Introduction
Butyric acid plays a key role in maintaining gut health in animals, as a major source of energy to colonic mucosa and an important regulator of gene expression, differentiation, inflammation and apoptosis in host cells (Pajak et al., Reference Pajak, Orzechowski and Gajkowska2007; Hamer et al., Reference Hamer, Jonkers, Venema, Vanhoutvin, Troost and Brummer2008). Butyrate supplementation improved feed efficiency, digestibility of nutrients and growth in young calves (Guilloteau et al., Reference Guilloteau, Zabielski, David, Blum, Morisset, Biernat, Wolinski, Laubitz and Hamon2009). During absorption in the rumen, around 0.9 of butyric acid is oxidized to ketone bodies (Britton and Krehbil, Reference Britton and Krehbil1993). Development of the ketogenic capacity of ruminal epithelium occurs as the animal ages and genes encoding the enzymes controlling ketogenesis are expressed independently of intra-ruminal butyric acid concentration (Lane et al., Reference Lane, Baldwin RL and Jesse2002). Thus, butyrate supplementation should be provided to young ruminants after birth when the ruminal butyrate concentration is quite low: in adult ruminants, butyric acid is one of the main products of ruminal fermentation, and its proportion in total fermentation acids varies between 0.05 and >0.2 (Aschenbach et al., Reference Aschenbach, Penner, Stumpff and Gäbel2011). However, the effects of butyrate depend not only on animals’ age but also on the experimental model (in vivo or in vitro) and supplementation doses used (Guilloteau et al., Reference Guilloteau, Martin, Eeckhaut, Ducatelle, Zabielski and Van Immerseel2010).
Tributyrin (TB), composed of butyric acid and glycerol, is a triglyceride naturally present in butter. In the rumen, TB can be metabolized into three free butyric acid molecules by microbes. Compared with sodium butyrate, TB has more favourable pharmacokinetics because of its more potent and direct effect on cells (Chen and Breitman, Reference Chen and Breitman1994). Although TB has been used as a feed additive to assess its effects on performance and metabolism in Holstein calves (Araujo et al., Reference Araujo, Terré, Mereu, Ipharraguerre and Bach2016), research on its positive effects on metabolism in adult ruminants is still quite limited. Thus, the current study aimed to evaluate the positive effects of TB supplementation in adult Small Tail ewes through both in vitro and in vivo trials, particularly to assess the effects on short-chain fatty acid (SCFA) concentrations, fibrolytic enzyme activities, nutrient degradation and methane emissions.
Materials and methods
The in vitro trial was conducted at the Key Laboratory of College of Animal Science, Anhui Science and Technology University (Fengyang, China), while the in vivo trial was conducted at the Experimental Station of Anhui Province Modern Agriculture Technology System in Cattle and Sheep (Bengbu, China).
In vitro trial
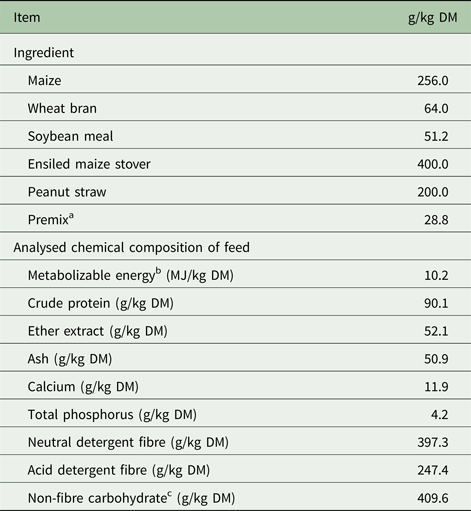
Nine 12-month-old, non-pregnant, ruminally cannulated Small Tail ewes (initial body weight 55 ± 5.0 kg, mean ± standard deviation) were confined in a 27-m2 concrete-floor pen, in which 12 feed bunks and two watering points were provided for ad libitum consumption. Ewes were fed twice daily (at 07.00 and 19.00 h) ad libitum with a total mixed ration (TMR) consisting of 256 g maize meal/kg, 64 g wheat bran/kg, 51 g soybean meal/kg, 29 g premix/kg, 400 g ensiled maize stover/kg and 200 g peanut straw/kg.
