The history of congenital interventional cardiology has seen numerous groundbreaking innovations typically related to the introduction of a new device or a novel treatment technique. Similarly, imaging of cardiac defects has changed dramatically over the past decades, although some of the advancements have seemed to omit the catheterisation laboratories. Rotational angiography, one of the imaging techniques for guidance of cardiac catheterisation currently referred to as “advanced”, in fact was described already in 1960s.Reference Snider, Klopfenstein, Mendelsohn and Crow1 More recently its improved version, including three-dimensional reconstruction (3DRA), became a valuable intra-procedural imaging tool in interventional cardiology and neuroradiology.Reference van der Stelt, Siegerink, Krings, Molenschot and Breur2 Dr Evan Zahn was one of the pioneers of 3DRA in the field of congenital cardiology, setting an example for many to follow. With his innovative publication and subsequent lecture at 2011 Pediatric and Adult Interventional Cardiac Symposium (PICS-AICS) on “The Emerging Use of 3-Dimensional Rotational Angiography in Congenital Heart Disease” he motivated many to explore benefits of this modality to strive for improved procedural outcomes and reduced patients’ burden of cardiac catheterisationReference Zahn3. I was one of those to take Dr Zahn’s thoughts and implement them into routine workflow.Reference Góreczny, Dryżek and Moszura4–Reference Góreczny, Dryżek, Moll and Moszura6 However, almost a decade after Dr Zahn shared his important work, despite tremendous efforts by teams from Utrecht, (Netherlands) and Columbus (Ohio, United States of America) to popularise 3D imaging in catheterisation laboratory during dedicated meetings, two-dimensional (2D) angiography does not seem to be threatened in many, otherwise-progressive, laboratories. During the recent 30th Japanese Pediatric Interventional Cardiology (JPIC) meeting I had the opportunity to ask Dr Zahn why giving up knowledge is almost never a good idea, what is technology’s natural order of things, and why the technology has to be more than just exciting, pretty, and new.
Sebastian Góreczny: What is your experience with 3D guidance for cardiac catheterisation?
Dr Evan Zahn: Most of our experience with 3D guidance with cardiac catheterisation revolves around 3DRA.Reference Berman, Khan, Gutierrez and Zahn7 We certainly use a great deal of computed tomography (CT) and magnetic resonance imaging (MRI) and stereolithography (SLA) printed models for planning procedures, in another words before we get to the cath lab.Reference Phillips, Nevin, Shah, Olshove, Garg and Zahn8 We really haven’t been very active in importing images to the cath lab even though we greatly value what 3D assessment of anatomy, particularly complex anatomy, brings to the table.Reference Fagan, Truong and Jone9,Reference Hascoët, Warin-Fresse and Baruteau10 Very early on, when I was still in Miami, we got very interested in obtaining 3D angiograms. We started off simply looking at single ventricle patients, where it is easy to acquire images because of slow, non-pulsitle blood flow, and we gradually moved on to more complex patients trying to figure out the correct recipes to obtain the best image quality we could.Reference Glöckler, Koch and Halbfaß11,Reference van der Stelt, Krings, Molenschot and Breur12 I was always very interested, from the beginning, in using this technology to assess the right ventricular outflow tract (RVOT). This was several years ago, just before we started Melody (Medtronic) valve in the United States, but we knew, while that would be dealing with relatively simple RVOT like conduits and eventually bio-prosthetic valves, someday in the future we’d be dealing with more complex RVOT, things like trans-annular patch, and other very irregular shapes. We felt that assessing these with just 2D angiography would be somewhat challenging and would not be as precise as we would like. We really aimed our sights at how we assess high-flow situations of irregular, unpredictable shapes like the so-called “native” RVOT in the cath lab and that led us to 3DRA. We have had a fairly broad experience with that, both in Miami and now in Los Angeles, and it is our sense at least, that while you don’t need 3DRA on every patient, we have found it to be greatly beneficial in many situations.Reference Zahn3,Reference Berman, Khan, Gutierrez and Zahn7 It has really become a part of our routine workflow for things like trans-catheter implantable valves, aortic interventions, particularly complex arch interventions and coarctation. We find it incredibly useful, and it gives us much more data, than we could have ever hoped for from a simple 2D angiogram. We learned a lot early on even looking at a simple structures like the pulmonary artery after a Fontan procedure.Reference Zahn3 Just looking from the front with a little angulation or the side with a little angulation wasn’t really enough, when the stenosis was from the back to the front and you needed to be looking from a superior-inferior perspective (Fig 1). Three-dimensional imaging gives you things like this that we can’t really get in the cath lab with any other technology short of importing CT or MRI images. So mostly, when I use 3DRA, I will typically perform this injection early in the procedure and utilize what I have learned from it to guide any subsequent angiograms, interventions, catheter guidance, etc.
