INTRODUCTION
Parasitic infections are non-randomly distributed in space (Ostfeld et al. Reference Ostfeld, Glass and Keesing2005). For instance, physical habitat structure can influence the distribution and abundance of organisms, and thus affect interspecific interactions such as host-parasite associations (Sousa and Grosholz, Reference Sousa, Grosholz, Bell, McCoy and Mushinsky1990). Several illustrations can be found in the literature. Disease was influenced by altitude in amphibian-fungal disease (Pounds et al. Reference Pounds, Bustamante, Coloma, Consuegra, Fogden, Foster, La Marca, Masters, Merino-Viteri, Puschendorf, Ron, Sánchez-Azofeifa, Still and Young2006), patch size and connectivity in tick-borne disease (Allan et al. Reference Allan, Keesing and Ostfeld2003), deforestation in mosquito-borne malaria (Molyneaux, Reference Molyneaux, Aguirre, Ostfeld, Tabor, House and Pearl2002) and basin-shape in Daphnia-fungal disease (Hall et al. Reference Hall, Smyth, Becker, Duffy, Knight, MacIntyre, Tessier and Cceres2010). Lotic systems (rivers) are very particular ecosystems in that they have a dendritic shape and continuous unidirectional water flow. In any river, the physical properties change drastically from its source to its mouth in ways that could affect infection patterns. Several theories exist in river ecology to explain such changes. The River Continuum Concept (RCC) developed by Vannote et al. (Reference Vannote, Minshall, Cummins, Sedell and Cushing1980) predicts the way in which biotic changes occur longitudinally instream in accordance to downstream changes in hydrologic and geomorphic properties. On the other hand, discrepancies have been explained by considering alternative factors such as heterogeneity of habitats, stochastic disturbance and hierarchical scaling (e.g., Perry and Schaeffer, Reference Perry and Schaeffer1987; Townsend, Reference Townsend1989; Benda and Dunne, Reference Benda and Dunne1997; Gomi et al. Reference Gomi, Sidle and Richardson2002). Only a handful of studies have addressed spatial patterns of parasite distributions in rivers (e.g. Kennedy, Reference Kennedy, Esch, Bush and Aho1990; Barker et al. Reference Barker, Marcogliese and Cone1996; Weichman and Janovy Jr, Reference Weichman and Janovy2000; Barger and Esch, Reference Barger and Esch2001; Kennedy, Reference Kennedy2001; Barger, Reference Barger2006; Loot et al. Reference Loot, Reyjol, Poulet, Simkova, Blanchet and Lek2007), but mostly focused on the similarities and heterogeneities in richness and abundance at the component community and infracommunity levels. In addition to those, a few have focused on the environmental determinants of myxozoan infections such as sediment type, temperature and flow rate in salmonid whirling disease (e.g. Krueger et al. Reference Krueger, Kerans, Vincent and Rasmussen2006; Hallett and Bartholomew, Reference Hallett and Bartholomew2008). So far, very little is known about disease distribution in rivers and the processes and environmental factors structuring infection foci in these habitats. Such information will undoubtedly be valuable for disease risk assessment in freshwater ecosystems.
In a river, a common process originating from the unidirectional river flow, stream drift, causes the displacement and downstream dispersion of typically benthic invertebrates. This process could promote a gradient in infection levels, leading to decreasing numbers of parasites per host as one moves upstream from the river mouth, in 2 different ways. First, the constant unidirectional drift of small organisms downstream (see e.g. Müller, Reference Müller1954; Waters, Reference Waters1961; Hynes, Reference Hynes1970; Townsend and Hildrew, Reference Townsend and Hildrew1976; Elliott, Reference Elliott2003; Lagrue et al. Reference Lagrue, Kaldonski, Motreuil, Lefèvre, Blatter, Giraud and Bollache2011) should lead to both free-living infective stages (miracidia, cercariae) and small intermediate hosts harbouring parasites (e.g. metacercariae infecting benthic amphipods and snails carrying intramolluscan stages) invariably moving downstream. Along the river, drifting infected invertebrate hosts and free-living stages may encounter pools or still reaches to settle (Hynes, Reference Hynes1970) and successfully infect an available host; alternatively, they become food for other organisms or perish. There is evidence that acanthocephalan-infected amphipods have a higher tendency to enter into the drift (McCahon et al. Reference McCahon, Maund and Poulton1991; Maynard et al. Reference Maynard, Wellnitz, Zanini, Wright and Dezfuli1998). This means that not only infective stages are lost from upstream sites, but also that they may accumulate in downstream reaches, possibly leading to disproportionately high infection levels in downstream sites.
