INTRODUCTION
Over recent decades, consumption of soil, called geophagy, has been described for various frugivorous and omnivorous animals. Among vertebrates, examples for this widespread behaviour can be found in birds (Brightsmith & Muñoz-Najar Reference BRIGHTSMITH and MUÑOZ-NAJAR2004, Diamond et al. Reference DIAMOND, BISHOP and GILARDI1999, Gilardi et al. Reference GILARDI, DUFFEY, MUNN and TELL1999) and many mammals (Blake et al. Reference BLAKE, GUERRA, MOSQUERA, TORRES, LOISELLE and ROMO2010, Kreulen Reference KREULEN1985, Montenegro Reference MONTENEGRO2004). Several explanations for geophagy have been suggested (reviewed in Diamond Reference DIAMOND1999, Klaus & Schmid Reference KLAUS and SCHMID1998) and usually at least some medicinal benefit has been associated with the consumption of soil (Krishnamani & Mahaney Reference KRISHNAMANI and MAHANEY2000). However, the most common explanation for geophagy has been associated with nutrient supplementation or detoxification of plant secondary compounds (Abrahams & Parsons Reference ABRAHAMS and PARSONS1996, Holdø et al. Reference HOLDØ, DUDLEY, MCDOWELL and TOMASI2002, Mahaney & Krishnamani Reference MAHANEY and KRISHNAMANI2003, Voigt et al. Reference VOIGT, CAPPS, DECHMANN, MICHENER and KUNZ2008). The nutrient-supplementation hypothesis assumes that animals ingest clay material for its nutrient content (Brightsmith & Muñoz-Najar Reference BRIGHTSMITH and MUÑOZ-NAJAR2004, Emmons & Stark Reference EMMONS and STARK1979, Kreulen Reference KREULEN1985). The detoxification hypothesis argues that ingested clay may help in buffering toxic, carcinogenic, teratogenic or nutrient-binding effects of plant secondary compounds, such as tannins or alkaloids (Brightsmith et al. Reference BRIGHTSMITH, TAYLOR and PHILLIPS2008, Diamond Reference DIAMOND1999, Gilardi et al. Reference GILARDI, DUFFEY, MUNN and TELL1999).
In western Amazonia, frugivorous and omnivorous bats visit muddy depressions in forested areas for geophagy (Bravo et al. Reference BRAVO, HARMS, STEVENS and EMMONS2008, Reference BRAVO, HARMS and EMMONS2010; Tuttle Reference TUTTLE1974, Voigt et al. Reference VOIGT, DECHMANN, BENDER, RINEHART, MICHENER and KUNZ2007). In particular, pregnant and lactating frugivorous bats of Phyllostomidae subfamily Stenodermatinae have been observed visiting these mineral licks in high numbers. During reproduction, frugivorous bats may suffer from severe nutrient stress, because many fruits are low in physiologically relevant minerals. One example is sodium (Na) that is responsible for a wide set of body functions, such as nerve impulses or the homeostatic balance (National Research Council 2005). This nutrient stress may be pronounced for fruit-eating bats living in nutrient-depleted ecosystems, such as the Amazonian lowlands (Terborgh Reference TERBORGH1992). Therefore, geophagy serving as mineral supplementation for frugivorous bats has been suggested by several authors (Bravo et al. Reference BRAVO, HARMS, STEVENS and EMMONS2008, Reference BRAVO, HARMS and EMMONS2010; Voigt et al. Reference VOIGT, DECHMANN, BENDER, RINEHART, MICHENER and KUNZ2007). However, bats in times of high nutritional demand may also benefit from geophagy by buffering toxic plant secondary compounds in their fruit diet (Voigt et al. Reference VOIGT, CAPPS, DECHMANN, MICHENER and KUNZ2008). Thus far, it is unclear whether free-ranging bats consume water or clay material at mineral licks. If bats only drink water, they most likely supplement their nutrient-depleted fruit diet with essential nutrients dissolved in the water. Alternatively, if bats ingest additionally clay, it is more likely that they detoxify plant secondary compounds in their diet. In this study, we tested the hypotheses that frugivorous bats visit mineral licks to drink water and to ingest clay.
To evaluate whether frugivorous bats drink water or ingest clay or soil-enriched water at mineral licks, we conducted video observations and analysed chemical elements in bat blood and faeces. We predicted that frugivorous bats captured at mineral licks should have an altered blood composition in relation to frugivorous bats captured in the nearby forest. Further, we predicted that soil tracer elements in bat faeces would indicate soil consumption, if frugivorous bats consume soil or soil-enriched water at mineral licks.
