficient infective stages must reliably reach the definitive host. Where parasite transmission relies on ingestion of infective stages (e.g. cestodes, trematodes), specificity may be determined and maintained passively via the feeding habits of the host (Adamson and Caira, 1994). This is not the case for monogeneans with active infective stages in their direct life-cycle. With few exceptions, the larvae of monogeneans (oncomiracidia) face the challenge of finding, recognizing and then attaching to the definitive host if the life-cycle is to be completed. However, compared to their hosts, oncomiracidia are tiny, swim slowly (if at all) and survive for only a short time after hatching (Whittington et al. 2000). Despite apparently unfavourable odds, the prevalence of many host-specific Monogenea in nature bears testament to the fact that they do succeed. While success is likely to be the result of an interplay of factors operating over different scales, the larva's ability to detect and effect responses to stimuli, both environmental and host generated, must be of fundamental importance.
Factors including light periodicity (e.g. Kearn, 1973), variations in light intensity (e.g. Kearn, 1982), chemicals in host mucus and tissues (e.g. Kearn, 1986) and mechanical disturbance (e.g. Whittington and Kearn, 1988) have all been shown to elicit egg hatching in monogeneans. Hatching in response to 1 or more of these cues may assist larval transmission from egg to host by minimizing the temporal or spatial distance between the two. For instance, larvae may hatch at a time that coincides with a predictable aspect of host behaviour (e.g. Kearn, 1973), or may remain unhatched inside the egg conserving valuable energy reserves until a host is nearby (Whittington and Kearn, 1988). After hatching, physical capabilities of the larvae, such as whether or not they can swim and how long they live, will also influence successful host location.
Comparisons of egg hatching in unrelated monogeneans from the same fish host species are limited to 2 studies involving 2 sites: Whittington (1987a) for Leptocotyle minor (Microbothriidae) from the skin and Hexabothrium appendiculatum (Hexabothriidae) from the gills of the common dogfish, Scyliorhinus canicula (Scyliorhinidae); and Kearn et al. (1992) for Benedenia seriolae (Capsalidae) and Heteraxine heterocerca (Heteraxinidae) from the skin and gills, respectively, of Japanese yellowtail, Seriola quinqueradiata (Carangidae).
This study compares the hatching strategies of 3 unrelated species of monogenean from 3 different sites on the same host species, the southern fiddler ray, Trygonorrhina fasciata (Rhinobatidae): Calicotyle australis (Monocotylidae) from the cloaca; Pseudoleptobothrium aptychotremae (Microbothriidae) from the skin; and Branchotenthes octohamatus (Hexabothriidae) from the gills. Larval behaviour for each species after hatching was examined and analysed in relation to larval morphology. Particular emphasis is given to the association between hatching strategy, larval behaviour and morphology in each species’ quest to infect their shared host species and complete their life-cycle.
MATERIALS AND METHODS
Host collection and maintenance
Eight T. fasciata were caught by hand in shallow water at Kingston Point, Seacliff (35 °1′59″S, 138 °31′29″E), near Adelaide, South Australia between May and October 2004. Rays were transported alive to The University of Adelaide (UA) and transferred to a 2000 l aquarium containing recirculating, aerated seawater. To simulate a natural day/night cycle, a timer was used to regulate lighting in the aquarium facility (lights on at 06.00 h, lights off at 18.00 h): aquarium room ambient temperature was between 18 and 20 °C. To promote an intense parasite infection, a piece of plastic mesh (60×50 cm) was secured inside the tank to trap monogenean eggs (see Ernst and Whittington, 1996). Rays were fed daily on chopped pilchard.
Egg collection and incubation
Monogenean eggs were collected by isolating individual infected rays from the UA aquarium for up to 12 h in a 60 l bin containing approximately 40 l of fresh seawater aerated by an air stone. The light regime under which the eggs were incubated was consistent with the time they were collected. For example, eggs that were to be incubated in complete darkness were collected in darkness and eggs that were to be incubated under constant illumination were collected during periods of illumination. Following isolation, rays were returned to the main aquarium. The water from each 60 l bin was filtered through a 63 μm Nitex mesh sieve and the residue examined for eggs laid by parasites in vivo. The eggs of C. australis, P. aptychotremae and B. octohamatus are morphologically distinct allowing straightforward identification. Eggs were incubated in single species batches.
Using a fine wire loop, eggs of each monogenean species were transferred to small Perspex wells (internal diameter 9 mm; volume approximately 1 ml) containing fresh filtered seawater (FSW), filtered through Whatman qualitative paper. The eggs of C. australis are particularly sticky and adhere readily to any surface. Therefore, each egg was transferred separately in a droplet of FSW. Batch sizes depended on the number of eggs recovered on each collection day for each species. Each batch of eggs within a Perspex well was gently immersed, using forceps, into a larger glass crystallizing dish (40 mm diameter×30 mm deep; volume approximately 30 ml) filled with FSW, with the exception of P. aptychotremae eggs which were maintained in the Perspex wells only because the small, light coloured eggs (and larvae within) were difficult to see in larger volumes of water. To eliminate the chances of larvae being lost or damaged at the air/water interface after hatching, each crystallizing dish was covered with a glass plate (for C. australis and B. octohamatus batches) and a cover-slip was placed over each Perspex well (for P. aptychotremae batches). All air bubbles were excluded by overfilling each dish with FSW (see Whittington and Kearn, 1986).