Second, upstream reaches consist of less stable populations that tend to have lower densities of several organisms like plankton (e.g. Greenberg, Reference Greenberg1964; O'Farrell, Reference O'Farrell1993; with exceptions in large rivers, see Basu et al. Reference Basu, Kalff and Pinel-Alloul2000; Allan, Reference Allan2007) and other invertebrates (e.g. Perry and Schaeffer, Reference Perry and Schaeffer1987; Milner et al. Reference Milner, Taylor and Winterbourn2001), because of drift, of lower resources and primary productivity, of lower temperature, and of greater physical disturbances (higher current speed and shallower waters occur upstream) following the RCC. It is well known, from theoretical models (Anderson and May, Reference Anderson and May1978; May and Anderson, Reference May and Anderson1978) and from empirical studies (e.g. Arneberg et al. Reference Arneberg, Skorping, Grenfell and Read1998; Morand and Poulin, Reference Morand and Poulin1998; Arneberg, Reference Arneberg2001; Sures and Streit, Reference Sures and Streit2001; Hansen and Poulin, Reference Hansen and Poulin2006), that parasite transmission and abundance are strongly dependent on host density. Therefore, because of a decreasing downstream-to-upstream gradient in host density, we would also expect a parallel longitudinal gradient in infection levels along the river.
In this study we tested for the existence of a decreasing gradient in infection levels with increasing distance upstream by examining the abundance of 4 trematode species with different life histories that infect upland bully (Gobiomorphus breviceps (Stokell, 1939)), a native New Zealand fish, along a river.
MATERIALS AND METHODS
Host – parasite system
We studied 4 trematodes, Coitocaecum parvum Crowcroft, 1944 (Opecoelidae), Apatemon sp. Szidat, 1928 (Strigeidae), Stegodexamene anguillae Macfarlane, 1951 (Lepocreadiidae) and Telogaster opisthorchis Macfarlane, 1946 (Cryptogonimidae). All four share the same first intermediate host, the New Zealand mudsnail Potamopyrgus antipodarum (Gray, 1843), but thereafter follow different life-cycle pathways. Cercariae of Coitocaecum parvum leave the snail and crawl on the substrate to infect an amphipod [Paracalliope fluviatilis (Thomason, 1879)] as intermediate host. Later, infected amphipods are ingested by upland bully, which represents the definitive host. Cercariae of Apatemon sp., Stegodexamene anguillae and Telogaster opisthorchis all leave the snail host and swim to locate and infect the upland bully via skin penetration, where they encyst as metacercariae within internal tissues. Subsequently, these 3 species are transmitted by predation on infected bullies by definitive hosts. Apatemon sp. infects ducks (Anas platyrhynchos, Bronwen Presswell, personal communication) as definitive hosts whereas both S. anguillae and T. opisthorchis use eels (Anguilla australis and A. dieffenbachii; only the latter occurs in the studied river). The upland bully is a mostly territorial benthic fish (McDowall, Reference McDowall1990), such that their infection levels reflect local processes.