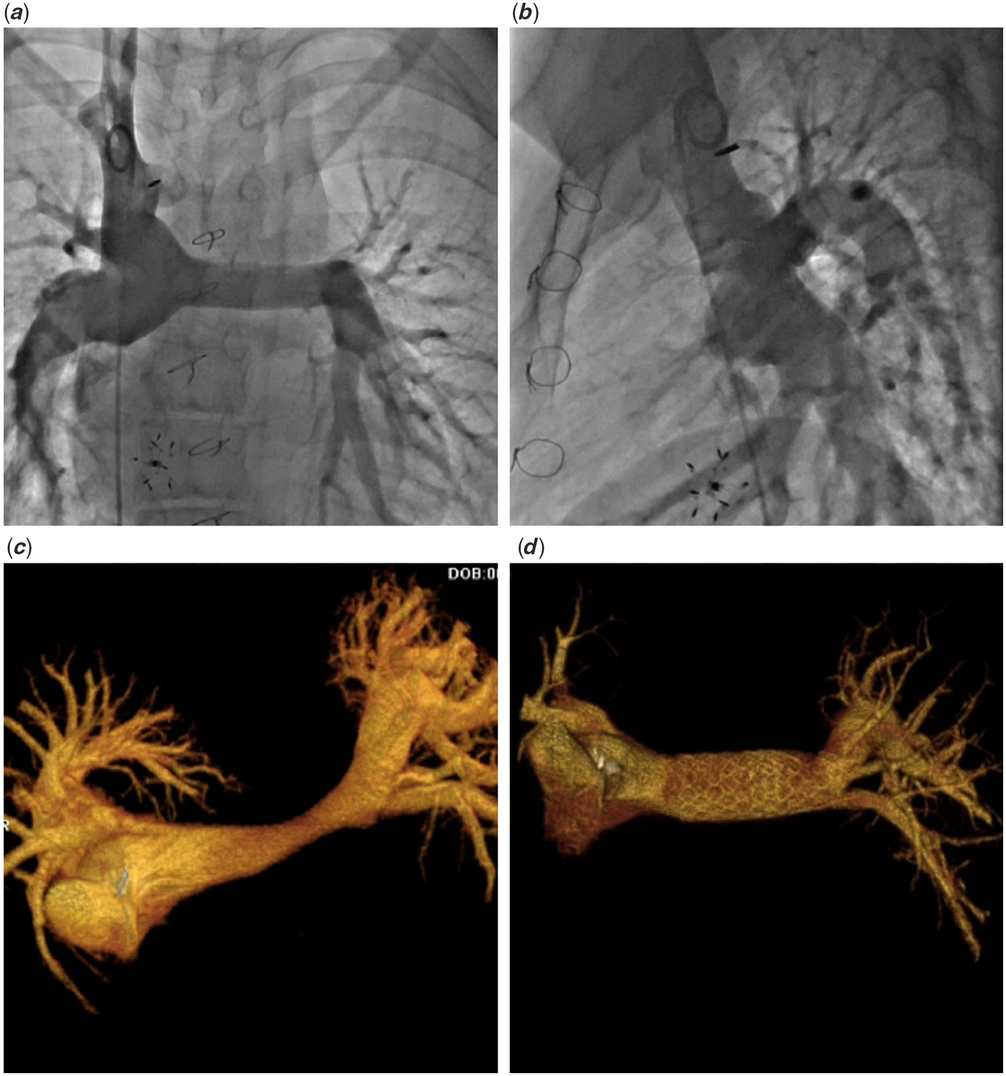
Figure 1. A traditional bi-plane two-dimensional angiography in a patient after total cavo-pulmonary connection shows unobstructed flow through both pulmonary arteries (a and b). Three-dimensional reconstruction from rotational angiography provides views unattainable with 2D angiography allowing for diagnosis of a dog-bone like left pulmonary artery stenosis (c) and evaluation of stent implantation (d).