Eggs were incubated in a controlled temperature cabinet at 22 °C. Experimental light regimes were achieved with a programmed timer connected to an 18 W Grow-lux tube fitted to the cabinet ceiling. Eggs were examined daily for signs of development using a dissecting microscope and the FSW in each dish was replaced. Water was replaced at different times during the day or night to avoid the possibility of imposing an artificial hatching cue. For this brief inspection period, egg dishes were removed from the incubator. All work during ‘dark cycles’ was conducted in a darkened room with the aid of a torch covered with a red filter, as several studies have shown that low-level red light has no effect on egg hatching or larval behaviour in monogeneans (Kearn, 1973; Ernst and Whittington, 1996; Chisholm and Whittington, 2000). During reversed light regimes, eggs incubated in darkness during daylight hours were covered with a black box at the start of the ‘dark cycle’ to prevent accidental exposure to light when the door of the incubator was opened (see Chisholm and Whittington, 2000).
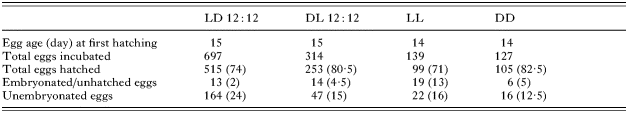
When hatching commenced, eggs were checked every 2 h (C. australis) or 3 h (P. aptychotremae) and the number of larvae hatched within each time-period was recorded. Eggs were monitored until all signs of hatching had ceased, or for a maximum of 14 days. Hatching success and egg viability was determined at the completion of each experiment by counting: the total number of eggs incubated; the proportion that embryonated and hatched successfully; those that embryonated but failed to hatch (C. australis only); those that failed to embryonate (C. australis only).
Experimental light regimes
The effect of 4 light regimes on egg hatching was investigated for C. australis: a light/dark (LD) 12[ratio ]12 cycle (i.e. light on 06.00 h, light off 18.00 h); a reversed light/dark (DL) 12[ratio ]12 cycle (i.e. light on 18.00 h, light off 06.00 h); a continuous light (LL) cycle; and a continuous dark (DD) cycle. Egg hatching for P. aptychotremae was only observed under LD 12[ratio ]12 because infections disappeared from our captured hosts after the first hatching trials and despite attempts to re-establish the parasite culture, no eggs were available for further experiments. Because no B. octohamatus eggs hatched spontaneously during the first experimental trial (LD 12[ratio ]12), subsequent trials under alternative light regimes were not conducted for this species.
Effects of mechanical disturbance and host skin secretions on egg hatching
Test and control batches of eggs of all 3 species were incubated under LD 12[ratio ]12 as described above. The effect of mechanical disturbance on egg hatching was examined by subjecting eggs to water currents generated by a Pasteur pipette and by tapping the dishes containing eggs lightly on the stage of a dissecting microscope. Control batches of eggs of the same age were left undisturbed.
To investigate the effect of host skin secretions on egg hatching, ‘ray water’ (fresh FSW that had been poured over the ventral surface of a live ray) was added carefully to test batches of eggs using a Pasteur pipette taking care to avoid water currents. Fresh FSW was similarly added to control batches of eggs of corresponding ages. Each batch was observed for signs of hatching for 5 min following treatment, again for 5 continuous minutes after 10 min and thereafter at 15 min intervals for 2 h.
Effect of shadows on egg hatching
The effect of shadows on egg hatching was tested on several batches of P. aptychotremae eggs after the 14-day observation period in LD 12[ratio ]12 was completed. Shadows were cast over egg batches using a piece of cardboard. The duration of shadowing ranged from 2 s to 15 s. No further P. aptychotremae eggs were available for more rigorous testing on eggs of different ages.
Larval behaviour
After hatching, the behaviour of 30 C. australis larvae, 9 P. aptychotremae larvae and 200 B. octohamatus larvae was recorded for 5 min at regular intervals from within 1 h of hatching until all larvae had settled or were dead. Larvae of each species were maintained in sealed glass dishes containing FSW at 22 °C and observations were made using a dissecting microscope with low-level white light from above. The response of larvae to illumination was investigated inside a darkened cabinet by shining the focused beam of a hand-held torch on alternate sides of the 40 mm diameter glass crystallizing dish containing the larvae in FSW. Larval swimming speed was also determined by recording the time taken by a larva to swim the diameter of the dish. To test for a response to host skin secretions, ‘ray water’ was added carefully as described previously and FSW was added to control dishes.
Limited observations on host behaviour
Trygonorrhina fasciata is a demersal ray species endemic to Southern Australia. These rays are frequently seen by divers and caught by fishermen in shallow coastal waters, often near jetties or adjacent to seagrass beds (Last and Stevens, 1994). However, nothing has been documented of their behaviour within these habitats. Efforts were therefore made to observe rays in the wild prior to capture and also while rays were captive in the UA aquarium.
