Introduction
The purpose of this review is to provide an overview of ultraviolet (UV) irradiation and its application for the control of microorganisms. The first work on the inactivation of microorganisms by UV light was published in 1892 (Ward, Reference Ward1892), with the ‘germicidal’ effects of UV wavelengths reported in 1903 (Barnard and Morgan, Reference Barnard and Morgan1903). In 1927, Rivers and Gates used UV light to inactivate virus in suspension and proved the efficacy of the method through subcutaneous inoculation of rabbits (Rivers and Gates, Reference Rivers and Gates1927). The use of UV light to inactivate microorganisms in the environment began when Wells and Wells (Reference Wells and Wells1938) described the use of UV to inactivate microorganisms in hospital operating rooms. Wheeler et al. (Reference Wheeler, Ingraham, Hollaneder, Lill, Gershon-Cohen and Brown1945) used UV light to ‘disinfect’ Army and Navy barracks for the control of airborne rubella virus and Streptococcus pyogenes and in 1947, the use of UV irradiation reportedly reduced the spread of airborne viral pathogens (‘measles’) in classrooms (Perkins et al., Reference Perkins, Bahlke and Silverman1947). In 1961, Riley demonstrated the efficacy of UV light for the control of airborne tuberculosis by showing that untreated ventilated air from wards housing infectious TB patients produced infection in guinea pigs whereas air irradiated with UV light did not (Riley, Reference Riley1961). Thereafter, the research moved from qualitative to quantitative measures of the effect of UV light on microorganisms.
In one of the first papers to quantify the genetic damage induced by UV, Miller and Plagemann (Reference Miller and Plagemann1974) calculated that a dose of 7 mJ cm−2 induced the formation of 1.7 uracil dimers per mengovirus plaque-forming unit and that dimer formation increased the production of empty viral capsids, altered protein structures and increased RNase activity. Four years later, in 1978, Sarasin and Hanawalt reported that a 10 mJ cm−2 dose resulted in seven pyrimidine dimers per simian virus 40 (SV40) genome (Kowalski, Reference Kowalski2009). With the exception of water treatment applications, relatively little research on the effect of UV light on microorganisms was published from the mid-1970s to 2000. However, concerns about bioterrorism, antibiotic-resistant bacteria and airborne spread of emerging and reemerging pathogens (e.g., pandemic influenza and the severe acute respiratory syndrome (SARS) corona virus) have stimulated renewed interest in the use of UV light as a microbial inactivant (Walker and Ko, Reference Walker and Ko2007).
The UV spectrum
The electromagnetic spectrum includes energies possessing both electrical and magnetic properties. Classified according to wavelength and photonic interaction with matter (ionizing or non-ionizing), these wavelengths vary from very high vibrational energy (1×10−7 nm) to infinitely long wavelengths and encompass all known forms of energy, from high-energy gamma rays (3×10−3 nm), microwaves to lower-energy radio waves (3×1013 nm) (Van Heuvelen, Reference Van Heuvelen1982). UV wavelengths lie between the high-energy X-rays (⩽100 nm) and the lower-energy visual spectrum (>400 nm). Wavelengths longer than 300 nm begin the visible region of the electromagnetic spectrum, followed by the infrared spectrum beginning at 700 nm, microwave frequencies (starting at 3×106 nm) and the radar/radio frequencies (3×108 nm and beyond). There is no clear delineation between X-rays and the UV spectrum; rather it is the nature of their interaction with matter that best defines the end of X-rays and the beginning of the UV spectrum. The energy–matter interactions of wavelengths less than 100 nm result in ionization (a change in atomic charge) of the exposed matter. As the wavelengths increase, the energy–matter interaction results in less ionization and more electron excitation (electrons jumping to higher-energy levels) as the energy is absorbed by molecules.