As we examined the elemental composition of available water sites in the area, we predicted that water sampled at mineral licks should show higher values of physiologically relevant elements than water sampled at nearby sites. Additionally, we compared the nutrient concentration in fruits from Costa Rica and Ecuador, predicting that fruits from rain forests in the Ecuadorian Amazon which grow on ancient nutrient-poor soils would be more depleted in essential nutrients than those of the more recent Costa Rican rain forests growing on nutrient-rich volcanic soils.
METHODS
Study sites
We conducted our study between October and December 2008 at the Tiputini Biodiversity Station (TBS, 0°38′S, 76°8′W), located in a lowland Amazonian primary rain forest, Orellana Province, Ecuador. For video observations and sampling of bats, we chose nine mineral licks and 15 control sites in the forest. Control sites were randomly selected at open locations such as trails or gap openings at the TBS. Additionally we collected and analysed elements in fruits that are known to be consumed by bats.
For comparative purposes, we collected fruit samples in 2009 at La Selva (LS) Biological Station near Puerto Viejo de Sarapiqui in Costa Rica (10°26′N, 85°59′W). LS served as a control site in our study, because no mineral licks are known to exist in Central America, even though local bat assemblages include many frugivorous bats, sometimes even the same genera or species as at TBS (Rex et al. Reference REX, KELM, WIESNER, MATT, KUNZ and VOIGT2008).
Video observations at mineral licks
We observed bats at the mineral licks in Ecuador by using a digital video camera (Sony Digital Handycam DCR-H C39E PAL, Sony Europe) with the night-shot function active. For illumination, we used an ECOLINE IR-Illuminator (Security-Center, Germany). At each of the nine licks, we recorded the behaviour of visiting bats from shortly after sunset (c. 18h00) until 20h00–24h00, depending on battery capacity and weather conditions. We performed video observations directly via a handheld camera or as automated observations with a tripod. Focus points of observation were chosen according to one of three criteria: (1) sites were visited by other animals, such as scraping or drinking spots of spider monkey (Ateles belzebuth, É. Geoffroy) or tapir (Tapirus terrestris, Linnaeus); (2) sites with a good overview of the entire mineral lick; and (3) sites with high bat activity based on our netting experience.
Collection of fruit samples
We collected potential bat fruits, from a total set of 32 ripe fruits (Table 1), in the vicinity of the two biological stations LS (n = 15) and TBS (n = 17). All fruits were cut into pieces and dried at 50 °C for about 72 h. All dried samples were kept at ambient temperature in closed ZipLock™–bags until analysis.
Table 1. Fruits consumed by bats and collected at the study sites in Costa Rica (CR; n = 15) and Ecuador (E; n = 17). The number of different individuals that have not been identified to the species level but that contributed as separate species to the analyses is given in parentheses after the genus name.

Collection of bat blood and faeces
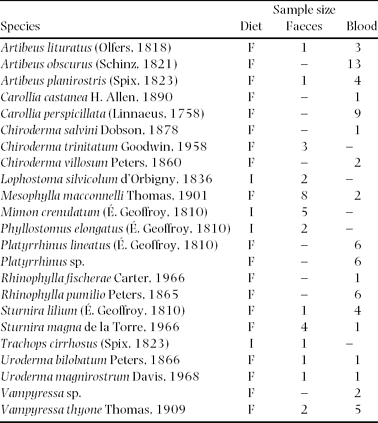
In 31 nights, we captured bats between 18h00 and 21h00 at nine mineral licks and 15 control sites by using ground-level mist nets (length 6–12 m; 70 dernier/2 ply, 36-mm mesh, five shelves; R. Vohwinkel, Velbert, Germany). We identified bats based on their morphology using two field keys (Timm & LaVal Reference TIMM and LAVAL1998, Tirira Reference TIRIRA2007). We held all bats individually in cloth bags for approximately 1 h to collect faeces. We recorded the following data using a standard protocol: species, body mass (accuracy 0.5 g; Pesola spring balance, Baar, Swiss), sex, age (juvenile, subadult, adult), and reproductive status (reproductively active, inactive, lactating, post-lactating) as outlined in Handley et al. (Reference HANDLEY, WILSON and GARDNER1991) and Kunz et al. (Reference KUNZ, ADAMS, HOOD, Kunz and Parson2009). We measured the length of forearm using a vernier caliper (accuracy 0.1 mm; SPI 2000 caliper, West Chester, Pennsylvania, USA). Thirty-one faecal samples were stored singly in Eppendorf tubes filled with 70% ethanol until further analysis (Table 2). We used ethanol blank samples to account for possible impurities or contamination. From each of 68 bats, we took 50–120 μl of blood, depending on body mass of the species by piercing the antebrachial vein (Kunz & Nagy Reference KUNZ, NAGY and Kunz1988) using sterile 23–30-gauge needles (Microlance, Becton Dickison S.A., Spain). All blood samples were dried at 50 °C for approximately 48 h and kept at ambient temperature in closed Eppendorf tubes until analyses. We released all bats at the site of capture after species identification and processing as noted above.