RESULTS
Egg hatching in C. australis
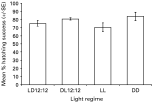
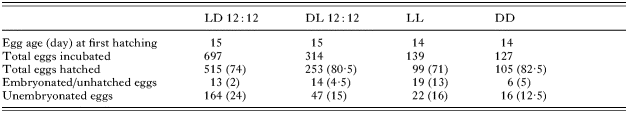
A total of 1277 C. australis eggs was incubated under 4 experimental light regimes at 22 °C (Table 1). Eyespots were visible from Day 11 and hatching commenced between Days 14 and 15 (Table 1). Hatching success ranged between 71% (LL) and 82·5% (DD) (Table 1). There was no significant difference in the mean hatching success for each light regime (Fig. 1).

Fig. 1. Mean percentage hatching success for Calicotyle australis eggs incubated under 4 experimental light regimes at 22 °C. Details of light regimes as listed in Table 1.
Table 1. Hatching success for Calicotyle australis eggs incubated under 4 experimental light regimes at 22 °C (LD 12[ratio ]12 (light on 06.00 h, light off 18.00 h), DL 12[ratio ]12 (light on 18.00 h, light off 06.00 h), LL (continuous light), DD (continuous darkness). Values in parentheses denote the percentage hatching success of total number of eggs incubated.)
LD 12[ratio ]12
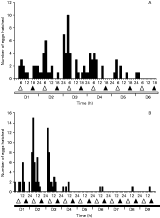
All hatching occurred during periods of illumination before 13.00 h, with 98% of larvae emerging in the first 4 h of light (Fig. 2A). Approximately 42% of eggs hatched on Day 1, with most of this hatching evenly spread over the first 4 h of light (Fig. 2A). Over half the eggs (54%) hatched on Day 2, but in contrast to Day 1, a distinct hatching peak (44%) was observed in the first 2 h period of illumination (Fig. 2A). Hatching continued for 4 days.

Fig. 2. Number of Calicotyle australis larvae hatching at 2-h intervals from (A) 697 eggs incubated in LD 12[ratio ]12 and (B) 314 eggs incubated in DL 12[ratio ]12 at 22 °C. Horizontal bars indicate alternating 12 h periods of illumination (white) and darkness (black).
DL 12[ratio ]12
A reversal of the light regime appeared to have little effect on the hatching pattern of C. australis (Fig. 2B). Again, hatching was confined to periods of illumination, with approximately 98% of eggs hatching during the first 4 h of light (18.00 h–22.00 h) (Fig. 2B). More eggs hatched on Day 2 (59%) than on Day 1 (38%), but on both days, hatching was greatest in the first 2 h period of illumination (Fig. 2B). Hatching continued for 3 days.
LL
Eggs collected and incubated under LL (Fig. 3A) did not hatch exclusively within the hours corresponding to dawn and dusk as described above for LD 12[ratio ]12 (Fig. 2A) and DL 12[ratio ]12 (Fig. 2B). Instead, 62% of larvae emerged during hours corresponding to darkness i.e. between 18.00 h and 06.00 h. Hatching peaked each day in the 6 h period between 24.00 h and 06.00 h, with 50% of all eggs hatching during this time (Fig. 3A). Hatching continued between 06.00 h and 12.00 h, representing 28% of total eggs hatched, but few eggs (10%) hatched in the 6 h period between 12.00 h and 18.00 h. No larvae emerged between 16.00 h and 18.00 h on any day. The highest number of larvae emerged on Day 3 (33%), although hatching was generally spread evenly over Days 1 to 4. Hatching continued for 5 days (Fig. 3A).

Fig. 3. Number of Calicotyle australis larvae hatching at 2-h intervals from (A) 139 eggs incubated in LL and (B) 127 eggs incubated in DD at 22 °C. Horizontal bars indicate constant illumination (white) and constant darkness (black). Triangles indicate the onset of natural illumination ([utri ]) and darkness ([utrif ]) respectively. D1–D9 denote the number of consecutive days of each experiment.
DD
The hatching pattern of eggs collected and incubated under DD (Fig. 3B) was similar to that observed for LL (Fig. 3A). Almost half the eggs (48%) hatched in the 6 h period between 24.00 h and 06.00 h, corresponding to the hatching peak described above for LL. Again, 28% of eggs hatched between 06.00 h and 12.00 h, but under DD, a higher proportion of larvae (19%) emerged in the 6 h period between 12.00 h and 18.00 h (Fig. 3B). Clearer distinction between hatching versus non-hatching periods was apparent for eggs incubated under DD, as no hatching occurred during the 6 h period between 16.00 h and 22.00 h on any day. Hatching continued for 9 days, although most eggs (92%) hatched within the first 3 days. The highest number of larvae emerged on Day 2 (41%) (Fig. 3B).
Egg hatching in P. aptychotremae
A total of 1290 P. aptychotremae eggs was incubated under LD 12[ratio ]12 at 22 °C, but only 480 (37%) of these eggs hatched. The first larvae emerged after 8 days and egg hatching was confined to the last 3 h of the illumination period i.e. between 15.00 h and 18.00 h (Fig. 4). While the majority of eggs (74%) hatched during the first 4 days, hatching in some batches continued for up to 14 days, after which monitoring stopped (Fig. 4).