A completely randomized design was applied to five runs of in vitro batch cultures, and 0.5 g experimental substrate (Table 1) was weighed into 90 bottles/run with 18 bottles for each treatment, to offer TB (Sigma Aldrich, St. Louis, MO, USA) at 0 (control), 2, 4, 6 and 8 g/kg on a dry matter (DM) basis. In each run, 50 ml freshly prepared buffer solution (pH 6.85, Menke and Steingass, Reference Menke and Steingass1988) were added to the bottles. Rumen fluids collected from nine ewes were filtered through two layers of muslin and mixed in equal volume ratios, and then 25 ml rumen fluid were added to the bottles to serve as a donor of mixed rumen microorganisms. All bottles were purged with anaerobic nitrogen gas (N2) for 5 s, sealed with butyl rubber stoppers and Hungate screw caps, and then incubated at 39 °C for 72 h. Three fermentations without substrate and TB supplemented were used as blanks in each run. If necessary, through analysing the concentrations of microbial protein and total SCFA in the blanks, the variation between runs caused by different rumen fluid inoculum in different periods could be checked.
Table 1. Ingredient and chemical composition of the basal diet

DM, dry matter.
a Per kilogram of premix contained 1 54 400 international unit (IU) of Vitamin A, 94 000 IU of Vitamin D3, 3 38 200 IU of Vitamin E, 120.0 mg iodine, 280.0 mg copper, 2240.0 mg iron, 1740.0 mg manganese, 1370.0 mg zinc, 60.0 mg selenium, 16.8 mg cobalt, 50.0 mg of rumen protection of Met and Lys.
b Metabolizable energy was estimated according to NRC (2001).
c Non-fibre carbohydrate (g/kg) = 1000 – (NDF + CP + EE + Ash).
After 72 h of incubation, head-space gas pressure was measured by using a pressure transducer to estimate the cumulative gas volume, and production of hydrogen (H2), methane (CH4) and carbon dioxide (CO2) were analysed. All culture fluids were filtered with nylon bags (48.0 µm pore size) and then 10 ml filtered fluids were prepared for analysis of pH, SCFA concentration and fibrolytic enzyme activities. The residual contents of the substrate were collected for analysis of DM, crude protein (CP), neutral detergent fibre (NDF) and acid detergent fibre (ADF).
In vivo trial
Forty-five 12-month-old Small Tail ewes were assigned randomly to five groups with nine animals each (live weight 55 ± 5.0 kg, mean ± standard deviation). The ewes were kept in individual cages (1.5 × 1.5 m2) on a perforated wooden floor without litter. During the experiment, water was available a d libitum. The ewes were fed individually twice daily at 07.00 and 19.00 h with TMR (Table 1), which was formulated at a constant concentrate-to-forage ratio (40:60). The TMR was supplemented with TB at 0, 2, 4, 6 and 8 g/kg on a DM basis, according to previous research (Araujo et al., Reference Araujo, Terré, Mereu, Ipharraguerre and Bach2016) in which TB was supplemented at a level of 3 g/kg DM in the calf ration. The offered and refused rations were recorded to assess the effect of TB supplementation on DM intake.
The in vivo trial lasted 18 days, including 15 days to allow diet adaptation and the last 3 days to collect 12 rumen fluid samples: rumen fluid was sampled four times on each of these days at 6-h intervals. The sampling times were moved forward by 2 h each day compared with the previous day, so that after 3 days there were samples for every 2-h interval in 24 h for each ewe. Samples were collected from the rumen according to the methods of oral tube collection described by Sorensen and Schambye (Reference Sorensen and Schambye1955). The rumen fluids collected at different sampling time points were pooled in equal portion, hand-mixed thoroughly and filtered through four layers of muslin. After pH measurement, the rumen fluids were centrifuged at 1000g for 30 min at 4 °C, and the supernatant was separated and immediately stored at −20 °C for analysis of SCFA concentrations and fibrolytic enzyme activities.