Although there are several classification schemes (Jagger, Reference Jagger1967), the UV spectrum may be most simply divided into four general classifications based on the wavelength's interaction with molecules: (1) ‘vacuum ultraviolet’ (VUV), (2) ultraviolet ‘C’ (UVC), (3) ultraviolet ‘B’ (UVB) and (4) ultraviolet ‘A’ (UVA). The VUV spectrum includes wavelengths <200 nm. The most energetic wavelengths within the UV spectrum, VUV readily interacts with oxygen atoms and their interaction with organic molecules is detrimental even at low doses; however, these wavelengths exist only in a vacuum due to the high energy. UVC encompasses wavelengths between 200 and 280 nm. This spectrum is also called the ‘germicidal’ spectrum because of its biocidal effects on bacteria (Jagger, Reference Jagger1967). UVB ranges from 280 to 315 nm and is the wavelength responsible for ‘sun burning’ the skin and the synthesis of Vitamin D (Goodsell, Reference Goodsell2001). UVA ranges from 315 to 400 nm and is the primary light produced by black light fixtures (Stowe, Reference Stowe2005). Both UVA and UVB are used in industry, e.g., to activate organic polymers used in laminates and to produce medical devices (Stowe, Reference Stowe2005). UVA and UVB, but not UVC, wavelengths, are long enough to pass through the earth's atmosphere to the earth's crust and can penetrate a short distance into the world's oceans (Jagger, Reference Jagger1967). The focus of this review is UVC and its effect on microorganisms. UVC is sometimes termed ‘ultraviolet germicidal irradiation’ (UVGI) to distinguish it from the non-germicidal wavelengths, UVA and UVB (Kowalski, Reference Kowalski2009).
UVC is biologically important because unsaturated organic compounds, i.e., compounds that are not fully saturated with hydrogen atoms or are composed of conjugated bonds, efficiently absorb wavelengths between 200 and 280 nm. Conjugated bonds hold two electron pairs, with each electron in the pair possessing an independent and opposite spin of equal energy. When a photon of UV radiation energy strikes an electron, it is induced to rise to an excited (higher-energy) level. This disruption of stable electrons can travel the entire organic structure, raising a bonded electron out of a bonding pair, and resulting in an unstable conformation. In conjugated bond-containing structures, the entire structure acts as a chromophore, i.e., the π orbitals are shared throughout the ringed structure (Jagger, Reference Jagger1967). Because of this, the entire structure absorbs the UV energy (photon) and this extra energy is drained off into the weakest bond, thereby causing conformational changes to occur (Jagger, Reference Jagger1967).
Conjugated organic structures include nitrogen-containing ring structures such as pyridines, pyrimidines, flavins and the aromatic amino acids (Jagger, Reference Jagger1967). Because these structures act as chromophores, these wavelengths can also be used for nucleic acid analysis. This process is based on the difference in absorption between nucleic acids and proteins. Essentially, this methodology relies on that fact that peptide bonds exhibit double-bond characteristics, whereas aromatic amino acids absorb UV (Jagger, Reference Jagger1967).
Measuring UV radiation: radiometry and actinometry
Measurement is the heart of science and the basis on which effects are evaluated. There are two basic ways to measure the intensity and duration of UV radiation: radiometry and actinometry.
Radiometry measures irradiance, the UV energy striking a surface from all forward angles at a point in time expressed as energy (watts) per unit area (Bolton and Linden, Reference Bolton and Linden2003). A radiometer is a sensor with an electronic readout device that displays the sensor readings. Sensors are wavelength specific and have a cosine response that accounts for the incident angle of the light source as it strikes the sensor's surface. (Note: to ensure accurate UV measurements, radiometers need to be calibrated annually.) Since UV sensors typically have a measurement area of 1 cm2, the amount of UV energy arriving at the surface is commonly measured in watts per m2 or mW per cm2. In biological experiments, it is necessary to account for both the intensity of the UV light energy (irradiance) and the length of exposure (time). Therefore, radiometers are often equipped to measure cumulative exposure over time (mW per cm2).
Actinometry is a second method of measuring UV light energy. Actinometry is based on chemical systems that undergo light-induced reactions at specific wavelengths for which the quantum yield is accurately known (Kuhn et al., Reference Kuhn, Braslavsky and Schmidt2004). The quantum yield is a measure of molecular UV-absorption efficiency of a chemical and is described as the ratio of the number of chemical changes per unit time to the number of photons absorbed per unit time (Kuhn et al., Reference Kuhn, Braslavsky and Schmidt2004):
where Φ is the quantum yield, Nc is the number of molecules chemically reacting and Np, is the number of photons absorbed.
For example, iodide/iodate solution is an actinometer commonly used in the UVC spectrum because it absorbs energy between 200 and 300 nm. The concentration of the product (triiodide) is directly proportional to the intensity, i.e., the number of photons absorbed, as measured on a spectrophotometer at 352 nm (Rahn et al., Reference Rahn, Bolton and Stefan2005). This is not to be confused with the radiometric UV dose that is independent of photon absorbance.