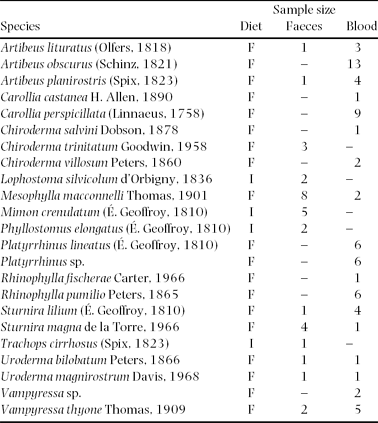
Table 2. Alphabetical list of insectivorous (I) and fruit-eating bats (F) from which faecal and blood samples were collected around Tiputini Biodiversity Station in Ecuador.
Collection of water samples
At TBS we collected 50-ml samples of water from puddles at each of six mineral licks and four control sites, such as rivers, streams and ponds that are potentially accessible for the bats. We sampled unfiltered water from the surface of the puddles in order to evaluate the actual water bats are confronted with at potential drinking sites. This water was stored in a polytetrafluoroethylene (PTFE) vessel mixed with 1 ml of 65 wt. % ultrapure nitric acid for preservation and was kept at ambient temperature until analysis.
Measurement of elemental composition
For elemental analyses in blood, fruit and faeces samples, we dissolved the samples using a total digestion apparatus (Picotrace, Bovenden, Germany; Ruppert Reference RUPPERT1987). A series of 32 solid samples was simultaneously digested in PTFE vessels using a mixture of ultrapure fluoric acid (40%), perchloric acid (70%) and nitric acid (65%) at elevated pressure and temperature (170 °C, all acids cleaned by sub-boiling distillation). During evaporation, acid fumes were removed directly from the samples using clean airflow. Residues of evaporation were dissolved in nitric acid and hydrochloric acid and water at 150 °C. After cooling, the clear solutions were transferred into a 10-ml volumetric flask. The homogenized solutions were stored in polyethylene bottles. During the digestion procedure, we assumed minimal risk of contamination, because ultraclean, sub-boiling distilled acids were used, and because the system was closed during the pressure phase and was sheltered during evaporation. Elemental analyses of the digested samples as well as of the water samples were conducted using inductively coupled plasma emission spectrometry (ICP-OES; Optima 3300 DV, Perkin Elmer) and by inductively coupled plasma mass spectrometry (ICP-MS Elan DRC II; Perkin Elmer SCIEX). The elements Na, potassium (K), magnesium (Mg) and iron (Fe) in faeces and blood samples were selected because they are physiologically relevant nutrients in mammals. Concentrations of Na, K, Mg, Fe, calcium (Ca) and phosphorus (P) from fruit and water samples were also analysed. To evaluate whether bats consume clay at mineral licks, we used the elements aluminium (Al), titanium (Ti), yttrium (Y), cerium (Ce), lanthanum (La) and neodymium (Nd) in bat faeces as markers to detect soil consumption (Calabrese & Stanek Reference CALABRESE and STANEK1995, Calabrese et al. Reference CALABRESE, STANEK, PEKOW and BARNES1997). These elements are insoluble in water and thus are only sparsely contained in plant products, such as fruits. Bats with a strict fruit diet should not come into contact with these elements when only feeding on fruits that grow on trees or tall shrubs.
Statistics
We applied a Mann–Whitney U-test to reveal significant differences in the elemental composition between fruit samples from La Selva (Costa Rica) and TBS (Ecuador), as well as for differences in the elemental composition of water samples from mineral licks and control sites.
We transformed blood-sample data using the log(x+1) function to reduce the effects of numerically dominant categories. Differences in blood composition were tested with samples of two frugivorous-bat groups for both sexes, collected at mineral licks and from open-forest sites, using the statistical software Primer v6 (Primer-236 E 2000 Ltd., Plymouth, UK). We computed the Bray–Curtis similarities for a MDS (multi-dimensional scaling) plot to document evidence of groupings. This plot illustrates the relationship between the two frugivorous groups, by representing the samples as points in two-dimensional space, where the relative dissimilarities of the samples are shown by the relative distances between points. We verified differences in elemental composition of blood by using a two-way crossed analysis of similarity (ANOSIM) permutation test (Clarke & Warwick Reference CLARKE and WARWICK1994).