Chemical analysis
Residual content analysis
Following AOAC (2012) procedures, in vitro residual contents and in vivo feed offered and refused were analysed for DM (ID 930.15) and CP (ID 984.13). Both NDF and ADF were analysed (Van Soest et al., Reference Van Soest, Robertson and Lewis1991) with heat-stable α-amylase (Sigma no. A3306; Sigma Chemical Co., St. Louis, MO, USA) and corrected for residual ash content. In vitro apparent degradations of DM, CP, NDF and ADF were calculated from Eqn (1):
Rumen fluid pH and short-chain fatty acid analysis
The filtered rumen fluid pH was measured immediately using a pH meter (Model pHs-29A, Jingke Leici Co. Ltd, Shanghai, China). After thawing at room temperature, the rumen fluid samples (1 ml) were mixed with 0.3 ml of 25% (w/v) meta-phosphoric acid solution for 30 min and then centrifuged at 15 000g for 10 min at 4 °C. Following the method of Yang et al. (Reference Yang, Tamminga, Williams, Dijkstra and Boer2005), concentrations of acetate, propionate, butyrate, valerate and branched chain SCFAs including iso-butyrate and iso-valerate in the supernatants were measured using a gas chromatograph (GC522, Wufeng instruments, Shanghai, China).
Fibrolytic enzyme assays
Reducing sugar (expressed as glucose) release was determined as described by MacKenzie and Bilous (Reference MacKenzie and Bilous1982) with glucose used as the standard, and one unit of enzyme activity was defined as the amount of enzyme that released 1 µm of reducing sugar/min in 1 ml fluid.
Gas generation assay
A pressure transducer interfaced with a computer was used to measure accumulated head-space gas pressure; the values could be entered directly into the computer and used to estimate the cumulative gas volumes. Before the onset of the in vitro trial, a standard curve was made to describe the quantitative relationship between gas volumes and pressures in bottles with 75 ml buffer solutions at 39 °C. The production of H2, CO2 and CH4 were measured following the method described by Zhang and Yang (Reference Zhang and Yang2011).
Statistical analysis
In vitro and in vivo data were analysed using PROC MIXED model of SAS 9.4 (Statistical Analysis for Windows, SAS Institute Inc., Cary, NC, USA). Linear and quadratic effects of treatments indicated by orthogonal contrasts were used to evaluate effects of TB supplementation. Duncan's multiple range test was conducted to determine the significance level of the particular comparison between treatment means. Differences were considered significant at P ⩽ 0.05. The model including random and fixed effects was as follows:
where Y ij is the dependent variable, μ is the overall mean, R i is the random effect (for in vitro random effect of run, i = 5; for in vivo random effect of ewe, i = 9), T j is the fixed effect of TB (j = 0, 2, 4, 6, 8) and ε ij is the error term.
Results
Effects of substrate supplementation with tributyrin on in vitro fermentation, nutrient digestibility and gas production characteristics
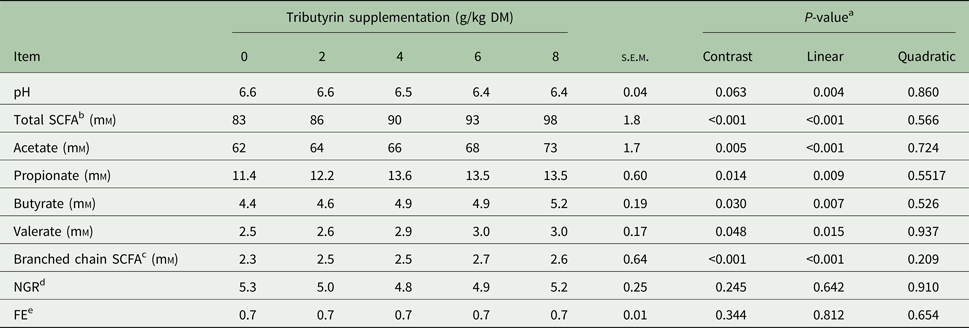
Substrate supplementation of TB (Table 2) linearly decreased in vitro pH of the filtered rumen fluid (P = 0.005) and increased concentrations of total SCFA (P < 0.001), acetate (P < 0.001), propionate (P < 0.001), butyrate (P = 0.007), valerate (P = 0.015) and branched chain SCFA (P < 0.001). However, TB supplementation had no effect on in vitro ratio of non-glucogenic to glucogenic SCFAs (NGR) and fermentation efficiency (FE).