There are important differences between the two methods and it should be recognized that each provides results based on different parameters. Actinometry is the preferred method of measuring UV exposure in the field of photochemistry and photochemists express UV exposure in units of quantum yield. Actinometry is neither convenient nor practical in photobiology and photobiologists measure UV using radiometry and express exposure in joules per cm2. This difference in the expression of UV exposure units presents a fundamental problem in communicating results across disciplines.
UV radiation is also sometimes described in terms of fluence rate, the energy (mW) passing through a cross-sectional area (cm2), with UV dose defined as the fluence rate per unit time in seconds (s), i.e., mW per cm2 (Bolton, Reference Bolton2000). A joule is expressed as energy×time, and therefore UV dose is expressed as joules per cm2. Because radiometers are in common use and more readily implemented than chemical actinometry, the term ‘ultraviolet dose’ is sometimes used interchangeably with ‘fluence rate’, regardless of the object's ability to absorb UV radiation. Therefore, depending on the field or discipline, the terminology used may not strictly conform to the definitions of the International Union of Pure and Applied Chemistry Working Party on Ultraviolet Disinfection (Bolton and Linden, Reference Bolton and Linden2003).
Considerations in experimental UV254 photobiology
Photobiology describes the interaction between light and living matter. Bolton and Linden (Reference Bolton and Linden2003) outlined the basic requirements in the design of bench scale UV inactivation apparatus for wastewater experimentation, but these standards also apply to other types of UV light experimentation involving inactivation of microorganisms. Specifically, bench-scale UV light inactivation experiments should be reproducible across laboratories and contain the basic elements of good experimental design. Results that provide a foundation for forward progress will also use accepted terminology to describe the experimental design and results. Thus, the terminology presented in this review is based on the recommendations of the International Union of Pure and Applied Chemistry Working Party on Ultraviolet Disinfection (Bolton and Linden, Reference Bolton and Linden2003).
The single most significant physical factor in UV254 inactivation is lamp design and performance (VanOsdell and Foarde, Reference VanOsdell and Foarde2002). In microbiology, the majority of work involving photobiology involves ‘germicidal’ UVC, with UV254 being the wavelength considered to possess the strongest inactivating effect. Importantly, VanOsdell and Foarde (Reference VanOsdell and Foarde2002) noted that for a UV254 system to efficiently inactivate pathogens, the bulb design (type of quartz, internal gas composition, operating temperature and ballast type) must be matched with the environmental conditions (temperature and relative humidity).
The first mercury vapor arc lamp was developed by Wheatstone in 1835 (cited by Kowalski, Reference Kowalski2009). Other metallic gases (zinc, iron or xenon) can be energized into UV emission, but excited mercury gas is the most efficient UV emitter and is used extensively in UV254 bulbs. Mercury gas UV emitters are produced as low-, medium- and high-pressure bulbs. Low- and medium-pressure mercury lamps consist of electrodes that produce electrons that collide with mercury atoms causing them to emit photons, predominately at 253.7 nm (Jagger, Reference Jagger1967). High-pressure mercury lamps, while similar in design, use electrodes capable of high voltage, thus allowing for an increase in the efficiency of emitted photons (Jagger, Reference Jagger1967).
Low-pressure bulbs (internal pressure of less than 1 bar) operate at a low surface temperature and emit monochromatic (UV254) wavelengths (Fig. 1). Medium-pressure bulbs (internal pressure slightly higher than 1 bar) operate at higher surface temperature and emit polychromatic light (Fig. 1). To eliminate undesired UV wavelengths, the synthetic quartz containing the vaporized mercury atoms can be treated with wavelength-dependent UV -absorbing components that block specific wavelengths from exiting the bulb. Proper temperature control must be maintained when using medium-pressure vapor lamps because the lamps produce heat and the output (irradiance) of these lamps is temperature sensitive. As a result, these lamps require additional power to increase the internal temperature and pressure of the lamp. This increased temperature allows for an increased UV light spectrum, but until the bulbs reach operating temperature, their output fluctuates. For this reason, under experimental conditions, a shutter system is needed so that light-emitting bulbs reach peak performance prior to target exposure (Bolton and Linden, Reference Bolton and Linden2003).