Could you point out the major benefits of 3D imaging?
Let’s go back and think what we do with 2D imaging. We take a picture from here, e.g. frontal projection and a picture from here, e.g. lateral projection, and we’re thinking about what a 3D structure is based on these 2D images. Most of the time maybe that is fine, but I think we are fooling ourselves if we think we have a clear picture of the anatomy. You have heard me speak, we have numerous examples where 2D angiography just misses things. We have published this with a high rate of underdiagnosing pulmonary artery stenosis.Reference Berman, Khan, Gutierrez and Zahn7 As we enter the age of more customisable devices, and complex interventions, we want to be able to more precisely choose the ideal device for a particular anatomy. I don’t think we will be always making 3D physical models for every case as it is too cumbersome.Reference Góreczny, Ivy and Anderson13 You are going to take an individual patient to the cath lab, you are going to do angiograms, and you are going to decide what device is best for them, what make, what shape, what design characteristics and what size. With 2D imaging we are going to have a problem when we start looking at increasingly complex anatomy with a consideration for intervention and guessing from a simple 2D image. There is overlap, you are not really profiling. What I get out of 3D and it is not just the 3D reconstruction, the pretty picture, I actually get as much or more from CT-like tomograms allowing me to slice through complex structures. I really feel that at some point people will really want that information. So where I really find this useful: complex aortas, aortas that have been rebuilt and are very tortuous.Reference Góreczny, Dryżek and Moszura4,Reference Moszura, Góreczny, Dryzek and Niwald5,Reference Stenger, Dittrich and Glöckler14 –Reference Sandoval, Aristizabal and Zabal-Cerdeira17 It is very hard to get those profiled with 2D angiography, and with 3D it is very simple (Fig 2). Right ventricular outflow tracts, again very tortuous, easy to get that picture and pulmonary arteries, particularly when they are behind or related to a reconstructed aorta, like after Norwood operation.Reference Góreczny, Dryzek and Moszura18–Reference Góreczny, Zablah, McLennan, Ross and Morgan21 We know that compression here is from front to back, which means the best way to look at that is from head to toe and you just can’t do that with 2D angiography. That is where I find it most useful.

Figure 2. Three-dimensional reconstruction from rotational angiography in a patient after Norwood procedure with arch obstruction provides multiple views allowing for better understanding of the lesion including relation not the nearby branches (a–c). Additional “cut plane” tool reveals the full extent of the narrowing (d).
What are the limitations of current 3D guidance techniques?
I think the biggest limitation at this point is that we are obtaining static images. People need to understand what they are looking at. I get asked all the time, “how do you know that if you take a quantitative measurement of something in systole and in diastole and obviously with 3D reconstruction or even a tomographic slice from that accusation, you are looking at a point in time, much like in a CT scan or an MRI?” I think you have to understand what you are looking at. I love to imagine a point in the future where we are looking at four-dimensional rotational angiography where we actually have these 3D structures that are moving with the cardiac cycle, that are gated. I know that technology is available now but not widely.Reference Lescher, Gehrisch, Klein and Berkefeld22 We certainly are not doing that routinely now. The other limitation is that in terms of overlying fluoroscopy, I don’t think it is incredibly useful to overlay a static image on your dynamic fluoroscopy, your beating fluoroscopy, especially within the heart and especially with some patients where we have tremendous variability in systole and diastole.Reference Góreczny, Dryżek and Moszura23 I would say, for me at this point, the biggest limitation is probably that I am dealing with static images. I think for people just starting out with 3D technology the limitation is that there is a learning curve to acquire the images, which I think has been greatly over exaggerated. We do lots of things in the cath lab that at first look cumbersome and then become second nature. I think people have stayed away from 3DRA because they feel there is this huge learning curve, and it takes time. In our cath lab, setting up for a 3DRA really does not take any longer than for a 2D angiogram but that took a little time to get that proficient at it so you have to invest yourself and your team in accepting that there is a short learning curve.