Study area, fish sampling and parasite collection
The Manuherikia River is a 7th order stream with an average discharge of 15 cumecs (m3s−1) and is a tributary of the Clutha River, the largest river in New Zealand in terms of volume (Murray, Reference Murray1975). In August 2010, we sampled the lower section of the Manuherikia River (Fig. 1), along a ∼70 km stretch downstream from Falls Dam. The altitude difference was 328 m from the junction with the Clutha River to the uppermost site (Table 1). Predominant sediment size changed from boulders and cobble (sites 10–7) to very coarse gravel with patches of fine gravel (sites 6–4), to fine gravel with patches of coarse gravel (sites 3–1). We sampled upland bully fish longer than 3 cm standard length (6–33 specimens per site) by electrofishing at 10 sites along the river numbered sequentially from downstream to upstream (Fig 1, see Table 1 for coordinates). After capture, the standard length of each fish was measured, and they were then euthanised by spinal cord severing, and frozen at −20 °C. Fish were subsequently thawed and dissected for parasite recovery according to a standardized protocol. Briefly, the digestive tract, liver, gall bladder, spleen, kidney, mesenteric tissue, gonads and muscle tissue were examined under a stereomicroscope for parasites. Coitocaecum parvum was recovered from the intestine whereas metacercariae of the other 3 species (Apatemon sp., S. anguillae and T. opisthorchis) were recovered from the liver, mesenteric tissue, gonads and muscle. Geographical distances between sampling sites were measured as river distances using ArcGIS 9.3.
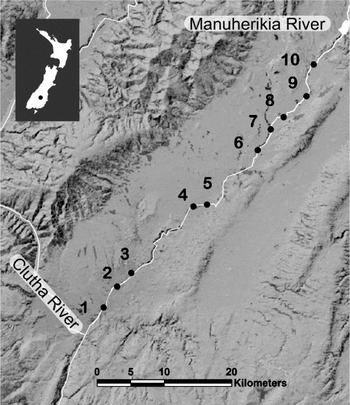
Fig. 1. Studied section of the Manuherikia River, and junction with the Clutha River, on the South Island (New Zealand), showing the 10 sampling sites numbered sequentially from downstream to upstream.
Table 1. Summary of sites and their characteristics for the fish parasite survey in the Manuherikia River: site coordinates, distance from the junction with the Clutha River, altitude, number of fish sampled, and mean fish size measured as standard length (and standard deviation) per site
Statistical analyses
We tested whether fish size and fish sex ratio changed along the river using a linear regression and a χ 2 test, respectively. In order to test for accumulation of trematode infection in the lower sections of the river, generalized linear models (GLM) were performed with a negative binomial error structure fitting data on individual fish for each trematode species separately, using the abundance (number of parasites per host) of each species as the response variable. Trematode abundance data followed different distributions (either Gaussian or negative binomial) depending on the site along the river; however, the negative binomial provided the best fit to data pooled across all sites. As predictor variables the distance from the junction with the Clutha River (continuous variable), fish size (continuous variable) and the interaction fish size × distance were used. Altitude was highly correlated with distance, therefore it was excluded from the analysis. For Apatemon sp. this analysis was run twice, including and excluding site 10 as it might represent an outlier influencing the result of the first analysis. An additional analysis including species as a factor was performed to test for the interaction species × distance that would demonstrate different responses (different slopes for the relationship of abundance with distance) of the 4 species. All analyses were conducted in R freeware v. 2.13.1 (R Development Core Team, 2010), using the MASS, AED and CAR libraries (alpha = 0·05).
RESULTS
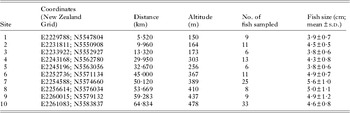
A total of 10 999 parasites were recovered from 131 fish along the river. Coitocaecum parvum reached the highest mean intensity in the river (50·3 ± 56·2 s.d.), followed by Apatemon sp. (42·1 ± 46·6 s.d.), S. anguillae (14·2 ± 22·4 s.d.) and T. opisthorchis (13 ± 16·7 s.d.). The most numerically dominant species (highest abundance) at sites 1, 3–4 was C. parvum, at sites 2, 5 and 10 it was Apatemon sp.; T. opisthorchis was dominant at sites 6–7, 9 whereas S. anguillae dominated at site 8. Fish size changed along the river (R 2 = 0·090; F (1,129) = 12·9; P < 0·001), with larger fish occurring upstream. However, the relationship was not truly linear (Fig. 2), and is best described by a third-order polynomial regression (R 2 = 0·236; F (3,127) = 13·1; P < 0·001), suggesting that still-water stretches or food abundance may not occur evenly along the river (a deep gorge exists between sampling sites 3 and 4). In contrast, sex ratio was similar (χ 2 = 18·58; d.f. = 19; P = 0·484) among sites along the river.