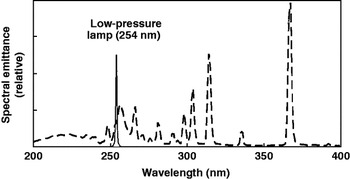
Fig. 1. Spectral output of low- and medium-pressure UV lamps. Low-pressure bulbs (solid line) are monochromatic (UV254), whereas medium-pressure lamps (dashed line) are polychromatic (200–400 nm). Figure adapted from Bolton and Linden (Reference Bolton and Linden2003). Reprinted by permission of the publisher.
Under experimental conditions, the structure of the UV-emitting apparatus must be designed to provide a spatially homogeneous field of irradiation. This can be accomplished through the use of a collimating tube (Bolton and Linden, Reference Bolton and Linden2003; Thurston-Enriquez et al., Reference Thurston-Enriquez, Haas, Jacangelo, Riley and Gerba2003; Shen et al., Reference Shen, Fang, Bergstrom and Blatchley2005). However, depending on the experimental design, a collimating tube may not be required. That is, in the absence of reflected UV energies and with irradiance from one plane only, the cosine sensors will account for any incident irradiation. Sensors for measuring UV and holding treatment samples should be secured to a thermally and physically stable exposure stage. For accurate measurements of UV exposure, sensors must be placed at the same distance from the energy source as the irradiated sample and absorbance of UV by the sample matrix must be taken into account. Bolton and Linden (Reference Bolton and Linden2003) consider it necessary to stir the solution to ensure uniform UV dose, but small volumes or a matrix that does not readily absorb UV need not be stirred.
Lambert–Beer law
In brief, this law states that some of the UV254 energy to which the target is exposed will be absorbed by the surrounding environment and this absorption must be taken into account. For microorganisms in suspension (liquid or aerosol), the average UV254 intensity may be calculated as follows (Thurston-Enriquez et al., Reference Thurston-Enriquez, Haas, Jacangelo, Riley and Gerba2003):
where I average is the average UV254 intensity (milliwatts per square centimeter), ae is the absorbance of the virus suspension to the base e,
I 0 is the UV254 intensity after passing through solution and
L is the depth (cm) of the solution irradiated by the UV254 energy.
For liquid media, the amount of UV energy absorbed by the solution can be determined by measuring the amount of UV light that passes through the matrix using a quartz cuvette equal to the depth of the sample (Lambert–Beer's law). Jagger (Reference Jagger1967) provides information on the absorption coefficients for various solutions, but only empirical data will provide the researcher the information needed to determine if stirring is necessary.
Mechanism of inactivation by UV radiation
Inactivation of microorganisms by UV light is initiated at the quantum level. The quantum yield is the number of photons or the photon density, impacting a surface area. Each photon carries an amount of energy called a quantum (ε) determined from quantum mechanics (Jagger, Reference Jagger1967; Kowalski, Reference Kowalski2009):
where ε is the energy in one photon, h is the Planck's constant, 6.626×10−34 joules (J) and ν is the frequency in hertz (Hz).
Much of the quantal information was determined in the 1960s, when researchers on UV radiation focused on the mechanism of inactivation. During this time, researchers estimated the quantum yields required for dimerization of nucleic acids (Shore & Pardee Reference Shore and Pardee1956; Kleczkowski, Reference Kleczkowski1963). Thus, Kowalski (Reference Kowalski2009) calculated that UV light at a wavelength of 253.7 nm has a frequency of 1.18×1015 Hz and 7.819×10−19 J of energy per photon. It follows that each joule contains 1.279×1018 photons and a UV light dose of 1 mJ cm−2 will produce 1.279×1015 photons cm−2. Thus, a microorganism with a diameter of 0.1 μm, i.e., a cross-sectional area of 3.14×10−14 m2 (3.14×10−12 cm2), will be subjected to the passage of about 401,000 photons per second (Kowalski, Reference Kowalski2009).
