Published online by Cambridge University Press: 06 May 2004
Sexual transmission occurs commonly in microparasites such as viruses and bacteria, but this is an unusual transmission route for macroparasites. Here we present evidence which suggests that a nematode parasite of Wood Mice (Apodemus sylvaticus) may be sexually transmitted and we have classified the nematode using molecular data. Wood Mice were collected annually in the course of work on their reproductive physiology. Larval nematodes were found in the epididymides of 19·6% of males. It seems likely that they would be transmitted to females at ejaculation. To identify these larval nematodes, which we were unable to do using morphological features, we sequenced the 18S rDNA. Sequence comparisons with the molecular phylogeny of Blaxter et al. (1998) demonstrated that they were bursate nematodes (Order Strongylida). The relationships between strongylid taxa were poorly resolved by 18S rDNA. However, both distance and parsimony analyses grouped the nematode with the superfamily Metastrongylidea in a clade containing Filaroides and Angiostrongylus sp. Importantly, the sequences were distinct from those of Heligmosomoides polygyrus and Angiostrongylus dujardini, two common strongylid nematodes of Apodemus. We were therefore unable positively to identify these worms by matching their sequences with those from morphologically identifiable adult strongylid nematodes infecting Apodemus. These results demonstrate that an as yet unidentified strongylid is quite commonly found in large numbers in the male reproductive tract of Wood Mice. Further work is required to understand the biology and transmission dynamics of this interesting system.
Sexual transmission of nematodes appears to be rare in animals. In a recent review of sexually transmitted diseases, microparasites such as viruses, fungi, bacteria, spirochaetes and protozoa accounted for 87% of the 149 cases of well-established sexual transmission, while macroparasites (helminthes, nematodes, arthropods) were reported to be sexually transmitted in just 13% (Lockhart, Thrall & Antonovics, 1996). Of the 18 nematode examples recorded, 16 were from invertebrates while 2 involved duck or snake hosts. There are two reports of sexual transmission of macroscopic parasites in mammals. Both of these involved quite unusual situations: sexual transmission of Strongyloides sp. via anal sex in homosexual men (Sorvillo et al. 1983), and transmission of schistosomiasis by individuals with schistosomal granulomas in the genital region (Attili, Hira & Dube, 1983).
Here we describe the occurrence of large numbers of nematodes in the epididymis of Wood Mice (Apodemus sylvaticus) from Oxfordshire, and we identify the nematode order to which they belong, using molecular methods. Their discovery was largely fortuitous. In the course of an investigation of the breeding season of Wood Mice, animals were live-trapped in Wytham Wood, Oxfordshire. The immediate objective was to find out whether or not spermatozoa were being produced. The simplest way of establishing this was to tease apart the epididymides in physiological saline to see whether spermatozoa were present. One of us (J.R.C.) was astonished to find nematodes amongst the sperm and within the lumen of the epididymis of the first Wood Mouse to be examined.
Wood Mice were caught with Longworth traps by the method of Chitty & Kempson (1949) in Wytham Wood, Oxfordshire, in early Spring 1983–1985 and 1998. The animals were killed in the laboratory by cervical dislocation. The head, body and tail of one epididymis were separately teased apart in physiological saline, the preparations examined under a dissecting microscope at magnifications of ×60, ×120 and ×250, and the presence or absence of nematodes recorded. The occurrence of sperm was scored on a scale of 0 to 5 (0, none; 1, very few; 2, a few; 3, moderate number; 4, abundant; 5, very abundant). Corpses were preserved in Bouin's fluid. Testes and seminal vesicles were later carefully removed and weighed on a torsion balance. Their weight, and the sperm score, provide a guide to the sexual maturity of the Wood Mice. Serial sections (7 μm) of the remaining epididymides and the testes were made by standard paraffin wax methods. They were stained with Ehrlich's haematoxylin and eosin, and, at a magnification of ×150, the number of nematodes in the head, body and tail of these epididymides recorded. The testes sections were also searched for nematodes.
Using a dissecting microscope, individual nematodes, collected with Eppendorf pipettes from the teased, saline preparations of the epididymis, were placed in 0·5 ml Eppendorf tubes, and washed twice with sterile physiological saline to remove contaminating tissues and sperm. Nematodes were digested for 2 h by adding 10 μl of sterile 10 mM Tris (pH 7·8) and 200 μg proteinase K at 55 °C, and 2 μl of digested material was used in each PCR reaction. Serge Morand (Université de Perpignan, France) and Carlos Feliu (Universidad de Barcelona, Spain), kindly provided us, for comparative purposes, with alcohol-preserved adult specimens of two common strongylid nematodes of Wood Mice, Angiostrongylus dujardini (Order Strongylida, Superfamily Megastrongyloidea), found in the heart of Apodemus sylvaticus (Drozdz & Doby, 1970; Asakawa & Tenora, 1996) and Heligmosomoides polygyrus (Order Strongylida, Superfamily Trichostrongyloidea), a common gut-dwelling nematode of Wood Mice (Elton et al. 1931). DNA was also prepared from the alcohol-preserved specimens of A. dujardini and H. polygyrus. Individual worms were digested for 4 h at 55 °C in 100 μl of sterile 10 mM Tris (pH 7·8) containing 2 μg/l of proteinase K, extracted twice with phenol, once with chloroform, and then ethanol-precipitated with an acrylamide carrier (Gaillard & Strauss, 1990). DNA was re-suspended in 20 μl of sterile TE (pH 8·0).
The primers used for amplifying and sequencing 18S rDNA are shown in Table 1. Primers SSU-F2 and SSU-R2 were designed to be invertebrate specific. These primers had mismatches with the Wood Mouse rDNA sequence to avoid amplification of contaminating Wood Mouse tissue. A. dujardini and H. polygyrus rDNA was amplified in a single reaction using primers SSU-F1 and SSU-R2, and sequenced using internal primers. PCR cycling conditions were as follows: 94 °C, 3 min; 35×94 °C, 1 min; 50 °C, 1 min; 72 °C, 3 min; 72 ° C, 3 min with the Mg2+ concentration at 3·0 mM. The nematode DNA was amplified and sequenced in 2 pieces using semi-nested PCR. In the first round of PCR we used primers SSU-F1 and SSU-R2. PCR cycling conditions were as described above. Then 1 μl of product from this reaction was used as template for the secondary reactions. We amplified 2 overlapping smaller fragments using primers SSU-F1/SSU-D-R and SSU-F2/SSU-R2. PCR cycling conditions were as above but only 30 cycles were used, and the Mg2+ concentration was 2·5 mM. The amplified fragments were cleaned using spin columns (Qiaquick spin columns, Qiagen) and sequenced in both directions using the BigDye cycle sequencing kit (PE Biosystems).
Table 1. Oligos used for amplification and sequencing of nematode 18S RNA (Degenerate bases are marked using IUPAC-IUB ambiguity codes.)