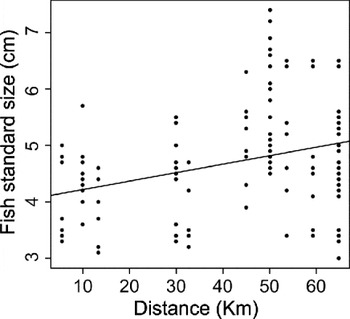
Fig. 2. Linear regression of standard length of upland bully, Gobiomorphus breviceps, as a function of distance from junction with the Clutha River.
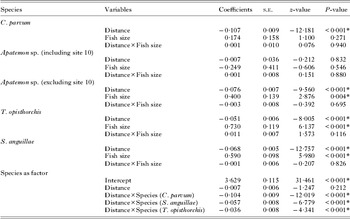
Overall, increasing distance upstream had a negative effect on the abundance of 3 out of 4 parasite species (see Table 2 and Fig. 3). Apatemon sp. was the exception, with a negative effect of distance upstream only becoming apparent if the upstream-most site was removed from the analysis (P< 0·001; Table 2). Abundance dropped at site 6 and remained low further upstream in most cases (Fig. 3). Fish size had a positive statistically significant effect on infection levels for T. opisthorchis and S. anguillae, and Apatemon sp. when site 10 was ignored. The interaction fish size × distance was not significant in any case (Table 2); whereas the interaction species × distance was significant for all levels of the factor. Thus different trematode species showed slightly different abundance gradients with distance (Table 2; Fig. 3, note the different scale for the y-axes).
Fig. 3. Mean abundance of each trematode species per site versus distance from the downstream end of the river. (A) Coitocaecum parvum; (B) Apatemon sp.; (C) Telogaster opisthorchis; (D) Stegodexamene anguillae. Circle size is scaled to sample size. Note different scales of the y-axes.
Table 2. Summary of the statistical results for the Generalised Linear Models evaluating the effect of distance from the river mouth (junction with the Clutha River) and fish size on the abundance of four trematode species in the upland bully. A statistical summary of the interaction distance × species for the analysis on pooled data (species as a factor) is also included.
DISCUSSION
The goal of our study was to determine whether there was a gradient in the distribution of trematode abundance in a river. As predicted, our results supported the existence of such a longitudinal gradient in trematode abundance along the Manuherikia River with a decreasing downstream-to-upstream continuum. This result applied to 3 out of 4 different trematode species, suggesting that this might be a common pattern in river populations.
Multiple scenarios could explain a longitudinal infection gradient in rivers
In addition to the unidirectional water current favouring the dispersal and drift of organisms downstream, predicted changes in physical, chemical and biotic conditions along the length of a river following the RCC could in many ways contribute to the strength of the observed gradient in infection. For instance, a salinity and eutrophication gradient caused differences in parasite community structure, composition and abundance of some species between upstream and downstream sites in 2 polluted estuaries in Canada (Blanar et al. Reference Blanar, Marcogliese and Couillard2011). Other factors such as productivity, temperature, current speed, habitat or abundance of intermediate hosts could be influential in structuring infection foci in the Manuherikia River. Data from a study by the Otago Regional Council (2011) showed that median concentration of dissolved reactive phosphorus (DRP) was lowest upstream (corresponding to site 10 of the present study) and highest at Ophir (site 4). DRP increased towards downstream because it was not used in algal growth due to nitrogen limitations (Otago Regional Council, 2011). In the same study, water quality was rated as excellent in the upper sites and good from site 5 to downstream. Habitat in the main stem was graded as excellent at every site studied. Based on the above, physical and chemical conditions did not vary greatly in the ∼70 km studied stretch. Indeed, DRP values (see Table 6 in Otago Regional Council, 2011) showed an average 4-fold increase from the uppermost (Loop road, site 10 in our study) to the most downstream site in the main stem (at Galloway, site 1 here) whereas mean trematode abundance increased 112-fold (C. parvum), 5-fold (T. opisthorchis) and 26-fold (S. anguillae) in the same ∼70 km stretch studied. So, the increase in parasite abundance towards downstream was considerably larger than that of nutrients.