In some of the examples you mentioned, if not all, relevant information may be obtained from pre-catheter imaging
All of the examples I gave can be obtained from pre-cathater imaging. The question becomes, when you want to intervene on something in the cath lab, what’s the best way to image this both before and after a given intervention. Let’s take a common thing, left pulmonary artery stenosis, with a single ventricle after a Norwood stage 1 procedure. It looks great on anterior-posterior (AP), looks OK on the lateral but when you look at it from top to bottom, it looks like a ribbon, and it is compressed. Additionally, you want to know the influence that stenting this lesion might have on the left main stem bronchus; we know this is important. Yes, you can see all that on CT, but the problem is when you want to do your intervention in the cath lab and then evaluate if your intervention worked, what’s your post-intervention angiogram going to be? If your AP and lateral looked fine, the problem was on your CT, then you do this intervention, you put a stent in, how are you going to look at it? Are you going to wait and send the patient to the CT scanner? Again, I think using CT and MRI in the cath lab is genius and I think we should do more of it, but what is easier for my workflow is 3DRA and I find that very beneficial.Reference Góreczny, Dryżek and Moszura23–Reference Góreczny, Moszura, Lukaszewski, Podgorski, Moll and Dryzek26 I find it as beneficial for post-intervention as I do for pre-intervention for aortas. We have had several cases where we caused small but not insignificant aneurysms after interventions completely missed on 2D angiography but with 3DRA we can see it quite clearly (Fig 3). It is much more sensitive for pseudo or aneurysm formation. Again, we get away with using limited knowledge most of the time, but in my opinion people are giving up knowledge, which is almost never a good idea. We have now gotten the dose down, the technique down, the contrast load down, so there is no longer a good reason for me to say, well I’d rather go with less knowledge.Reference Góreczny, Moszura and Dryzek20,Reference Manica, Borges, Medeiros, Fischer Ldos, Broetto and Rossi Filho27 –Reference Ehret, Alkassar and Dittrich30 That’s just never been a good idea.

Figure 3. A bi-plane two-dimensional angiography in a patient after stenting of aortic coarctation shows unobstructed flow through the stent (a and b). Three-dimensional reconstruction from rotational angiography reveals vessel dissection (white arrow) not visible in traditional angiography (c and d).
It has almost been a decade since your lecture on 3DRA in congenital interventions at 2011 PICS in Boston, and still in many centres worldwide 2D imaging is the main way of guidance for cardiac catheterisation. What is the reason for that?
It is a very intriguing question, and I certainly don’t think I have the answer. I have some ideas. I have been a little bit fascinated by this. When I first saw that we could obtain a 3D image in the cath lab, in fact I didn’t see this in cath lab, I saw it in neuro-radiology suites, and my first thought was “Oh my gosh, we need to have this! What specialty needs this more than congenital heart disease, where there is so much variability and it is so complex?” I would have guessed there would have been, in fact probably in that lecture I had a slide that said, I predict that in 10 years we will be just doing the majority of our cases using 3D, single plane angiography, and clearly I misjudged the field. I think part of it is the start-up and the learning curve. I think people are busy and they are learning new procedures and things like that. There are several lesions where at least 3DRA has not really proved beneficial, shunt lesion in particular, things like ventricular septal defects, were it would be really nice to have a 3D accurate image from an angiogram. You would not have to guess the gantry angles depending on where the whole was in the septum, if you are trying to close it. Shunt lesions are somewhat heard to capture consistently. Guessing what the field as a whole is thinking, I guess they just don’t see the value in 3DRA just yet. People forget; when digital acquisition came along, in the late 1980s and early 1990s, it didn’t take 10 years but it took some time to be universally accepted and widely adopted in clinical practice. Some of the icons of the field at that time, I was very young and I remember speaking to them: “This will never pan out. This 30 frames per second (now we do 15 and 7 frames) is not good enough for congenital heart disease. The heart rate is too fast, we’re going to miss too much information and we will all be going back to 60 frames per second cine angiography”. Clearly that didn’t happen. Part of it may just be that the field is a little bit slow to adapt, to change. I can’t help but think that people just haven’t seen the utility in it, which is hard for me to understand because I clearly do.