Fig. 4. Number of Pseudoleptobothrium aptychotremae larvae hatching at 3-h intervals from 1290 eggs incubated in LD 12[ratio ]12 at 22 °C. Horizontal bar as in Fig. 2. D1–D14 denote the number of consecutive days of the experiment.
Effects of mechanical disturbance and host skin secretions on egg hatching for C. australis and P. aptychotremae
The effect of mechanical disturbance and host skin secretions was tested on eggs that had started spontaneous, rhythmic hatching under an LD 12[ratio ]12 regime. Eggs were subjected to treatments at times that were outside the daily hatching pattern observed for each species. No hatching was induced by either stimulus in either species and no hatching occurred in the controls.
Effect of shadows on egg hatching for P. aptychotremae
No hatching occurred either during or subsequent to shadows cast over eggs aged 22 days.
Hatching in B. octohamatus
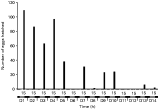
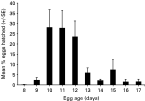
Over 7000 B. octohamatus eggs were incubated under LD 12[ratio ]12 at 22 °C. No spontaneous hatching occurred and no hatching was induced by the addition of host skin secretions to FSW containing batches of B. octohamatus eggs aged between 5 and 17 days. However, ~85% of eggs hatched when mechanically disturbed (Fig. 5). Often the slightest movement of dishes containing eggs was sufficient to promote instant hatching. Of the eggs that hatched, the majority (83%) did so when disturbed between Days 10 and 12 after laying (Fig. 5). However, some hatching occurred as early as Day 8 and some eggs failed to hatch until Day 17, despite daily disturbance (Fig. 5). Hatching was not influenced by the time of disturbance which was varied each day, although eggs were not disturbed during darkness. No hatching occurred in the controls.

Fig. 5. Relationship between egg age (days) and egg hatching success when eggs of Branchotenthes octohamatus were mechanically disturbed. Egg hatching is expressed as the relative proportion of eggs to hatch from each batch after disturbance on each day. Eggs were incubated under LD 12[ratio ]12 at 22 °C. Total eggs incubated ~7500; hatching success ~85%.
Larval behaviour
Calicotyle australis
The ciliated C. australis larvae (~190 μm long) had a mean swimming speed of 4·8 mms−1 based on 15 (of 30) larvae studied less than 2 h old. Calicotyle australis larvae have pigmented eye spots. From the time of hatching until settlement, larvae were photo-responsive. In an otherwise dark environment, the focused beam of a torch elicited a positive photo-response. However, under continuous, low-level white light from above, positive phototaxis was not overt and other behaviours, described below, were identified. The addition of host skin secretions to the water containing larvae had no detectable effect on their behaviour, irrespective of age, or on their longevity.
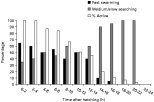
Two broad categories of swimming behaviour were identified: (1) fast ‘swarming’ involved fixed, repetitive patterns such as tight vertical spirals, horizontal zigzagging, on-the-spot spinning and 3-dimensional figure-of-8 formations, and (2) medium to slow ‘searching’ involved horizontal and vertical meandering through the water column and gliding over the bottom and sides of the dish, interspersed with occasional pauses. During each 5-min observation period, the frequency of each of the 8 behaviours was noted. After the observation period, the score for each behaviour was converted to a percentage, based on the total number of observations within that time-period. The relative proportion of time spent in each broad behavioural category was determined by combining the percentage values relevant to each category to provide a view of behavioural change with larval age. The percentage of larvae that were active during each 5 min observation interval was also recorded.
Immediately after hatching, all larvae were active. Fast ‘swarming’ was most frequent (~65% of the time), although medium to slow ‘searching’ also occurred (~35%) (Fig. 6). As larvae aged there was a shift in the proportion of time spent in these 2 broad behavioural categories and the proportion of active larvae declined. Only half the larvae were still active 10–12 h after hatching when ‘swarming’ and ‘searching’ behaviours were divided equally (Fig. 6). The number of active larvae continued to decline with age and fast ‘swarming’ was replaced by slow ‘searching’ as the dominant behaviour. All larvae had ceased activity between 22 and 24 h after hatching.

Fig. 6. Relationship between swimming behaviour, activity level and larval age for Calicotyle australis maintained at 22 °C. Larvae were observed for 5 min every 2 h from the time of hatching until all larvae were inactive. The relative frequency of each behaviour is presented as a percentage of each 5-min observation period, based on a total of 30 larvae.
Pseudoleptobothrium aptychotremae
These larvae are very small (~110 μm long), transparent and extremely fragile. The transfer of these larvae to a separate dish after hatching (i.e. away from unhatched eggs) frequently resulted in their loss or inflicted damage but sufficient data were obtained for comparative purposes.
Based on 9 larvae <2 h old, ciliated P. aptychotremae larvae had a mean swimming speed of 4·8 mms−1. Pseudoleptobothrium aptychotremae larvae lack pigmented eye spots and showed no detectable response to illumination. The addition of host skin secretions to FSW containing larvae had no apparent effect on their behaviour regardless of age, or on their longevity.