Sequences obtained were aligned by eye using the alignment of Blaxter et al. (1998) as a template. The alignments used are available from one of us (T.J.C.A.) on request. We used PAUP* 4.0 for all analyses, and conducted both parsimony and distance-based analyses.
Differences between means for body and organ weights, and sperm score, of Wood Mice were examined by the t-test. Data for numbers of worms in the 3 parts of the epididymis were examined by analysis of variance. Some data had unequal variances, or were not normally distributed. In these cases measurements were transformed into their logarithms for the purpose of statistical analysis.
Forty-six Wood Mice were caught and examined. Nine had nematodes in the epididymides, giving a prevalence of 19·6%. Data on the infected and uninfected Wood Mice, given in Table 2, indicate that the animals were overwintered adults reaching sexual maturity (Flowerdew, Gurnell & Gipps, 1985). There are no statistically significant differences in body, testes and seminal vesicle weights, nor in sperm score, between infected and uninfected Wood Mice. The nematode is shown in Fig. 1. In the sections of the epididymides the worms were about 450 μm long and 20 μm wide. In the living state, they wriggled vigorously, making some forward (or backward) progression. With these simple, microscopic preparations, little can be made out of their anatomy, except for the presence of the pharynx, the rest of the alimentary canal, and paired amphids (Fig. 1). In those cases where nematodes were immediately juxtaposed to the epididymal epithelium, there was an indentation of the epithelial surface (Fig. 1B). It was not possible, in these preparations, to determine whether spermatozoa had been ingested by these nematodes. While the prevalence of infection was 19·6% the intensity of infection, based on counts of nematodes in one epididymis from each animal, was from 1 to 582. The nematodes occur in all parts of the epididymis (Table 3). The mean numbers for the head, body and tail of the epididymis are not significantly different. None were observed in the sections of testes.