Table 2. Effects of tributyrin supplementation on in vitro fermentation characteristics at 72 h

DM, dry matter; SCFA, short-chain fatty acids; NGR, ratio of non-glucogenic to glucogenic SCFAs; FE, fermentation efficiency.
a Contrast, supplemental effect of tributyrin; Linear, linear effect of tributyrin; Quadratic, quadratic effect of tributyrin.
b Total SCFA = acetate + propionate + butyrate + valerate + branched chain SCFA.
c Branched chain SCFA including iso-butyrate and iso-valerate.
d NGR calculated according to Orskov (Reference Orskov1975): NGR = (Acetate + 2 × Butyrate + Valerate)/(Propionate + Valerate).
e FE = (0.622 × Acetate + 1.092 × Propionate + 1.56 × Butyrate)/(Acetate + Propionate + 2 × Butyrate) (Abdl-Rahman, Reference Abdl-Rahman2010).
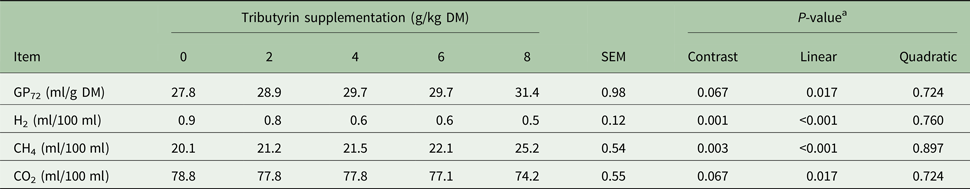
As shown in Table 3, TB supplementation linearly increased in vitro apparent digestibility of DM (P < 0.001), CP (P < 0.001), NDF (P < 0.001) and ADF (P = 0.001), as well as in vitro gas production (P = 0.017) (Table 4). It decreased production of H2 (P < 0.001) and CO2 (P = 0.017), but increased CH4 production (P < 0.001).
Table 3. Effects of tributyrin supplementation on in vitro apparent degradations of dry matter (DM), crude protein (CP), neutral detergent fibre (NDF) and acid detergent fibre (ADF) at 72 h

a Contrast, supplemental effect of tributyrin; Linear, linear effect of tributyrin; Quadratic, quadratic effect of tributyrin.
Table 4. Effects of tributyrin supplementation on in vitro gas production characteristics at 72 h

DM, dry matter; GP72, gas production at 72 h; H2, hydrogen gas; CH4, methane; CO2, carbon dioxide.
a Contrast, supplemental effect of tributyrin; Linear, linear effect of tributyrin; Quadratic, quadratic effect of tributyrin.
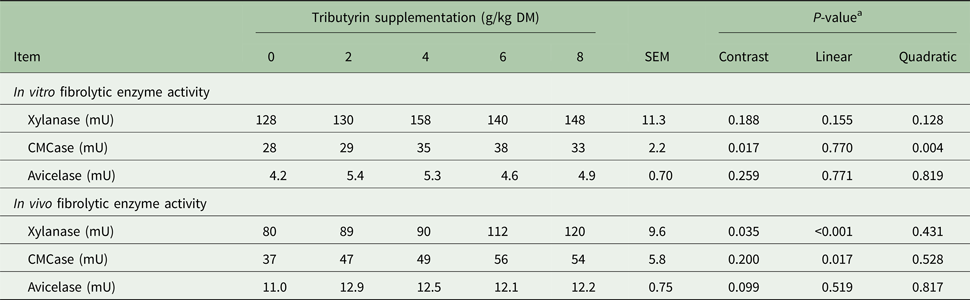
Effects of tributyrin supplementation on both in vitro and in vivo fibrolytic enzyme activities
Substrate supplementation of TB (Table 5) quadratically increased in vitro carboxymethyl cellulase (CMCase) activity (P = 0.004). Feed supplementation of TB linearly increased activity of xylanase (P < 0.001) and CMCase (P = 0.017). Compared with the control, TB supplementation tended to increase in vivo avicelase activity (P = 0.099).
Table 5. Effects of tributyrin supplementation on both in vitro and in vivo fibrolytic enzyme activities

DM, dry matter; CMCase, carboxymethyl cellulase.
a Contrast, supplemental effect of tributyrin; Linear, linear effect of tributyrin; Quadratic, quadratic effect of tributyrin.