What will be the role of other imaging tools like holography or virtual reality for guidance of cardiac catheterisations in the coming years?
I don’t know. I’m intrigued to see; I have been following from afar. Unless it offers the operator something remarkably unique when compared with other 3D imaging, I don’t know what the penetrance will be and how well it will be adapted. It’s certainly intriguing. Again, with these new technologies, intravascular ultrasound (IVUS) is a good example; IVUS is a fantastic technology and has helped millions of people in the adult cardiology world but it never really found its home in congenital heart disease. So the technology is there, but IVUS does not get used in most congenital heart labs.Reference Zbroński, Tomkiewicz-Pająk, Kochman and Huczek31 I think holography may have a huge role, but it will have to prove its benefit to the operator in treating his or her patient. Clearly that hasn’t happened yet with 3DRA or else it would have been widely adopted. People haven’t bought into that it will help them and their patients. I believe doctors will migrate to anything that they think helps their practices, anything that helps them to be better operators and take better care of patients. But the technology has to be more than just exciting and pretty and new, except for a small few who are advancing it; it has to be helpful. I think all of these imaging technologies need to prove their usefulness and quite frankly I think many of them will and holography maybe in that basket as well.
What about virtual reality?
Similar sentiments. The surgeons have been looking at virtual reality (VR) for awhile and it has yet to become mainstream, but that doesn’t mean it won’t. Certainly as a training tool, I think VR will play a very important role. We are way behind other fields in terms of using it. It’s coming.Reference Rymuza, Grodecki, Kamiński, Scisło and Huczek32–Reference Tandon, Burkhardt and Batsis34 I can imagine for learning the technique for closing a premature Patent Ductus Arteriosus, where very delicate hand movements are required, or if you are a surgeon learning to perform coronary anastomosis for a switch. I can’t help thinking that doing it virtually will be very beneficial. I can tell you, this is far from advanced VR but it is a form of VR, just working with 3D SLA models when we are doing the Alterra trial (Multicenter Study of Congenital Pulmonic Valve Dysfunction studying the SAPIEN 3 THV with the Alterra Adaptive Prestent, Edwards Lifesciences), before I go into every case, I put the model on a cath table (Fig 4).Reference Zahn, Chang, Armer and Garg35 We fix it to the table, I do a few implants, I have the fluoroscopy going, I can see, I do it, I look at the model. Did I put it right? When I do those cases, I feel like I have already done it. I am a fairly experienced operator, and I find that hugely beneficial. So I can’t imagine somebody just starting out or maybe mid-career won’t find it beneficial to have their patients’ CT or MR or 3D echo in a VR set-up, practice whatever intervention they are going to do so that when they do it the next day or in the afternoon of that day, that they have already done it in a sense. I just think they will be better operators.

Figure 4. Still fluoroscopic frames illustrate the test deployment of a self-expanding Alterra Adaptive Prestent (Edwards Lifesciences) within a three-dimensional soft rubber model of the right ventricular outflow tract (a–d). The model allows for the evaluation of the extent of device contact with vessel wall (e).
You have mentioned 3D printing in the context of Alterra trial, but some postulate that with the currently available software you can simulate an intervention without the need for an actual 3D print out. Would you agree with that?