Shortly after hatching, fast ‘swarming’ incorporating tight vertical spirals, horizontal zigzagging and on-the-spot spinning was the dominant swimming behaviour. However, within 4 h of hatching, all larvae had ceased activity. Lack of movement and their transparency made the larvae impossible to find and we could not determine whether the larvae had settled but remained alive or were dead and had started to decompose.
Branchotenthes octohamatus
These larvae (~170 μm long) are unciliated. They are also without pigmented eye spots and larvae showed no detectable photo-response. The addition of host skin secretions to FSW containing larvae did not alter their behaviour regardless of age, nor appear to affect their longevity.
Over 200 B. octohamatus larvae that hatched simultaneously when mechanically disturbed from eggs aged 14 days, were monitored from the time of hatching until all had died. Following the appropriate stimulus, the larvae extended their bodies to push off the operculum and then escaped from the egg completely. While emerging from the egg and immediately following hatching, larvae thrashed vigorously from side to side. If mechanical disturbance was persistent, the larvae continued to behave in this way. However, when the disturbance subsided and conditions in the vessel were still, the larvae stopped moving. Activity only resumed with further disturbance. Occasionally, larvae made leech-like movements across the bottom of the vessel. With increasing age, the level of activity stimulated by each disturbance event was less frenetic and of shorter duration. After 24 h approximately half the larvae remained responsive and some larvae survived up to 50 h (2·5 days) after hatching. Larvae were lifted readily off the bottom of the vessel by water currents from a Pasteur pipette.
Host behaviour
All T. fasciata specimens caught during the course of our work were first spotted either settling into the sediment, lying motionless on the sandy bottom, partially covered in sediment or cruising slowly just above the seafloor. In captivity, the rays behaved similarly, spending much of their time resting on the floor of the tank. Periods of inactivity were interspersed with bouts of active swimming in the tank, both during the day and night. Frequently, the rays were observed to ‘flutter’ just above the tank floor before coming to rest. In the natural environment, this behaviour may resuspend sediment, which would then settle on the ray covering it either partially or fully. It is interesting that this behaviour occurred despite the absence of sediment in the tank.
DISCUSSION
Each monogenean species from T. fasciata (Rhinobatidae) has evolved a different egg hatching and subsequent host finding strategy. Calicotyle australis (Monocotylidae) from the cloaca hatches spontaneously with a strong diurnal rhythm that is likely to be under circadian control. The larvae that emerge are ciliated, photo-responsive and can survive for up to 24 h after hatching. Pseudoleptobothrium aptychotremae (Microbothriidae) from the skin may have a ‘bet-hedging’ strategy. Some eggs hatch spontaneously and rhythmically, but their low hatching success rate suggests that other eggs may require a different cue provided by the host. The larvae that emerge are also ciliated but show no photo-response and may only survive for a few hours after hatching. Branchotenthes octohamatus (Hexabothriidae) from the gills has a ‘sit-and-wait’ strategy dependent entirely on mechanical disturbance for eggs to hatch. The larvae that emerge are unciliated and show no photo-response but may survive for more than 2 days after hatching.
Hatching rhythms similar to that of C. australis have been reported for a number of monocotylids from the shovelnose ray, Rhinobatos typus (Rhinobatidae): Neoheterocotyle rhinobatidis (gills), Troglocephalus rhinobatidis (gills) and Merizocotyle icopae (nasal tissue) (Chisholm and Whittington, 2000). Eggs of these species also hatch spontaneously and rhythmically, releasing ciliated larvae. Hatching in the monocotylid, Dictyocotyle coeliaca, from the body cavity of the cuckoo ray, Raja naevus (Rajidae), is also spontaneous but arhythmic (Kearn, 1975). A close phylogenetic relationship is shared by C. australis and D. coeliaca (see Chisholm et al. 1995).
Until now, Leptocotyle minor from the skin of Scyliorhinus canicula was the only microbothriid investigated for egg hatching (see Whittington, 1987a). Unlike those of P. aptychotremae, eggs of L. minor did not hatch spontaneously, but instead hatched in response to host skin secretions. P. aptychotremae had a low hatching success and perhaps host skin secretions also promote hatching in this species. A ‘bet-hedging’ strategy may have evolved in which some eggs hatch with a daily rhythm whereas other eggs may only hatch when exposed to a specific host induced cue. However, no eggs hatched when we added ‘ray water’ to egg batches and it is possible that the hatching stimulant was not provided at the appropriate concentration to elicit a response (e.g. Pike, 1990). No hatching response to shadows was detected. It is also possible that P. aptychotremae eggs may have continued to hatch beyond the 14-day study duration, resulting in higher hatching success. A protracted, 7-week hatching period was reported by Kearn (1975) for D. coeliaca and hatching over several consecutive days is regularly observed among monogeneans (e.g. Kearn, 1973; Ernst and Whittington, 1996; Chisholm and Whittington, 2000). Hatching over many days increases the chances of larvae finding a host, if a host is not nearby at the time of emergence.