We applied a Mann–Whitney U-test to reveal significant differences in the soil tracer concentrations between faecal samples of frugivorous bats captured at mineral licks and of co-existing insectivorous bats that have never been recorded to visit mineral licks (Table 2). We used faeces of insectivorous bats that never visit mineral licks as a control group, as we cannot rule out that frugivorous bats captured away from mineral licks might have visited the licks before. This was necessary because the retention time of clay or soil material in the digestive system of an animal is potentially long, as Gilardi et al. (Reference GILARDI, DUFFEY, MUNN and TELL1999) reported for mineral-lick-visiting parrots. We calculated the upper 95% confidence interval of the median (95% CI) of the insectivorous group to establish threshold values that indicated soil consumption by frugivorous species. Since the faecal dataset is not normally distributed we used a non-parametric method to calculate the 95% CI of the median according to Olive (http://www.math.siu.edu/olive/ppmedci.pdf). All individuals with values above the upper 95% CI were considered to have consumed soil. Values within the 95% CI suggest that animals had not consumed significant amounts of soil recently. We set the level of significance at 5% and used two-tailed tests.
RESULTS
Video observations at mineral licks
During 13 nights (total of 48 h of recording), we never observed bats feeding directly on clay at scraping spots of other lick-visiting animals. It was difficult to obtain good overview recordings using the automated video observation, due to the size and illumination of the mineral licks. However, by focusing on smaller areas at the same mineral licks using the handheld camera, we detected and observed many bats drinking water from small depressions (Figure 1).

Figure 1. Frames of a video recording within a mineral lick, showing a bat approaching (from left to right) a puddle (in white margins in frame (1)). Frames (2) – (4) show the snout of the bat moving towards the water surface; see online supplementary material for full video.
Elemental composition of fruits
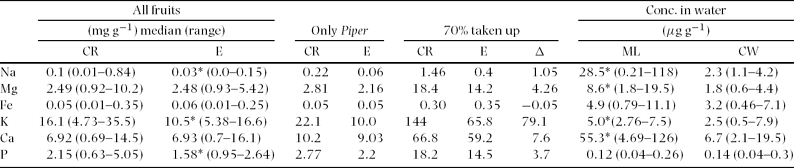
Fruits from Ecuador showed significantly lower concentrations of P, K and Na than fruits from Costa Rica (Mann–Whitney U-test, n = 32; phosphorus: U = 64; P = 0.016; potassium: U = 72; P = 0.037; sodium: U = 56; P = 0.007; Table 3).
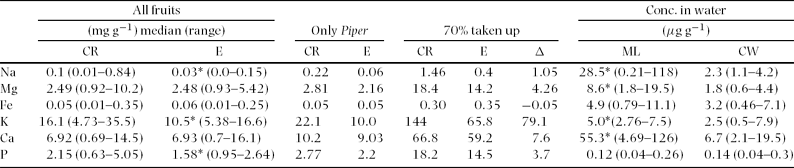
Table 3. Median mineral nutrient concentrations (mg g−1 of dry matter) of fruits collected from Costa Rica (n = 18; CR) and Ecuador (n = 20; E); (Mann–Whitney U-test, P: U = 64; P = 0.016; K: U = 72; P = 0.037; Na: U = 56; P = 0.007; * P < 0.05). The mineral concentration of Piper sp. infructescences was used to calculate the daily mineral shortfall of Carollia bats when consuming exclusively Piper infructescences. We estimated a daily fruit consumption of ~24g for C. brevicauda and assumed that 70% of consumed element amounts were taken up by the bat (70% taken up). Additionally, we calculated the difference in element content (Δ) between diets of Piper spp. fruits in Costa Rica and Ecuador as well as the average element concentration (μg g−1) of lick water (ML; n = 6 mineral licks) and control water sites (CW; n = 4 control sites) in Ecuador (Mann–Whitney U-test, n = 10, Na: U = 64; P < 0.001; Mg: U = 32; P < 0.001; K: U = 96; P = 0.007; Ca: U = 32; P < 0.001; * P < 0.05).