UV energy at 254 nm readily affects the double-bond stability between adjacent carbons. There are two types of molecular bonds occurring in conjugated organic structures, the sigma (σ) orbitals and the Pi (π) orbitals. The higher-energy sigma orbitals (shorter wave function) are located closer to the nucleus of the two bonded atoms. The Pi orbitals are of lower energy and are non-localized about the bonded pair. The lower-energy Pi orbitals are more stable and, therefore, have longer wave function (Kowalski, Reference Kowalski2009). Conjugated ring structures, like the pyrimidines, purines and the aromatic amino acids have large, non-localized Pi orbitals (Smith and Hanawalt, Reference Smith and Hanawalt1969). When an incoming UV254 photon strikes a Pi orbital, the photon's energy is converted to vibrational energy (Kowalski, Reference Kowalski2009). If this vibrational energy is sufficient, the Pi orbital is pushed into a transient unstable state that exists for a femtosecond (10−15 s). This unstable state must return to the ground state either by dissipation of the energy or through modification of the bond by rotation (Kowalski, Reference Kowalski2009).
Unsaturated organic compounds are essential to cell reproduction and cell metabolism. Unsaturated organic compounds vulnerable to UV254 inactivation include pyrimidines, purines and flavin. Pyrimidines provide the basic structure for nucleobases uracil (a component of tRNA), thymine (a component of DNA and tRNA) and cytosine (a component of DNA and RNA). Purines provide the basic structure for nucleobases adenine and guanine (in DNA and RNA) and the aromatic amino acids, phenylalanine and tyrosine. Flavin is an unsaturated organic compound found in flavin adenine dinucleotide (FAD), a molecule that is necessary for metabolic redox reactions. FAD is an important hydrogen acceptor in the cell and ATP is generated as a result of the oxidation of FADH.
Nucleic acids are composed of bases, sugars and phosphates. Photons affect DNA and RNA by inducing molecular transformation, i.e., photoproducts, of the genetic material. The sugars and phosphate groups do not absorb wavelengths above 210 nm, but conjugated bases have peak absorption of UV light energy at 260 nm, with pyrimidines being 10 times more sensitive to UV254 than purines (Jagger, Reference Jagger1967). It follows that uracil and cytosine in RNA and thymine and cytosine in DNA are the targets of UV254 inactivation. Whether the target is RNA or DNA, the mechanism of UV254 inactivation is hydration of the base or base dimerization (Jagger, Reference Jagger1967). There are six possible photoproducts induced by UV light: (1) thymine–thymine dimer, (2) cytosine–cytosine dimer, (3) cytosine−thymine dimer, (4) uracil–uracil dimer, (5) uracil–thymine dimer and (6) uracil–cytosine dimer (Kowalski, Reference Kowalski2009). The photoproducts requiring the least energy are the thymine-complex dimer and the uracil-complex dimer (Jagger, Reference Jagger1967; Kowalski, Reference Kowalski2009). Cytosine hydrate, another UV254-induced photoproduct, occurs in RNA and single-stranded DNA (Smith and Hanawalt, Reference Smith and Hanawalt1969). This structure requires more energy, but is formed when UV254 irradiation of cytosine yields 6-hydroxy-5,6-dihydrocytosine (O'Donnell et al., Reference O'Donnell, Boorstein, Cunningham and Teebor1994). In DNA, the thymine dimer is the photoproduct with the highest quantum yield (Kowalski, Reference Kowalski2009). These dimers occur when the hydrogen bonds linking the thymine bases are lost and the respective 5 and 6 carbon atoms are cross-linked.
The biological effects of UV254 exposure are reversible. In bacterial cells, dimer formation is reversible via absorption of wavelengths between 300 and 500 nm (photo reactivation) or by photolyase enzymes that split the dimers. DNA viruses utilize host cellular polymerase enzymes to excise dimers and replace the damaged DNA (Kowalski, Reference Kowalski2009). Generally, viruses do not produce their own photolyases. One exception is the fowlpox virus, the only virus known to code for its own photolysase enzyme production (Srinivasan et al., Reference Srinivasan, Schnitzlein and Tripathy2001).
The UV inactivation of microorganisms can be achieved with either monochromatic or polychromatic emitters. Monochromatic lamps producing primarily UV254 are routinely used to inactivate microorganisms. Compared to monochromatic lamps, polychromatic lamps may possess greater efficiency (Linden et al., Reference Linden, Thurston, Schaefer and Malley2007). For example, Eischeid et al. (Reference Eischeid, Meyer and Linden2009) reported low-pressure monochromatic UV lamp doses of 30, 50 and 80 mJ cm−2 resulted in (2 log), (3 log) and (4 log) reduction of adenovirus type 2 virus, respectively. In contrast, doses of 10 and 25 mJ cm−2 from medium-pressure polychromatic UV lamps resulted in (2.5 log) and (4.5 log) adenovirus reductions, respectively. Presumably these differences in inactivation reflect the fact that monochromatic UV wavelengths only caused genetic damage, whereas polychromatic wavelengths also affect aromatic proteins. That is, the structure and function of microbial proteins depend on their primary, secondary and tertiary structures, which reflect their constituent amino acids. It is estimated that one in 10 amino acids is susceptible to photochemical processes and, hypothetically, the photochemical alternation of any of these amino acids could affect protein structure and function (Jagger, Reference Jagger1967). Therefore, the efficiency of UV light inactivation could be increased through the use of medium- or high-pressure polychromatic bulbs, but this gain is generally offset by the additional expense of operating this equipment.