Fig. 1. Transverse sections of the epididymis of the Wood Mouse. (A) A larval nematode is present in the epididymal duct, together with numerous sperm; (B) Anterior end of nematode adjacent to an indentation of the epididymal epithelium. a, Amphid; e, epididymal tubule; n, nematode; s, sperm in the epididymis.
Table 2. Mean±S.E.M. of body and organ weights, and sperm score, for Wood Mice infected (n=9) or not infected (n=37) with larval nematodes (Larvae were counted in serial sections of one epididymis from each animal.)


The rDNA sequence obtained from the nematodes was 1638 bp. (GenBank Accession no. AY542281.) Fig. 2 shows their position in the phylogenetic framework generated by Blaxter et al. (1998). They are clustered with the strongylids. This grouping has 100% bootstrap support in both parsimony and Neighbour-joining trees. We conducted a further phylogenetic analysis of strongylid nematodes by including 24 additional sequences. In addition to the 4 strongylid taxa analysed by Blaxter et al. (1998), which comprised 3 species in the Superfamily Trichostrongyloidea, and 1 species, Syngamus trachea, classified in the Superfamily Strongyloidea, we included 3 nematodes in the Superfamily Trichostrongyloidea: Nematodirus battus (GenBank Accession no. U01230), Haemonchus placei (GenBank Accession no. L04154) and Heligmosomoides polygyrus (GenBank Accession no. AY542283, this study) and 17 species in the superfamily Metastrongyloidea. These included 14 sequences recently deposited in Genbank (Carreno & Nadler, 2003; GenBank Accession no AY295804–AY295807, AY295809, AY295810, AY295812, AY295815–AY295820) as well as Ostostrongylus sp. (Genbank Accession no. U81589), Parafilaroides sp. (GenBank Accession no. U81590) and Angiostrongylus dujardini (GenBank Accession no. AY542282, this study). In addition, we included 2 species of Dictyocaulus, a strongylid genus of uncertain affiliation (Hoglund et al. 2003) (GenBank Accession nos AY168856 and AY168861), and Necator americanus (Superfamily: Ancylostomatoidea) (GenBank Accession no. AY295811). H. polygyrus and A. dujardini, common strongylid nematodes of Apodemus sylvaticus, were included to try to match sequences from morphologically identifiable adult parasites with the unidentified nematodes we had found. The total alignment included a 1155 bp of which 77 sites were parsimony informative.