Elemental blood composition
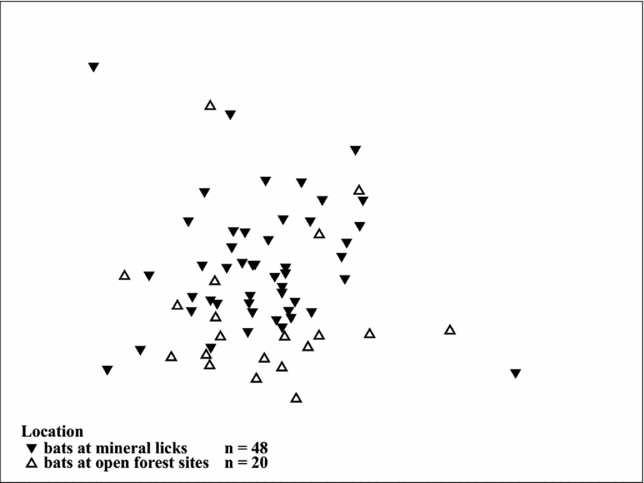
The two-way crossed ANOSIM permutation test revealed a significant global R value of 0.188 (n = 68; P = 0.025) for the two locations, suggesting significant differences in elemental composition of blood between the two groups. Of the 9999 permutations in our model, only 245 were equal to or greater than the global R, which indicates a high within-group similarity. The tests for the differences between sexes did not reveal a significant difference (Global R = 0.032; P = 0.246). Of the 9999 permutations, 2463 were equal to or greater than the global R. The Bray–Curtis dissimilarity matrix among all pairs of objects was used to construct a MDS plot for the two locations (Figure 2), which suggests groupings according to capture site (two-dimensional stress level = 0.19).
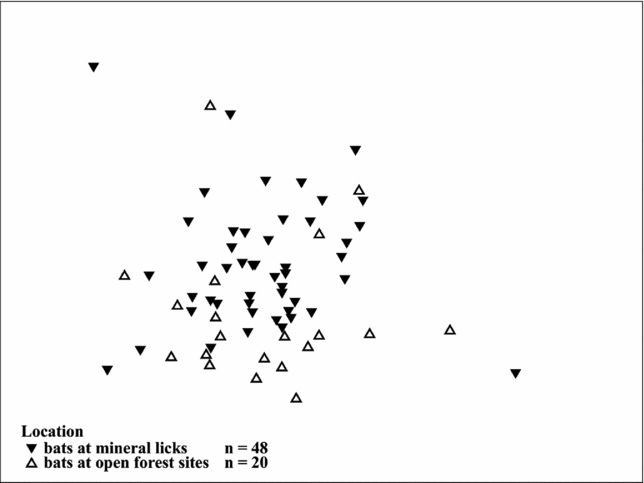
Figure 2. Two-dimensional MDS plots of soil tracer elements in blood of bats captured at mineral licks (filled triangles, n = 48) and nearby forest sites (open triangles, n = 20). The similarity matrix was computed using the Bray–Curtis Similarity Index (2D stress 0.19). The more similar the elemental compositions of bats, the closer the symbols appear on the plot.
Elemental composition of water
Water collected from puddles at mineral licks showed significantly higher concentrations of Na, Mg, K and Ca when compared with the concentrations of the control sites (Mann–Whitney U-test, n = 10, sodium: U = 64; P<0.001; magnesium: U = 32; P <0.001; potassium: U = 96; P = 0.007; calcium: U = 32; P < 0.001; Table 3). The concentrations of Fe and P did not differ significantly (Mann–Whitney U-test, n = 10, iron: U = 132; P = 0.1; phosphorus: U = 93; P = 0.404; Table 3).