Principles of inactivation by UV radiation
The inactivation kinetics of UV radiation can be described as a first-order chemical reaction. That is, the amount of reagent (UV irradiance) will equal the amount of product (modified conjugated bonds) in a given time period. The Stark–Einstein law states that if a photon is absorbed, then only one photon should be required for the formation of one photoproduct. This law is the foundation of the ‘one-hit’ (first-order) kinetics that have historically been used to describe UV254 inactivation (Hiatt, Reference Hiatt1964; Qualls and Johnson, Reference Qualls and Johnson1983; Thurston-Enriquez et al., Reference Thurston-Enriquez, Haas, Jacangelo, Riley and Gerba2003; Kowalski, Reference Kowalski2009).
Grotthus–Draper law
The Grotthus–Draper law states that photons must be absorbed for a photochemical reaction to occur (Kowalski, Reference Kowalski2009). Following this line of thought, Bolton and Linden (Reference Bolton and Linden2003) suggest that the term ‘ultraviolet dose’ should be used to describe the total energy absorbed by the target. Problematically, energy striking an object is not necessarily absorbed by the object (Jagger, Reference Jagger1967) and absorbed photons may not produce a photochemical reaction (Kowalski, Reference Kowalski2009). Currently, the only method to measure the absorptive efficiency of a conjugated organic molecule is actinometry.
Bunsen–Roscoe reciprocity law
The Bunsen–Roscoe reciprocity law states that microbial inactivation is dependent on dose and dose is the product of UV intensity expressed in mW per cm2 and exposure time expressed in seconds (Riley and Kaufman, Reference Riley and Kaufman1972).
where D is the UV dose, I is the irradiance (intensity) in mW per cm2 and T is the exposure time in seconds.
The Bunsen–Roscoe law is important and relevant to microbial inactivation because it shows that, although the UV irradiance drops as the target moves further away from the source (except in a vacuum), the desired UV dose can be achieved by increasing the exposure time. This law is fundamental because it allows for the comparison of results from experiments using different UV equipment types, wattages and conditions, when the exposure time is known.
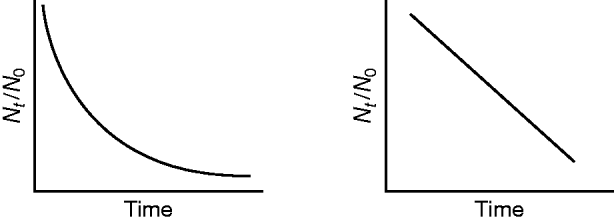
Chick's law
Chick's law states that as disinfectant contact time (t) increases, the ratio of viable microorganisms (Nt) to the total (N 0) microbes at time zero decreases. Chick's law was originally used to describe the relationship between chlorine and the inactivation of microorganisms in wastewater (Rubin and Elmaraghy, Reference Rubin and Elmaraghy1977). Figure 2 illustrates first-order inactivation kinetics where absorbance of one photon results in inactivation.

Fig. 2. Effect of disinfectant contact time on the proportion of remaining viable microorganisms (Chick's law). Non-transformed data are shown on the left; log10-transformed data on the right.
Since the 1950s, inactivation constants (k) derived using Chick's law have been used to measure the sensitivity of microorganisms to UV inactivation, with larger inactivation constant values (larger slope) indicative of greater susceptibility to UV inactivation. The following equation is a modification of the formula used by Tseng and Li (Reference Tseng and Li2005) to solve for the inactivation constant (k):
where k is the inactivation constant, N 0 is the quantity of microbes at time zero, N t is the quantity of microbes at UV254 exposure time ‘t’ and Dose is the ultraviolet light dose.