Fig. 2. Maximum parsimony tree showing the position of parasites from Apodemus sylvaticus in the nematode phylogeny. The tree was generated using a heuristic search, with TBR branch swapping. Bootstrap values (500 replicates) are shown only for the strongylid clade. The arrow shows the phylogenetic placing of the nematode from Apodemus, while the shaded box shows the position of the strongylid clade. Neighbour-joining trees also gave 100% bootstrap support for placing the Apodemus nematode in the Order Strongylida.
Both Neighbour-joining and parsimony trees are imperfectly resolved with quite low bootstrap values for many clades (Fig. 3A,B). However, a number of consistent features emerge that allow us to make tentative conclusions about the position of this nematode from Apodemus. (1) Both Neighbour-joining and parsimony trees indicate that Trichostrongyloidea, Strongyloidea and Ancylostomoidea are a monophyletic group, and that the present nematode does not belong to these superfamilies. (2) Both analyses show the nematode from Apodemus nested among the Metastrongylid taxa, suggesting affiliation with this superfamily. (3) Both analyses show the Apodemus nematode clusters in a clade with the 2 Angiostrongylus spp. and Filaroides martis, albeit with low bootstrap support. (4) All analyses show that the nematode is unrelated to H. polygyrus and distinct but quite close to Angiostrongylus dujardini, both strongylids occurring in Apodemus. We are therefore unable positively to identify this nematode by comparison with sequences from adults of known strongylid parasites infecting Apodemus.
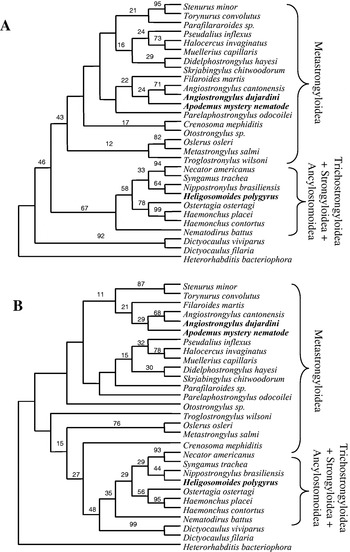
Fig. 3. Neighbour-joining (A) and parsimony (B) cladograms of strongylid taxa based on 18S rDNA sequences. Neighbour-joining trees were constructed using Jukes-Cantor corrected distance estimates. The trees were rooted using the rDNA sequence of Heterorhabditis bacteriophora. The parsimony trees were generated by excluding gaps, using the branch-and-bound option. Bootstrap values (500 replicates) are shown.
We have shown that larval nematode infections of the male reproductive tract of the Wood Mouse A. sylvaticus in Wytham Wood, Oxfordshire have a prevalence of 19·6%. These worms are highly overdispersed, numbers per host varying from one to many hundreds in sections of the single epididymis from each animal in which they were counted. We could not positively match their rDNA sequences with those from common adult nematodes infecting A. sylvaticus. However, the rDNA sequence data indicate that they are strongylids and provide some support for the idea that they fall within the Superfamily Metastrongyloidea.
The discovery of an unknown species of nematode in an extremely well-studied mammal is quite surprising. The parasites infecting Wood Mice have been extensively investigated (Elton et al. 1931; Lewis, 1968, 1987; Gregory, 1991, 1992; Gregory, Keymer & Clarke, 1990; Gregory, Montgomery & Montgomery, 1992; Quinnell, 1992; Brown et al. 1994a,b; Asakawa & Tenora, 1996), but nematodes have not previously been noticed in the reproductive tract of Apodemus. Lewis (1968) included an examination of the epididymis of Wood Mice for the presence of sperm, but did not record the occurrence of nematodes. However, with the prevalence of those we have found of 19·6% it would be easy for his Wood Mouse samples not to have included animals with this nematode.
The occurrence of helminths in the epididymis immediately raises the question of their life-history. What might be the route by which these worms have arrived in the epididymis, and what route do they take to enable the life-history to be completed? We discuss some possibilities below (see also Fig. 4).