Soil markers in faeces
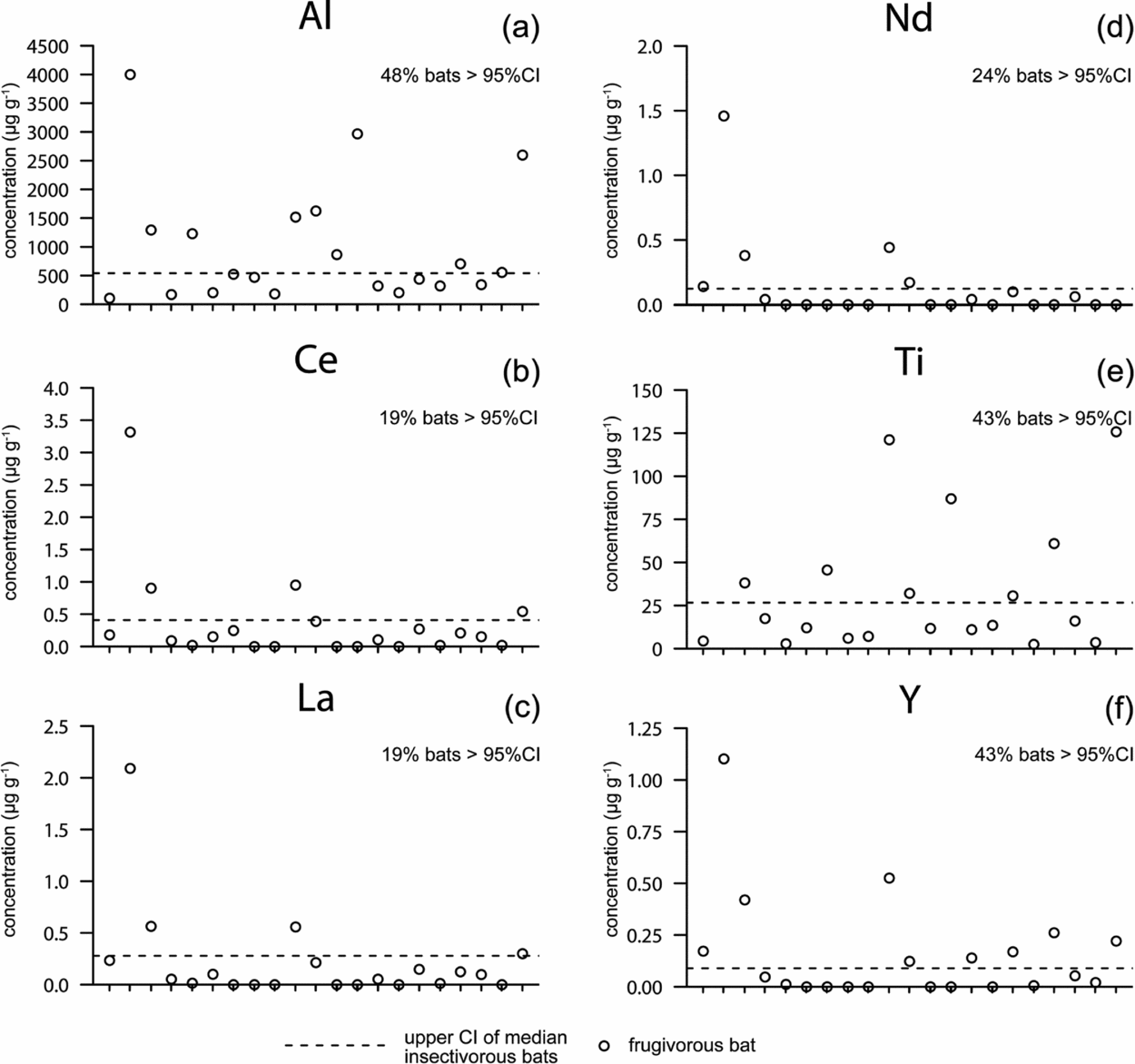
The calculated threshold (upper 95% CI of the median of insectivorous group) for soil consumption revealed that 19–48% of the frugivorous species exhibited soil-tracer concentrations in faeces that were higher than those of insectivorous species (Figure 3). Faeces of frugivorous species that visit mineral licks were significantly more enriched in the soil tracer aluminium than the droppings of insectivorous bats that do not visit mineral licks (Mann–Whitney U-test; n = 31, U = 40; P = 0.032).

Figure 3. Soil tracer concentrations (μg g−1 of dry matter) of the soil tracer elements Al = aluminium (a), Ce = cerium (b), La = lanthanum (c), Nd = neodymium (d), Ti = titanium (e), and Y = yttrium (f), in droppings of frugivorous bats (every tick on the x-axis is a sample, n = 21) captured at mineral licks in eastern Ecuador. The upper 95% confidence interval of the median (95% CI) of soil tracer concentrations in the faeces of insectivorous bats (n = 10) served as a baseline soil tracer concentration for bats not consuming soil. We considered frugivorous bats as soil consumers, when tracer concentrations in their faecal material were above the upper 95% CI of soil tracer concentrations in the faeces of insectivorous bats (see given percentages).