Several studies have investigated egg hatching in hexabothriids and a number of different hatching strategies have been identified. Eggs of Rajonchocotyle emarginata from the gills of Raja spp. (Rajidae) hatch spontaneously and rhythmically to release a ciliated, free-swimming larva (Whittington and Kearn, 1986). Hexabothrium appendiculatum from Scyliorhinus canicula (Scyliorhinidae) hatch only when stimulated by host skin secretions and a ciliated, free-swimming larva emerges (Whittington, 1987b). Squalonchocotyle torpedinis from Torpedo marmorata (Torpedinidae) hatch in the presence of host gill tissue (indicating a chemical hatching cue), but the larva is unciliated and cannot swim (Euzet and Raibaut, 1960). The egg hatching strategy and larva of Neonchocotyle pastinacae from the gills of Dasyatis pastinaca (Dasyatidae) most closely resembles B. octohamatus from T. fasciata because eggs hatch only when mechanically disturbed and the larva is unciliated (Ktari and Maillard, 1972).
Synchronization of circadian rhythms by the light-dark cycle is photic entrainment (Dunlap et al. 2004). Photic control of egg hatching rhythms has been demonstrated for many organisms including insect species such as crickets (e.g. Itoh and Sumi, 2000), as well as for some monogenean species. Daily egg hatching rhythms that correspond to light periodicity are reported for 16 monogenean species (Whittington et al. 2000). Of these, experimental manipulations to test whether observed hatching rhythms are likely to be endogenously or exogenously driven have so far been undertaken for 8 species in 4 families: Entobdella soleae (Capsalidae) (see Kearn, 1973), Polystoma integerrimum (Polystomatidae) (see Macdonald and Combes, 1978), Benedenia lutjani and B. rohdei (Capsalidae) (see Ernst and Whittington, 1996), Discocotyle sagittata (Discocotylidae) (see Gannicott and Tinsley, 1997), Neoheterocotyle rhinobatidis, Troglocephalus rhinobatidis and Merizocotyle icopae (Monocotylidae) (see Chisholm and Whittington, 2000). Without exception, these studies showed that egg hatching was specific to either the light or dark phase of LD 12[ratio ]12 and in most cases, hatching occurred with a sharp peak in the first few hours following the transition from light to dark or dark to light. We have observed the same for C. australis in which virtually all eggs hatched during the first 4 h of the light period. Eggs of P. aptychotremae also hatched only during the light phase of LD 12[ratio ]12, although hatching preceded the transition from light to dark in this species. Under constant conditions (LL and DD), the hatching rhythms of 5 species (B. lutjani, B. rohdei, N. rhinobatidis, T. rhinobatidis and M. icopae) mirrored those observed under LD 12[ratio ]12, suggesting that an alternative entraining agent(s) may bring about phase control in their hatching rhythms (Ernst and Whittington, 1996; Chisholm and Whittington, 2000). Calicotyle australis eggs incubated in LL or DD did not hatch with the same rhythm observed in LD 12[ratio ]12. Instead, hatching was earlier (peak between 24.00 h and 06.00 h) and occurred over a broader time-period. Even though temporal cues are equally absent in constant light and in constant darkness, light has been demonstrated to affect the operation of circadian pacemakers (Refinetti, 2000) and this may account for the slightly greater length of the non-hatching period in DD i.e. 16.00 h to 22.00 h (DD; Fig. 3B), c.f. 16.00 h to 18.00 h (LL; Fig. 3A). Both the LL and DD experimental regimes produced similar hatching rhythms that may reflect the endogenous free-running rhythm or circadian pacemaker for C. australis (see Refinetti, 2000). Under entraining light schedules (i.e. LD 12[ratio ]12 and DL 12[ratio ]12), the free-running rhythm was modified, and hatching followed the dark-light transition predictably. Kearn (1973) also found that eggs of E. soleae, from the flatfish host Solea solea, hatched with a distinct rhythm when incubated in LD 12[ratio ]12 but exhibited little evidence of rhythmicity when incubated in DD.
Accurate interpretation of egg hatching patterns can be difficult. Whether light serves solely as a synchronizer of an endogenous circadian rhythm or plays an additional role as a direct hatching stimulus was raised by Kearn (1973) for E. soleae. If the activity of an organism is triggered by lights switching on or off, the resulting activity rhythm may appear as photic entrainment, when in fact the circadian clock has been masked (Roenneberg et al. 2003). The experimental manipulations carried out by Gannicott and Tinsley (1997) for D. sagittata provided strong evidence that hatching was driven by the direct effect of the light to dark transition, although further study was recommended to fully understand the mechanisms involved. Because individual batches of C. australis in this study were not transferred from 1 lighting regime to another within the same experiment, we also cannot rule out the possibility that the strong hatching rhythm observed at the start of the illumination phase for eggs incubated in LD 12[ratio ]12 and DL 12[ratio ]12 was not a direct response to photic stimulus. However, light can be ruled out as a direct hatching stimulus for P. aptychotremae because hatching always occurred before the light-dark transition.
Our experiments suggest that egg hatching in P. aptychotremae is also likely to be under circadian control. Light may again be the entraining agent, even though P. aptychotremae larvae lack pigmented eye spots. Ciliary organs, believed to be photo-receptors, were identified by Lyons (1972) in the larva of E. soleae. Kearn (1973) surmised that these ‘photoreceptors’ may be responsible for monitoring day length, whereas pigmented eye spots may play a greater part in orientation with respect to light after hatching.