Fig. 4. Some possible nematode life-histories involving a larval stage in the Wood Mouse epididymis. Explanatory notes are shown adjacent to each diagram.
Venereal transmission at once suggests itself. This would tend to occur seasonally since Wood Mice are fertile in the spring and summer and less fertile or infertile in the autumn and winter (Baker, 1930). An unknown adult parasite in A. sylvaticus could produce larvae which migrate to the male reproductive tract, and are then transmitted to females during copulation and ejaculation (Fig. 4A). Our phylogenetic analyses suggest that these nematodes belong to the Superfamily Metastrongyloidea. Metastrongylids are frequently ovoviviparous so a life-cycle of this form might be one possibility. However, the rDNA sequences of the present nematodes do not match those of Angiostrongylus dujardin, an ovoviviparous metastrongylid dwelling in the heart of Wood Mice (Drozdz & Doby, 1970). Furthermore, no other adult metastrongylids have been recorded in A. sylvaticus, despite extensive field surveys (see Askawa & Tenora, 1996).
Venereal transmission could also be effected in another fashion as a supplementary route (Fig. 4B). Free-living forms of these nematodes could enter a new male host by the oral or percutaneous routes, used by many species of strongyles. They might then migrate to the reproductive tract for sexual transmission. Once in the female reproductive tract, the nematodes might undergo tissue migration to the gut where they could mature, produce eggs or larvae which pass out with the faeces to become free-living stages, followed by further such cycles. This hypothetical transmission route would be similar to that used by a variety of other nematode species (e.g. Toxocara spp. in dogs, Protostrongylus sp. in bighorn sheep) which, following infection, migrate as eggs or larvae to the reproductive tract and, crossing the placenta, pass into the developing embyos or fetuses (Douglas & Baker, 1959; Forrester & Senger, 1964). In this case, Wood Mouse transmission would be venereal through the male host, rather than from mother to offspring through the placenta.
Sexually transmitted disease in animals has been comprehensively reviewed by Lockhart et al. (1996). The majority of examples of venereal transmitted helminths are found in invertebrate hosts. A well-studied example is provided by Morand and colleagues who have described the occurrence of Nemhelix bakeri (Cosmocercidae) in the male genital tract and uterus of the snail Helix aspersa, and Agfa flexilis in the genital system of the slug Limax cinereoniger (Morand & Petter, 1986; Morand & Hommay, 1990). The location of these nematodes perhaps provides circumstantial evidence for the occurrence of vertical transmission. It is notable that a transverse section of the seminal receptacle of Helix aspersa showing a nematode amongst the host's sperm (Morand, 1988) is extraordinarily like what we have found in A. sylvaticus. The apparent rarity of sexual transmission among nematode parasites is peculiar. This transmission strategy would seem less wasteful than the predominant faecal or oral transmission routes in which only a tiny percentage of eggs produced ever come into contact with a new host.
There are other adaptive interpretations of the existence of the larval nematodes in the male reproductive tract that do not primarily involve venereal transmission. Rather little is known about the innate defence mechanisms of the male reproductive tract (Li et al. 2001). However, there is a blood-epididymis barrier (Hoffer & Hinton, 1984) suggesting that pathogens in the epididymis may be at least partially sheltered from immune attack. Larval nematodes may remain in this reproductive system to evade immunological rejection rather than as an adaptation for sexual transmission. One possibility is that A. sylvaticus may be a paratenic host for these nematodes, with a predator of Wood Mice (for example owls, kestrels, stoats, weasels or foxes) as the definitive hosts. Evasion of the immune reaction by migration to the reproductive tract could extend the longevity of these larval stages in the paratenic host (Fig. 4C).
The hypothetical life-cycles discussed above are all adaptive, with obvious transmission benefits. It is also possible that the occurrence of larval nematodes in the epididymis has no adaptive explanation. For example, it is quite conceivable that Wood Mice are not the natural hosts of these larvae and represent a dead end (Fig. 4D). In this case the larvae may have migrated to the epididymis because the physiological cues determining migration in the natural host differ from those in Wood Mice. A parallel situation occurs in the tapeworm, Taenia solium, when people rather than the natural pig intermediate host ingest T. solium eggs. This results in cysticercosis due to migration of cysticercoid larvae to the brain. This migration causes pathological changes, but being without any obvious transmission benefits, is a dead end for the larvae.
Regardless of which of these explanations is closest to the truth, larval nematodes in the epididymis will inevitably be ejaculated into the female Wood Mice during copulation. It is not known if these nematodes are subsequently established in the females. If other life-cycle stages of this nematode can be identified, then controlled laboratory experiments could be conducted to investigate whether venereal transfer of these larvae is an effective mode of transmission. The molecular classification presented here provides an essential step towards further investigations of the life-history of this nematode.
We are indebted to the late Dr Anne Keymer for encouragement at an early stage of this work. Some of it was carried out while John Clarke held a Leverhulme Emeritus Research Fellowship.

Table 1. Oligos used for amplification and sequencing of nematode 18S RNA

Fig. 1. Transverse sections of the epididymis of the Wood Mouse. (A) A larval nematode is present in the epididymal duct, together with numerous sperm; (B) Anterior end of nematode adjacent to an indentation of the epididymal epithelium. a, Amphid; e, epididymal tubule; n, nematode; s, sperm in the epididymis.

Table 2. Mean±S.E.M. of body and organ weights, and sperm score, for Wood Mice infected (n=9) or not infected (n=37) with larval nematodes

Table 3. Numbers of larval nematodes (mean±S.E.M.) in 3 parts of one epididymis taken from each of 9 Wood Mice

Fig. 2. Maximum parsimony tree showing the position of parasites from Apodemus sylvaticus in the nematode phylogeny. The tree was generated using a heuristic search, with TBR branch swapping. Bootstrap values (500 replicates) are shown only for the strongylid clade. The arrow shows the phylogenetic placing of the nematode from Apodemus, while the shaded box shows the position of the strongylid clade. Neighbour-joining trees also gave 100% bootstrap support for placing the Apodemus nematode in the Order Strongylida.

Fig. 3. Neighbour-joining (A) and parsimony (B) cladograms of strongylid taxa based on 18S rDNA sequences. Neighbour-joining trees were constructed using Jukes-Cantor corrected distance estimates. The trees were rooted using the rDNA sequence of Heterorhabditis bacteriophora. The parsimony trees were generated by excluding gaps, using the branch-and-bound option. Bootstrap values (500 replicates) are shown.

Fig. 4. Some possible nematode life-histories involving a larval stage in the Wood Mouse epididymis. Explanatory notes are shown adjacent to each diagram.