DISCUSSION
We tested the hypothesis that frugivorous bats ingest water for nutrient supplementation or soil material for detoxification at mineral licks of the Amazon rain forest. Similar to previous studies, we captured mostly reproductively active female frugivorous species visiting mineral licks (Bravo et al. Reference BRAVO, HARMS, STEVENS and EMMONS2008, Reference BRAVO, HARMS and EMMONS2010; Tuttle Reference TUTTLE1974, Voigt et al. Reference VOIGT, DECHMANN, BENDER, RINEHART, MICHENER and KUNZ2007, Reference VOIGT, CAPPS, DECHMANN, MICHENER and KUNZ2008). Although, we were not able to document large numbers of drinking bats with our automated camera setup, our video observations with hand-held cameras demonstrate unambiguously that bats drank water at mineral licks in the lowland Ecuadorian Amazon. We suggest that this water uptake is most likely unrelated to the daily water requirements, because frugivorous species usually consume large amounts of water when ingesting fruits (Morrison Reference MORRISON1980, Ruby et al. Reference RUBY, NATHAN, BALASINGH and KUNZ2000). The high water content of fruits is presumably sufficient for lactating bats when facing elevated water demands during milk production. Instead, frugivorous bats are probably attracted to the water-filled depressions at mineral licks, because the water is more enriched in nutrients such as sodium than water from local creeks, ponds or rivers. In support of the nutrient supplementation hypothesis, we found that the composition of physiologically relevant elements (Na, Mg, K and Fe) differed between blood collected from frugivorous bats at mineral licks and blood collected from bats at open-forest sites. Still, one has to be careful interpreting the results of the blood elemental comparison. Because bats are highly mobile, our study design allows that individuals captured far away from mineral licks may have visited mineral licks as well. Further, the calculated ANOSIM might be sensitive to the varying sample sizes of species. Not controlling for this variation might compromise our data by pseudoreplication and bias the results towards those species with large sample size, for example Artibeus obscurus. However, none of the species with large sample sizes showed extreme values and therefore, we consider our analysis as robust against such biases. Nevertheless, we have been able to document significant differences in the elemental blood composition between bats from the two locations. Based on these differences, we infer that traces of the nutrient-enriched water consumed by bats at mineral licks can be found in their blood samples, even though elemental composition of blood is probably regulated within narrow ranges. This finding is interesting given that extreme deviations of elemental concentrations from the range of physiological acceptable values may be detrimental or even fatal to mammals. Thus, our finding may provide further support for the hypothesis that bats take up nutrients at mineral licks by drinking water from small puddles. A key question related to this is whether fruits from plants growing in the Amazonian ecosystem provide sufficient quantities of essential mineral nutrients to meet the daily requirements of frugivorous bats during reproduction. Bravo et al. (Reference BRAVO, HARMS and EMMONS2010) reported that figs in Peru include less sodium than fruits of other tropical regions. Other studies in Peru showed that parrots with diets extremely low in sodium often supplement their diets by eating soils with high concentrations of sodium, most likely to meet their daily nutrient demands (Brightsmith & Muñoz-Najar Reference BRIGHTSMITH and MUÑOZ-NAJAR2004, Gilardi et al. Reference GILARDI, DUFFEY, MUNN and TELL1999). In the present study, we showed that nutrient concentrations of fruits collected from Ecuadorian rain forests had less sodium, potassium and phosphorus than fruits from Costa Rican rain forests. Although we may introduce a bias with dry-weight comparisons or a limited sample size, our findings are in accordance with the data of the Peruvian studies that suggest that the western Amazon region is generally depleted in mineral nutrients. This can be attributed to the unique geographical features of the western lowland Amazon. This region is essentially beyond coastal climate and thus marine input of sodium, because of its isolation from the Pacific Ocean by the Andes to the west, and the Atlantic Ocean to the east (Kaspari et al. Reference KASPARI, YANOVIAK and DUDLEY2008). Moreover, the soils of the Amazonian rain forest are ancient and have degraded and leached over millions of years (Terborgh Reference TERBORGH1992). A recent study by Dudley et al. (Reference DUDLEY, KASPARI and YANOVIAK2012) proposed a cross-taxonomic need for sodium especially in the Western Amazon region, where mineral licks are used to minimize the nutritional deficit. Thus, low concentrations of nutrients in plants and their associated fruits may be caused by the nutrient depletion of Amazonian soils (Jordan & Herrera Reference JORDAN and HERRERA1981, Stark Reference STARK1970). Mineral licks and mineral supplementation may become superfluous in areas with overall higher levels of mineral nutrients in their soils, such as in Costa Rica.
Based on nutrient data from the infructescences of Piper collected in Ecuador and Costa Rica, and the measured rates of water flux in Carollia brevicauda, a frugivorous bat species and Piper specialist (Voigt et al. Reference VOIGT, KELM and VISSER2006), we estimated the nutritional deficit in this species when consuming only Ecuadorian fruits. For the daily water flux of 30 ml d−1 for C. brevicauda (Voigt et al. Reference VOIGT, KELM and VISSER2006), we assumed that about 20% of this water flux originates from the oxidation of fat as metabolic water. Thus, C. brevicauda must consume approximately 24 ml d−1 of water from fruits to maintain its daily water balance. Given that fruits are comprised of about 70% water, we estimated that this fruit-eating species handles approximately 31 g of fruits each day. As a conservative approach, we assumed that a bat takes up about 70% of the nutrients of the consumed fruits. Thus, C. brevicauda in Ecuador would be expected to consume about 1 mg sodium, 8 mg calcium and 4 mg magnesium less than a respective Costa Rican individual on a daily basis. For sodium the daily minimum requirements of small mammals in their most demanding life periods have been reported to be 600 ppm on a dry matter basis (Dempsey Reference DEMPSEY2004). The estimated sodium values for Ecuadorian C. brevicauda when consuming exclusively Piper infructescences lay below these minimum requirements. However, our nutrient analysis of water from mineral licks suggests that a daily consumption of 1–2 ml of mineral-lick water may be sufficient to compensate for this deficit depending on the bioavailability of the dissolved nutrients.