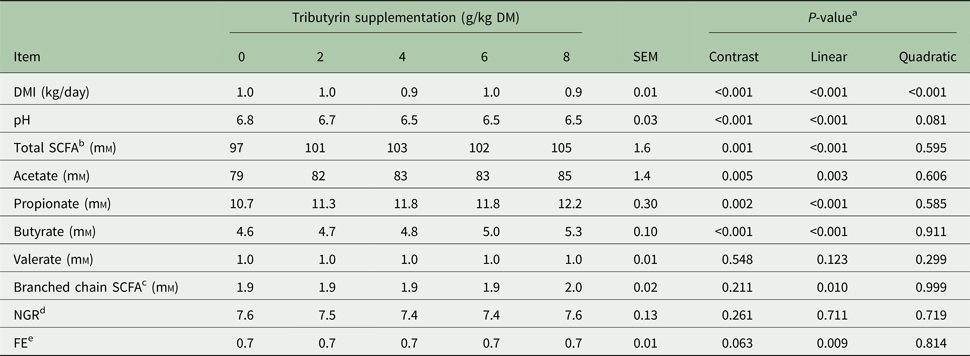
Effects of feed supplementation of tributyrin on dry matter intake and in vivo ruminal fermentation characteristics
Feed supplementation of TB (Table 6) decreased DM intake (P < 0.001) and ruminal pH (P < 0.001) but linearly increased concentrations of total (P < 0.001) and most individual SCFA (P ⩽ 0.01) except valerate (P = 0.123). Feed supplementation of TB had no effect on in vivo NGR but enhanced ruminal FE (P = 0.009).
Table 6. Effects of tributyrin supplementation on dry mater intake (DMI) and in vivo ruminal fermentation characteristics in adult ewes

DM, dry matter; SCFA, short chain fatty acids; NGR, ratio of non-glucogenic to glucogenic SCFAs; FE, fermentation efficiency.
a Contrast, supplemental effect of tributyrin; Linear, linear effect of tributyrin; Quadratic, quadratic effect of tributyrin.
b Total SCFA = acetate + propionate + butyrate + valerate + branched chain SCFA.
c Branched chain SCFA including iso-butyrate and iso-valerate.
d NGR calculated according to Orskov (Reference Orskov1975): NGR = (Acetate + 2 × Butyrate + Valerate)/(Propionate + Valerate).
e FE = (0.622 × Acetate + 1.092 × Propionate + 1.56 × Butyrate)/(Acetate + Propionate + 2 × Butyrate) (Abdl-Rahman, Reference Abdl-Rahman2010).
Discussion
Butyrate is a major microbial fermentation product in the rumen. In the current in vitro and in vivo trials, the same supplementary dosages of TB were used to avoid possible confounding effects of butyrate on fermentation characteristics.
Feed supplementation of TB decreased in vivo DM intake in ewes compared with the control group, possibly because: (i) TB used as a feed additive may modify the taste of feed and cause greater amounts of refusals, despite its stability and low odour; and (ii) TB might act as an energy source by undergoing β oxidation to acetyl-coenzyme A (acetyl-CoA) and generating adenosine triphosphate (ATP) for TB-supplemented ewes (Donohoe et al., Reference Donohoe, Garge, Zhang, Sun, O'Connell, Bunger and Bultman2011).
In both trials, decreased pH values might be due to the increased total SCFA concentration in the rumen and culture fluids (Burrin and Britton, Reference Burrin and Britton1986). Alternatively, SCFAs also play an important role as the major energy sources for ruminants. In the current study, both in vitro and in vivo total SCFA concentrations were positively related to TB supplementation, which might be attributed to the stimulating effects of TB on the concentrations of individual fatty acids. Li et al. (Reference Li, Wu, Baldwin, Li and C2012) found that exogenous butyrate stimulated native butyrate-producing bacteria population such as Butyrivibrio and Pseudobutyrivibrio, which are probably the predominant butyrate producers in the rumen microbial ecosystem. In addition, growth of ruminal Bacteroidetes, Firmicutes and Fibrobacteres, which are essential in converting carbohydrates to SCFAs, were also increased by exogenous butyrate (Li et al., Reference Li, Wu, Baldwin, Li and C2012).