Biotic factors such as the presence of non-indigenous introduced trout (Salmo trutta L. and Oncorhynchus mykiss Walbaum 1792) could also contribute to the gradient. Kelly et al. (Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009) showed that infection by C. parvum, S. anguillae and T. opisthorchis in upland bully was lower in those sites with high density of trout, through the so-called ‘dilution’ effect (loss of infective stages via penetration of non-suitable hosts), in tributaries of the Manuherikia River. The main stem is used by adult trout and includes also spawning and juvenile habitats. If a downstream-to-upstream gradient in trout density existed, it would also contribute to the results observed. The dendritic nature of the river can be relevant too. For instance, tributary confluences could affect by themselves the connectivity, distribution and abundance of organisms and disease (see Campbell Grant et al. Reference Campbell Grant, Lowe and Fagan2007; Carrara et al. Reference Carrara, Altermatt, Rodriguez-Iturbe and Rinaldo2012).
We cannot pinpoint which factors contributed to the observed gradient. However, changes in productivity, as well as succession in fish assemblages or in dominant species of the macroinvertebrate community may occur along the studied stretch of the river. In relation to the latter, epidemiological models assume that parasite transmission success depends on local host density (May and Anderson, Reference May and Anderson1978; Roberts et al. Reference Roberts, Dobson, Arneberg, de Leo, Krecek, Manfredi, Lanfranchi, Zaffaroni, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2002) and there exist thresholds below which parasite transmission can be disrupted (see Krkosek, Reference Krkosek2010). In the case of parasites with complex life cycles, several studies have shown that intermediate host densities are in most cases correlated with the abundance of parasites in the final hosts (e.g. Sures and Streit, Reference Sures and Streit2001; Hansen and Poulin, Reference Hansen and Poulin2006; de Montaudouin and Lanceleur, Reference de Montaudouin and Lanceleur2011). Therefore, the unidirectional river current in combination with a major process like invertebrate drift in lotic systems which influences the dynamics and distribution of invertebrate hosts could also have an effect on the trematodes exploiting them. We focus below on the processes behind the gradient but a corollary direction for future studies would be to disentangle the biotic, physical and chemical factors that may influence this gradient in infections under natural conditions.
Avian trematode, the exception to the pattern
The absence of a gradient in the abundance of Apatemon sp. (except when ignoring the uppermost site) is not surprising. Previous studies have demonstrated the influence of host dispersal ability on the distribution and genetic makeup of trematode parasites infecting mammals, fish and birds (Blouin et al. Reference Blouin, Yowell, Courtney and Dame1995; Criscione and Blouin, Reference Criscione and Blouin2004; Prugnolle et al. Reference Prugnolle, Liu, De Meeûs and Balloux2005; Louhi et al. Reference Louhi, Karvonen, Rellstab and Jokela2010; Blasco-Costa et al. Reference Blasco-Costa, Waters and Poulin2012). In particular, Criscione and Blouin (Reference Criscione and Blouin2004) found that parasites using both freshwater and terrestrial hosts in their life cycle (allogenic parasites) had less structured populations and higher gene flow among subpopulations than parasite species that used only freshwater hosts. Similarly, Louhi et al. (Reference Louhi, Karvonen, Rellstab and Jokela2010) found that avian parasite dispersal, mediated by definitive bird hosts, acts as the homogenizing force limiting genetic differentiation in the parasites. Our results suggest that although a decreasing trend in abundance can also be found in Apatemon sp. when excluding the uppermost site, it may be easily counteracted by local environmental factors (e.g. habitat suitability for the definitive host) or host traits (e.g. definitive host dispersal ability). Indeed, there is evidence (Marcogliese et al. Reference Marcogliese, Compagna, Bergeron and McLaughlin2001) that proximity to a bird definitive host colony and other habitat characteristics are positively associated with abundance of bird trematodes in the second intermediate host (the upland bully in this case). Thus, local factors and host traits may be the main players determining the abundance and distribution of Apatemon sp. and possibly other trematodes with allogenic life cycles in freshwater habitats. Generally, local factors are expected to alter the longitudinal gradients in rivers (see also T. opisthorchis at site 9; Fig. 3C).