Not necessarily. When I was training, the big discussion was: what is better, angiography or echocardiography? Echocardiography 2D echo was still relatively new with colour flow mapping that had just come out and people had just moved from M-mode Doppler. It sounds like a long time ago, because it was. But the reality of it is the people who knew what they were talking about would always take the position: it isn’t an either or, it is complimentary. I am just in the process of editing a textbook on 3D modelling, and I can tell you in that textbook we have about 13 chapters. When I think of 3D modelling, I don’t just think of plastic or rubber models. I am certainly thinking of computational modelling, software modelling with various computer-aided design (CAD) programmes. I think there is a whole lot we can do, not only basic things like putting devices into models, but predicting outcomes. We are able to use computational modelling to not just do the intervention but to see how will it look; how will the flow dynamics look after the intervention.Reference Biglino, Capelli, Bruse, Bosi, Taylor and Schievano36,Reference Gundelwein, Miró, Gonzalez Barlatay, Lapierre, Rohr and Duong37 People are working on all of these things, of course, which is why we are able to write a book about. I don’t think these new technologies are conflicting at all. I think there is something fun, and I don’t know enough right and left brain to speak like a physician on this, but just as an operator there is something very organic, very fundamental about having a model, a life size, made to scale model of whatever you are working on, be it the heart or anything else, in your hand, being able to turn it, look at it from any angles. I know we can do that on a screen, but it is still a screen; it is still a 2D image. There is something fundamental, for me and maybe that is just the way I learn, of having this in my hand and feeling the contour and feeling what the device feels like in that anatomy, that is very beneficial to me as an operator.Reference Sabiniewicz, Meyer-Szary, Potaż, Jagielak and Moszura38 Do I need that to close an atria septal defect? Probably not, but for some of these more complex procedures we all are engaging in, I think it is quite useful. I don’t think 3D physical modelling will go away; I think 3D printed modelling will get cheaper, faster. The printers are already cheaper; you can have it in your office now. Our turnaround time is the same day. If I give the guys in our office a CT, that afternoon I have a printed model, soft or hard, of whatever I want to see. I just think it is going to be very functional. The bigger issue with that is going to be reimbursement and how we will get paid for that time.
Having said a lot about different imaging techniques, which modality do you anticipate will be the key imaging tool in the next 5, 10 years?
Five years, I am not sure how much things will change. I will take a little bit longer vision and say, it’s very hard for me to imagine that in 10, 20, 25 years we are going to be shooting black and white biplane angiograms and that will be the standard of care. Let’s think about it, an angiogram that shows you very little if anything about the actual tissue we intend to intervene upon, we are just filling the vascular space, an angiogram that shows you very little if anything about the surrounding structures like airways. I think we will look back and think this was pretty primitive. I don’t know when that will come, but I think anybody who thinks we have reached our zenith, this is our peak with black and white 2D angiograms that we have been using for decades, is mistaken. It’s just the natural order of things that the technology moves forward. I think we will get to a place where different types of lesions lean on a variety of different advanced imaging techniques. I think that for shunt lesions, where there is a lot of movement and a lot of blood flow, perhaps the best tool will be 3D and 4D echo and echo fusion.Reference Hascoët, Warin-Fresse and Baruteau10,Reference Jone, Ross, Bracken, Mulvahill, Di Maria and Fagan39 –Reference Rodríguez-Zanella, Sandoval, García-Montes, Zabal-Cerdeira and Arias-Godínez41 People such as your centre (Colorado Children’s Hospital) have really led the way on that, and I think there is something to that. Echo marking, image fusion, I can’t help to think, that anything that makes us better and more precise is good.Reference Fagan, Truong and Jone9,Reference Hascoët, Warin-Fresse and Baruteau10,Reference Góreczny, Dryzek, Morgan, Lukaszewski, Moll and Moszura24,Reference McLennan, Góreczny, Jone, Haak and Morgan40 I think we will be stepping on some kind of pedal and either looking at a hologram or looking at a 3D image with an eye tracker, and we will want have to see things dynamically moving with the cardiac cycle.Reference Bruckheimer and Rotschild42 It has to get to that point. If you just look at other industries at some point it will come to that, where we see soft tissue that we actually care about, and where we see surrounding structures that we actually care about.Reference Borik, Volodina, Chaturvedi, Lee and Benson43,Reference Truong, Fagan, Deterding, Ing and Fonseca44 I would love to put a balloon expandable valve in pulmonary outflow tract and I have a better way to figure out if it will hurt the coronaries than just blowing up a balloon in it. I think we need better techniques and technology. To those who say “we don’t need all this”, I would say, we are not really at that point till every single operator has a 100% success rate with every intervention and 0% complication rate with every intervention. We are far from that. I can’t help but think that something as basic as seeing things clearer understanding things better, having more information, will be a vital part of getting us moving closer to this lofty goal.
Thank very much for your time!
Sure, my pleasure.
Acknowledgements
Dr Góreczny would like to thank the Polish-U.S. Fulbright Commission for supporting his research projects with a Senior Award Scholarship.
Disclosures
None.