In the current study, increased butyrate concentration might be related to the hydrolysis of TB by rumen microorganisms. Alternatively, butyrate synthesis by rumen microorganisms may be related to the utilization of acetate or compounds giving rise to acetyl-CoA such as pyruvate (Barker, Reference Barker1961).
Unlike acetic, propionic and butyric acids, branched chain SCFAs including iso-butyrate and iso-valerate are produced by the breakdown of protein and are essential for synthesis of cellular constituents by ruminal bacteria (Allison, Reference Allison1969). Elastase II activity related to protein digestibility in calves was enhanced by sodium butyrate supplementation (Guilloteau et al., Reference Guilloteau, Zabielski, David, Blum, Morisset, Biernat, Wolinski, Laubitz and Hamon2009), which is beneficial to generate branched chain SCFA.
The optimal value of NGR in the rumen is about 3.5, and a value lower than 3.5 indicates more efficient utilization of SCFA for gluconeogenesis (Abdl-Rahman, Reference Abdl-Rahman2010). In the current study, TB supplementation had no effect on the fermentation pattern in ewes indicated by the fairly constant NGR, which is consistent with previous research (Kowalski et al., Reference Kowalski, Górka, Flaga, Barteczko, Burakowska, Oprządek and Zabielski2015). Likewise, Malhi et al. (Reference Malhi, Gui, Yao, Aschenbach, Gäbel and Shen2013) reported that the fermentation pattern was not affected in goats infused intra-ruminally with 0.3 g/kg body weight of butyrate/day.
Xylanase, CMCase and avicelase are the primary fibrolytic enzymes for the hydrolysis of dietary carbohydrates in the rumen (Santra et al., Reference Santra, Karimo and Chaturvedi2007). Ruminal microorganisms such as bacteria, protozoa and phycomycete fungi may provide a wide range of fibrolytic enzymes to degrade feed fibre (Chen et al., Reference Chen, Wang, Wu and Liu2008). Thus, the enhanced fibrolytic enzyme activity in the current study might be attributed to the stimulating effects of TB on butyrate-producing bacteria (Guilloteau et al., Reference Guilloteau, Martin, Eeckhaut, Ducatelle, Zabielski and Van Immerseel2010; Li et al., Reference Li, Wu, Baldwin, Li and C2012), which is responsible for fibre digestion and utilization in the rumen (Mrazek et al., Reference Mrazek, Tepsic, Avgustin and Kopecný2006). In addition to the enhanced fibrolytic enzyme activities, in vitro apparent nutrient digestibility of substrate was increased by TB supplementation, which was consistent with a previous report by Huhtanen et al. (Reference Huhtanen, Miettinen and Ylinen1993) that apparent digestibility of dietary DM, CP and NDF was enhanced by the increased ruminal supply of butyrate in dairy cows. Thus, the results of the current study indicate the positive effects of TB supplementation on nutrient utilization and fermentation efficiency.
Methane production indicates an energy loss to ruminants. In the present study, increased CH4 production by TB supplementation is probably related to the reduced H2 and CO2, which were probably used by methanogenic bacteria to generate methane. Wang et al. (Reference Wang, Zhang, Wang and Meng2009) demonstrated that even though butyric acid concentration was high, up to 1800 mg/l, no significant inhibition effect was observed on the activity of methanogenic bacteria. The increased methanogenesis by TB supplementation in the present study could have potentially negative effects on animal performance.
Conclusions
The current study demonstrated positive effects of TB supplementation on total and most individual SCFA concentrations, fibrolytic enzyme activities, as well as in vitro apparent nutrient digestibility and in vivo fermentation efficiency. These results suggest that TB supplementation might exert a positive influence on rumen microbial metabolism in adult ewes, despite having an enhancing effect on methane production.
Financial support
The authors appreciate the financial support of the Natural Science Foundation of Anhui Provincial Education Department (project no. KJ2015ZD17 and KJ2015A293), Beijing Key Laboratory of Dairy Cow Nutrition, the Scientific Research Foundation of University of Science and Technology of Anhui (grant no. 1409) and Anhui Science and Technology Research Project – ‘Study on the Effective Breeding Techniques of the Dabie Mountains Yellow Cattle’ (1704a07020084).
Conflicts of interest
There were no conflicts of interest in the present study.
Ethical standards
This study was approved by the ethics committee of Anhui Science and Technology University (ECASTU-2015-P08).