Extent of the influence of drift
In our system, not only invertebrates (snails, amphipods and trematode free-living stages) can be subjected to drift. Hopkins (Reference Hopkins1970) reported that upland bully fry are passively dispersed downstream after hatching. After a couple of years, juvenile fish migrate upstream prior to establishing territories. The poor swimming ability of bully fry cannot prevent them from entering into drift, which should result in small or juvenile fish found more frequently downstream. Atkinson and Joy (Reference Atkinson and Joy2009) have shown that this is the case for an amphidromous New Zealand bully, Gobiomorphus hubbsi Stokell, 1959. Our results showed that upstream fish are, on average, a little larger than downstream ones, supporting Hopkins’ (Reference Hopkins1970) observations on fry drift and adding a non-amphidromous species (although amphidromy is considered to be ancestral to all bully species in New Zealand) to the list of species with such behaviour. In addition, we found that fish size was positively correlated with abundance of both T. opisthorchis and S. anguillae (2 of the 5 trematodes found at the metacercarial stage, but also Apatemon sp. when site 10 was excluded). This agrees with previous studies showing that metacercariae accumulate in fish over time (Poulin, Reference Poulin2000). Despite upstream fish being larger and larger fish generally harbouring more T. opisthorchis and S. anguillae, infection levels upstream were nevertheless lower.
The significance of invertebrate drift and unidirectional river current for parasites
Drift has been considered as being both beneficial (Hildrew and Townsend, Reference Hildrew and Townsend1980; Walton, Reference Walton1980) and detrimental (Wooster, Reference Wooster1998) to benthic invertebrates. Cercariae of most trematode species live for only 1 or 2 days at most, under ideal laboratory conditions; under natural conditions, they are thus unlikely to infect hosts far downstream from their site of emergence from snails. Behavioural studies of parasitized invertebrates, typically amphipods, show that the behaviour of infected individuals is different from non-infected ones, showing a higher tendency for drifting caused by hyperactivity (McCahon et al. Reference McCahon, Maund and Poulton1991; Maynard et al. Reference Maynard, Wellnitz, Zanini, Wright and Dezfuli1998). The reason for such behaviour change is still debated (see McCahon et al. Reference McCahon, Maund and Poulton1991; Jakobsen and Wedekind, Reference Jakobsen and Wedekind1998; Maynard et al. Reference Maynard, Wellnitz, Zanini, Wright and Dezfuli1998; Wellnitz et al. Reference Wellnitz, Giari, Maynard and Dezfuli2003). In addition, acanthocephalans can cause spatial segregation of infected and uninfected subpopulations of an amphipod host (Wellnitz et al. Reference Wellnitz, Giari, Maynard and Dezfuli2003), with larger numbers of infected amphipods drifting downstream and few moving upstream to compensate. Neither these authors nor Dezfuli et al. (Reference Dezfuli, Rossetti, Bellettato and Maynard1999) (who studied the same system) found a decreasing downstream-to-upstream pattern in infection (but the scale of their study was restricted to a 2 km stream section; see Dezfuli et al. (Reference Dezfuli, Rossetti, Bellettato and Maynard1999)). Since infected invertebrates tend to drift more and not all drifting infected invertebrates are caught by a fish predator, spatially structured host populations resulting from a ‘parasite-induced drift’ (Wellnitz et al. Reference Wellnitz, Giari, Maynard and Dezfuli2003) and regular drifting of free-living stages as a consequence of the unidirectional water current could also favour the establishment of higher infection areas in pools or still stretches downstream with higher host densities (also juvenile fish are more abundant downstream), where transmission is more likely. This could in turn favour genetic mixing within river populations with a higher genetic diversity downstream in those parasite species most influenced by invertebrate drift and river current dispersal (Blasco-Costa et al. Reference Blasco-Costa, Waters and Poulin2012).