One-stage versus two-stage inactivation
Hiatt (Reference Hiatt1964) published the first work detailing the kinetics of microbial activation and noted that the ‘first-order’ inactivation model, i.e., the single-hit hypothesis proposed by the Stark–Einstein law and described by a single inactivation constant (k), was accurate only if the exposed viral population was homogenous and inactivation did not require cumulative damage. Hiatt (Reference Hiatt1964) also described the classical inactivation curve dynamics of ‘shouldering’ and ‘tailing’. ‘Shouldering’ refers to an increase of UV254 dose with no corresponding increase in microbial inactivation, whereas ‘tailing’ is a decrease in UV254 dose with no corresponding decrease in microbial inactivation (Hiatt, Reference Hiatt1964). In contrast to the one-stage inactivation model, the two-stage model proposes that exposure of microbial populations to an inactivating agent may reveal two subpopulations: one subpopulation (f) more susceptible to inactivation and a second subpopulation (1–f) more resistant (Hiatt, Reference Hiatt1964; Cox, Reference Cox1976; Kowalski et al., Reference Kowalski, Bahnfleth and Carey2002).
where Nt is the quantity of virus in the test sample after treatment with Doset, N 0 is the quantity of virus in the unexposed control sample, f is the resistant fraction of the total initial virus population with inactivation rate K 2, (1–f) is the susceptible virus population fraction with inactivation rate K 1, k 1 is the inactivation rate of the inactivation curve for the ‘fast decay population’, k 2 is the inactivation rate of the inactivation curve for the ‘resistant population’ and Doset is the UV254 intensity×time.
In practice, the two-stage inactivation analysis should be utilized when the statistical analysis indicates that the data are better described by two-stage versus one-stage inactivation kinetics.
Applications of UV light to the inactivation of microorganisms
Laboratory use
The classic application of UV is the inactivation of microorganisms in biological safety cabinets. In many laboratories, turning on the UV lamp after using the cabinet is standard operating procedure. The current version of the National Science Foundation (NSF) International Standard 49 (Section 5.25.2) does not mandate the use of UV light in biosafety cabinets (Meechan and Wilson, Reference Meechan and Wilson2006). Further, the Centers for Disease Control and Prevention states that the use of UV light in biosafety cabinets is neither recommended nor necessary (CDC, Reference Chosewood and Deborah2009). UV inactivation of microorganisms on surfaces is ancillary to standard chemical disinfection and should not be relied upon as the sole method of disinfection.
Food processing
In the food-processing industry, germicidal UV light has shown potential for the surface disinfection of fresh-cut fruit and vegetables. In studies on carrots (Mercier et al., Reference Mercier, Arul, Ponnampalamm and Boulet1993), grapes (Nigro et al., Reference Nigro, Ippolito and Lima1998), sweet potatoes (Stevens et al., Reference Stevens, Khan, Lu, Wilson, Chalutz, Droby, Kabwe, Haung, Adeyeye, Pusey and Tang1999) and spinach leaves (Artés-Hernéndez et al., Reference Artés-Hernéndez, Escalona, Robles, Martínez-Hernández and Artés2009), treatment with UVC was shown to reduce product deterioration and prolong storage life. Alone or in combination with ozone, UV light-reduced microorganisms in water used to wash fresh-cut onion, escarole, carrot and spinach (Selma et al., Reference Selma, Allende, López-Gálvez, Conesa and Gil2008). UV light may provide a viable alternative to chemical sanitizers, e.g., titanium dioxide (TiO2) or chlorine. Currently, there is interest in developing non-thermal methods for the sterilization of juices, an objective in which UVC may play a role (Guerrero-Beltrán and Barbosa-Cánovas, Reference Guerrero-Beltrán and Barbosa-Cánovas2005).
Water treatment
The application of UV light to food preservation is a relatively recent development, but the use of UV light for the treatment of water has an extensive history: the first system for UV treatment of potable water went into operation in Marseilles, France in 1910 (cited by Kowalski, Reference Kowalski2009). UV treatment of water was not widely implemented at the beginning of the twentieth century for a variety of reasons, including high operating costs, issues with equipment reliability and maintenance, and the availability of cost-effective, chemical water treatment systems (Wolfe, Reference Wolfe1990). UV light is regarded as broadly effective against all human pathogens (bacterial, viral and protozoal) transmitted in water (Hijnen et al., Reference Hijnen, Beerendonk and Medema2006) and guidelines for the treatment of waste water and potable water with UV light have been established (Environmental Protection Agency, 2003). UV treatment of water (potable and wastewater) is increasingly common because the technology is readily available, the process is effective against a wide range of microorganisms, overdose is not possible, chemical residues or by-products are avoided and water quality is unaffected (Wolfe, Reference Wolfe1990; Hijnen et al., Reference Hijnen, Beerendonk and Medema2006).