The hatching of P. aptychotremae eggs in the 3 h preceding nightfall suggests that larvae ‘anticipated’ the advancing transition from light to dark and hatching at this time might therefore confer some benefit to the emerging larvae. By maintaining a fixed phase relationship between physiological processes and the environment, circadian rhythms allow precision timing of events (Sharma and Joshi, 2002). However, opportunistic responses to exogenous stimuli are also beneficial if they permit an organism to take advantage of a favourable environmental window (Dunlap et al. 2004). The instantaneous hatching of B. octohamatus eggs when mechanically disturbed represents a hatching strategy based entirely on an exogenous ‘opportunistic’ stimulus that may or may not be provided by a host.
The ability of an organism to respond to more than 1 cue may also prove advantageous where environmental periodicities are complex and/or not always regular in frequency such as in the marine environment (Dunlap et al. 2004). Ernst and Whittington (1996) and Chisholm and Whittington (2000) have suggested that a hierarchy of entraining agents and stimuli (e.g. tidal amplitude, tidal phase and temperature) may exist for monogenean eggs. But little is known about the mechanisms of non-photic entraining agents (Dunlap et al. 2004). Although light appears to be the entraining agent for C. australis, and possibly also for P. aptychotremae, non-photic synchronizers as well as other non-entraining environmental factors may interact with light (Roenneberg et al. 2003) to produce the hatching rhythms observed.
Differences between the 3 monogenean species from T. fasciata are not confined to their egg hatching strategies. Differences are also evident in the physical capabilities of the larvae that emerge from the eggs as well as in their host finding behaviour. The larvae of C. australis and P. aptychotremae are ciliated and free-swimming, whereas larvae of B. octohamatus are unciliated and cannot swim. This is an important difference because if no host is nearby when C. australis and P. aptychotremae larvae hatch, the larvae may be able to locate one. We have established that C. australis larvae can survive and remain active for up to 24 h in still water after hatching and P. aptychotremae larvae for at least 4 h. Experimental conditions i.e. incubation of eggs in a small volume of FSW and/or the transfer of larvae between dishes, may have affected larval longevity for P. aptychotremae. However, if egg hatching in this species is promoted by an, as yet, unidentified host stimulus, then close proximity to a host on hatching is likely. This might account for a shorter larval lifespan, as less time would be needed searching for a host. It is interesting to note that refringent droplets (likely lipid) visible in the body and ciliated cells of C. australis larvae (Glennon et al. 2006a), are not apparent in P. aptychotremae larvae (Glennon et al. 2006b), possibly reflecting differences in lifespan between the 2 species. Calicotyle australis larvae are photo-responsive after hatching, indicating that light may play a role in orientation for C. australis but not for P. aptychotremae whose larvae are unresponsive to light. Two broad categories of larval swimming behaviour were identified for C. australis and P. aptychotremae: fast ‘swarming’ and medium to slow ‘searching’. Omnidirectional swimming during ‘swarming’ should allow larvae to explore a large volume of water in a relatively short period of time and may increase the larvae's chances of randomly ‘bumping’ into a host. The repetitive nature of this behaviour suggests that ‘swarming’ may represent a collection of fixed action patterns. That is, once the stimulus that elicits each pattern is delivered, the larvae cannot modify it (Lorenz and Tinbergen, 1957).
Fixed action patterns, also known as releaser responses, are used by a number of parasite species in their search for a host (Sukhdeo and Sukhdeo, 2002 and references therein). Kearn (1980) described a simple, repetitive vertical swimming pattern for E. soleae. During each up and down phase, larvae would be transported away from the hatching site by horizontal water currents but on return to the seafloor, larvae might land on a resting host (Kearn, 1980). While the complex ‘swarming’ patterns we observed for C. australis and P. aptychotremae are different to the simple up and down pattern of E. soleae, both swimming behaviours, if fixed action patterns, have important potential to deliver a larva to a host without the need for orientation signals. In contrast to ‘swarming’, the slower ‘searching’ behaviour of C. australis and P. aptychotremae larvae is unlikely to comprise fixed action patterns, as swimming behaviour was modulated and larvae were able to change direction and swimming speed.
With increasing age C. australis larvae spent less time ‘swarming’. Kearn (1980) also noted a change in the behaviour of E. soleae larvae as they aged. It was proposed that the behavioural shift might reflect a change in emphasis from an early phase dominated by dispersal, to a later phase where host finding assumed greater importance (Kearn, 1980). The fast ‘swarming’ of C. australis larvae may also be part of an early dispersal strategy. However, a reduction in this behaviour as the larvae aged may also reflect energetic costs, which are likely to be high.