Host and parasite traits influencing an infection/abundance gradient
An additional question is to what extent such gradients in infection levels may be common to other parasitic groups, and what parasite and host traits may have an influence in addition to local environmental factors. As discussed above, dispersal ability of the definitive host (generally the most mobile in any trematode's life cycle) could alter the gradient. Other important host traits may include habitat preference (e.g. riffles versus backwaters, fast versus slow flows), feeding habits (e.g. filter-feeder versus detritivore versus active predator), or behaviour (e.g. gregarious versus solitary, territorial versus non-territorial). For example, S. anguillae and T. opisthorchis infecting eels as definitive hosts showed a similarly weak decrease in abundance with distance, whereas C. parvum that infects small territorial bully fish as definitive host showed a stronger effect.
Parasite traits, particularly those related to the life cycle, such as transmission mode and number of stages that entail suspension in the water column, could also influence the strength of the observed gradient. For instance, Cardon et al. (Reference Cardon, Loot, Grenouillet and Blanchet2011) investigated host and environmental features affecting the burden and pathogenicity of a copepod infecting its fish host. They found that the parasite burden along an upstream-to-downstream gradient was influenced by host factors, but not by environmental factors despite the target species being an ectoparasite. Most studies of parasites in fish with samples collected longitudinally in rivers involve pollution gradients (e.g. Krause et al. Reference Krause, McLaughlin and Marcogliese2010; Marcogliese et al. Reference Marcogliese, Gendron and Cone2009); therefore, they do not provide valid comparative estimates of infection gradients. We expect that this longitudinal gradient in parasite abundance in rivers would exist also for other parasites with complex life cycles.
In summary, trematode abundance showed a longitudinal gradient, decreasing from downstream to upstream sites, and processes such as unidirectional water current and invertebrate drift that influence the distribution of invertebrates and juvenile fish inhabiting lotic systems could account for it. Other factors such as gradients in physical, chemical and biotic conditions along the stream could also contribute to explain the gradient. There is a need for further research to better understand the role of unidirectional water current, invertebrate drift and environmental factors as well as parasite and host traits in determining infection patterns and connectivity among parasite populations in river systems. Baseline knowledge on natural patterns in rivers is required in order to produce more accurate modelling of disease incidence and spread in freshwater ecosystems (Poulin et al. Reference Poulin, Paterson, Townsend, Tompkins and Kelly2011; Thrush et al. Reference Thrush, Murray, Brun, Wallace and Peeler2011).
ACKNOWLEDGEMENTS
We would like to thank Kim Garrett, Abbas Akbaripasand and Dr Carlos Rouco for their assistance in the field. We are also grateful to Dr Martin Krkosek for statistical advice and to Dr Aneta Kostadinova and the members of the Parasitology Research Group for comments on an earlier version of the manuscript. The sampling in this paper complies with the current laws and animal ethics regulations of New Zealand.
FINANCIAL SUPPORT
This research was supported by a Marie Curie Outgoing International Fellowship for Career Development (I.B.-C., grant number PIOF-GA-2009-252124) within the 7th Framework Programme (FP7/2007-2013) of the European Commission.