A notable problem in most studies that have measured the composition of tropical fruits is that they focused on total availability of nutrients in fruits rather than on the bioavailability of mineral elements. Fruits that contain large amounts of secondary compounds such as phytate or oxalate can limit the bioavailability of nutrients (Kamchan et al. Reference KAMCHAN, PUWASTIEN, SIRICHAKWAL and KONGKACHUICHAI2004). One such example can be observed in figs, a food item largely preferred by the genus Artibeus. While figs are known to be rich in total calcium (Wendeln et al. Reference WENDELN, RUNKLE and KALKO2000), they often contain high levels of oxalate that not only bind the calcium in the fruit (Kamchan et al. Reference KAMCHAN, PUWASTIEN, SIRICHAKWAL and KONGKACHUICHAI2004), but may also reduce the amount of calcium available to the animal when it is consumed. Thus, many fig-eating bats may actually lack essential nutrients in their diet.
In addition to a higher demand for nutrients, reproductively active bats also have higher energy demands (Korine et al. Reference KORINE, SPEAKMAN and ARAD2004, Racey & Speakman Reference RACEY and SPEAKMAN1987, Voigt Reference VOIGT2003). Thus, an increase in fruit consumption in response to these demands should lead to an increase in secondary metabolites absorbed. Many of these compounds negatively affect their consumers, because they can be carcinogenic, teratogenic, toxic, or they may simply bind to elements as do phytate or oxalate (Heiser Reference HEISER1969, Kamchan et al. Reference KAMCHAN, PUWASTIEN, SIRICHAKWAL and KONGKACHUICHAI2004).
The elemental analysis of bat droppings in our study revealed higher concentrations of soil tracers in faeces collected from frugivorous species compared with our calculated threshold for soil consumption (95% CI of the median). As this threshold is based on insectivorous bats that were never observed at mineral licks, higher concentrations of soil tracers provide clear evidence for geophagy in frugivorous bats of the lowland Amazonian rain forest.
The soil tracer aluminium is a major structural component of clay minerals such as kaolinites or smectites (Mahaney & Krishnamani Reference MAHANEY and KRISHNAMANI2003). A higher enrichment of this element in faeces strongly suggests that fruit-eating bats consume clay minerals or other soil fractions while they are visiting water puddles at mineral licks. In a wide range of studies on birds and mammals, the consumption of clay minerals was linked to detoxification of plant secondary compounds. Brightsmith et al. (Reference BRIGHTSMITH, TAYLOR and PHILLIPS2008) and Gilardi et al. (Reference GILARDI, DUFFEY, MUNN and TELL1999) showed that clay minerals such as kaolinite buffer remarkable amounts of the toxin quinine, which is similar in structure to other compounds that occur in the diets of frugivorous parrots. Given these observations, it seems quite likely that bats could also benefit from the buffering and thus detoxifying effects of consumed clay minerals.
Our findings provide direct evidence that frugivorous bats obtain both essential mineral nutrients and soil material at mineral licks by drinking water from these sites. Two reasons may be causal for the drinking behaviour of female frugivorous bats during reproduction, apart from a demand for water: (1) female frugivorous bats may require more essential elements during reproduction than their diet of fruit can supply, and (2) possible exposure to higher levels of plant secondary metabolites may require the use of clay minerals as a detoxifying buffer. Although our correlative study design may allow other explanations, we suggest that in a mineral-depleted area, such as Western Amazonia, the drinking behaviour of frugivorous bats at mineral licks serves a dual function, namely nutrient supplementation and detoxification of plant secondary compounds in their diet. Our findings may help as a basis for future captive studies to quantify mineral and toxic levels of diets and to disclose their physiological consequences for bats in the wild such as mineral deficiency or intoxication.
SUPPLEMENTARY MATERIAL
For supplementary material for this article, please visit http://dx.doi.org/10.1017/S0266467412000740.
ACKNOWLEDGEMENTS
We wish to thank the administrative staff at the Tiputini Biodiversity Field Station and the La Selva Biological Station, as well as the governmental authorities of Ecuador and Costa Rica for granting permission to work. We also thank Lena John, Jaime Guerra for field assistance and Ursula Grunewald and Irina Ottenbacher who kindly did the sample preparation and the digestion for the geochemical analysis. All protocols were approved by the Animal Care and Use Committee from the Leibniz Institute for Zoo and Wildlife Research and from Boston University. This study was supported by a grant from the German National Council to CCV (Vo 890/15), and from Boston University's Center for Ecology and Conservation Biology.