In contrast to C. australis and P. aptychotremae, the larva of B. octohamatus cannot swim to locate a host. These larvae depend on a host coming to them, making the host finding strategy of B. octohamatus passive, rather than active. Yet, mechanical disturbance as a hatching cue is non-specific and may be generated by many spurious sources in the sea. The larvae therefore run the risk of hatching when a host is not nearby. However, in the event that hatching is falsely promoted, B. octohamatus larvae may be able to survive outside of the egg for up to 2·5 days while waiting for a host to arrive. These larvae contain large numbers of refringent droplets (likely lipid) dispersed throughout the body, in addition to a conspicuous mass of droplets posterior to the pharynx (Glennon et al. 2005). Such large energy reserves in non-swimming larvae suggest an adaptation to extended periods spent ‘waiting’ for a host, either prior to, or after hatching. Additionally, our observation that all eggs in a batch do not necessarily hatch at once may also prove advantageous. The chances of infecting a host may be increased if some eggs are available to hatch during a subsequent disturbance event that may be created by a potential host.
Monogenean species from the same host species might be expected to have similar hatching patterns as they share a common goal (Kearn, 1975). Whittington (1987a) found a similar strategy for L. minor (Microbothriidae) and H. appendiculatum (Hexabothriidae), from the skin and gills respectively of Scyliorhinus canicula. Eggs of these species hatched only in response to host skin secretions, yielding ciliated larvae. However, the hatching and host finding strategies of the 3 monogenean species from T. fasciata are very different. Different hatching strategies were also found by Kearn et al. (1992) for 2 unrelated monogeneans from Seriola quinqueradiata: Benedenia seriolae (Capsalidae) and Heteraxine heterocerca (Heteraxinidae) from the skin and gills respectively. Eggs of these species hatched spontaneously but at distinctly different times of the day and it was suggested that this difference may reflect different sites of host invasion, as some sites may be more accessible at certain times of the day (Kearn et al. 1992). The larval invasion sites are yet to be determined for monogeneans from T. fasciata, and we do not know if they differ between the 3 species. Branchotenthes octohamatus larvae may attach directly to the gills of T. fasciata. However, the migration of larvae over the body surface to the gills is also possible and has been documented in Urocleidus adspectus (Dactylogyridae) (see Cone and Burt, 1981). As P. aptychotremae lives on the skin of its host, larval invasion is likely to be direct. Calicotyle australis larvae may also attach first to the body surface and then migrate to the cloaca.
Whether active or passive, based on direct or indirect cues, fixed action patterns and/or modulated responses to stimuli, the success of any egg hatching and host finding strategy must, to a large extent, depend on the temporal and behavioural predictability of the host in its habitat. Other studies, reviewed by Chisholm and Whittington (2000), link spontaneous hatching rhythms of monogeneans to host phenology. For example, the flatfish host of E. soleae becomes inactive at dawn, so hatching in the first few hours of daylight is likely to increase the chances of a slow swimming larva making contact with a host (Kearn, 1973). Separate studies have shown that egg hatching strategies of the unrelated species Acanthocotyle lobianchi (Acanthocotylidae) from the skin (see Macdonald, 1974), Dictyocotyle coeliaca from the body cavity (see Kearn, 1975) and Rajonchocotyle emarginata (Hexabothriidae) from the gills (see Whittington and Kearn, 1986) of R. naevus, differ. Larvae of D. coeliaca (ciliated) hatch spontaneously but arhythmically, larvae of A. lobianchi (unciliated) hatch only in response to host skin secretions and larvae of R. emarginata (ciliated) hatch spontaneously and rhythmically. These different hatching strategies were thought to suggest a lack of a well-defined daily activity rhythm in this ray species (Whittington and Kearn, 1986). Irrespective of whether T. fasciata has a well-defined daily activity rhythm, the relative importance of any particular host behaviour in transmission success is likely to be different for each monogenean species because their strategies are so different. Southern fiddler rays may be less active at early morning and late afternoon and may therefore represent more accessible targets to C. australis and P. aptychotremae at these times, but this is yet to be substantiated. Trygonorrhina fasciata partially bury themselves in sediment while coming to rest on the sea floor, a behaviour that stirs up sediment and would provide the necessary mechanical disturbance for eggs of B. octohamatus to hatch. Furthermore, this behaviour may facilitate infection: newly hatched larvae may be suspended in the water layer surrounding the ray as a result of this activity and then be inspired through the dorsally located spiracles, permitting direct attachment to gills. Predation by nocturnal filter feeders is also suggested as a possible driving force behind diurnal hatching in some species (Whittington and Kearn, 1986; Ernst and Whittington, 1996).
In comparing egg-hatching and host-finding strategies of monogeneans from the same host species, it is important to consider that the strategies might not be equally efficient at uniting larva with host. Differential host finding efficiencies may be reflected in the prevalence and intensity of infections in the wild, or, in the ability of a parasite species to infect more than one host species (i.e. broader host specificity). Alternatively, other biological parameters such as fecundity and generation time may compensate for shortfalls experienced after hatching. Therefore, to gain greater insight into the selective forces that have shaped the lives of the 3 monogenean species from T. fasciata within their respective habitats, knowledge of egg-hatching and host-finding strategies must be placed in context with other life-history data.
This work was supported by funds from the School of Earth and Environmental Sciences of The University of Adelaide and an Australian Postgraduate Award to V. Glennon during her Ph.D. candidature (2004 onwards) and Australian Research Council grant no. DP0557697 for 2005–2007 awarded to I.D.W.