Bioaerosols
A wide variety of fungal, bacterial and viral pathogens may be transmitted by airborne droplets or droplet nuclei (Tang et al., Reference Tang, Eames, Chan and Ridgway2006; Blachere et al., Reference Blachere, Lindsley, Slaven, Green, Anderson, Chen and Beezhold2007). Airborne pathogens of humans include major emergent and re-emergent agents, e.g., Mycobacterium tuberculosis, influenza viruses, SARS corona virus, Aspergillus spp., Legionella spp. (Douwes et al., Reference Douwes, Thorne, Pearce and Heederik2003; Wong and Yuen, Reference Wong and Yuen2006; Escombe et al., Reference Escombe, Oeser, Gilman, Navincopa, Ticona, Pan, Martínez, Chacaltana, Rodríguez, Moore, Friedland and Evans2007). Likewise, some of the most economically significant pathogens of animals are transmitted via aerosols, e.g., foot and mouth disease and porcine reproductive and respiratory syndrome virus (Alexanderson et al. Reference Alexanderson, Brotherhood and Donaldson2002; Hermann et al., Reference Hermann, Muñoz-Zanzi and Zimmerman2009). Regardless of the actual level of risk bioaerosols present to the public, events of recent history have raised society's awareness and concern. In the market place, UV emitters designed for residential application, e.g., portable units or those for ductwork insertion, and commercial application, e.g., upper room air, ceiling mount or cooling-coil disinfection are available, despite the fact that data on the efficacy of these systems are sparse (Environmental Protection Agency 2006a, b, c, d).
UV light has successfully reduced the concentration of airborne microorganisms in targeted applications. Berg et al. (Reference Berg, Bergman and Hoborn1991) found that UV irradiation of air in operating rooms during surgery significantly reduced the number of viable airborne bacteria collected at the edge of the surgical site. Likewise, installation of UV light in air-handling units and ventilation systems reduced the concentration of airborne bacteria and fungi in indoor air (Menzies et al., Reference Menzies, Pasztor, Rand and Bourbeau1999; Levetin et al., Reference Levetin, Shaughnessy, Rogers and Scheir2001; Menetrez et al., Reference Menetrez, Foarde, Dean and Betancourt2010).
Moving beyond narrowly focused applications, the use of UV light for the routine inactivation of airborne microorganisms faces severe technical challenges. In the first place, the inactivation kinetics of most airborne pathogens is not known for the range of environmental conditions (temperature and relative humidity) under which such a system would need to function. This is a significant deficit because environmental conditions are known to affect UV light, e.g., as relative humidity increases, UV light becomes less efficient (Ko et al., Reference Ko, First and Burge2000; Peccia et al., Reference Peccia, Werth, Miller and Hernadez2001; VanOsdell and Foarde, Reference VanOsdell and Foarde2002; Lai et al., Reference Lai, Burge and First2004; Tseng and Li, Reference Tseng and Li2005; Walker and Ko, Reference Walker and Ko2007). Thus, acquiring baseline exposure doses to target UV exposure levels is the first priority. Beyond this, delivery of the inactivating dose uniformly and consistently to large volumes of air is a significant challenge given the current state of the technology. At present, UV inactivation of bioaerosols can only be considered one part of an overall biocontainment plan, rather than a stand-alone solution (Memarzadeh et al., Reference Memarzadeh, Olmsted and Bartley2010).
Conclusions
The use of UV light for the inactivation of microorganisms is appealing because it is a familiar, commercially available technology that does not involve the use of chemicals. Some applications are well developed, e.g., water treatment. Others show future promise, such as applications in food processing. One highly desirable application, the routine use of UV for the inactivation of microorganisms in aerosols, will require extensive development and ultimately may only function effectively in tandem with other technologies, e.g., with photocatalysis, where UV energies are used to induce the formation of metallic oxides and superoxides, rather than serving as the primary source of inactivation or with filtration where the UV energy is provided post-filtration.